Abstract
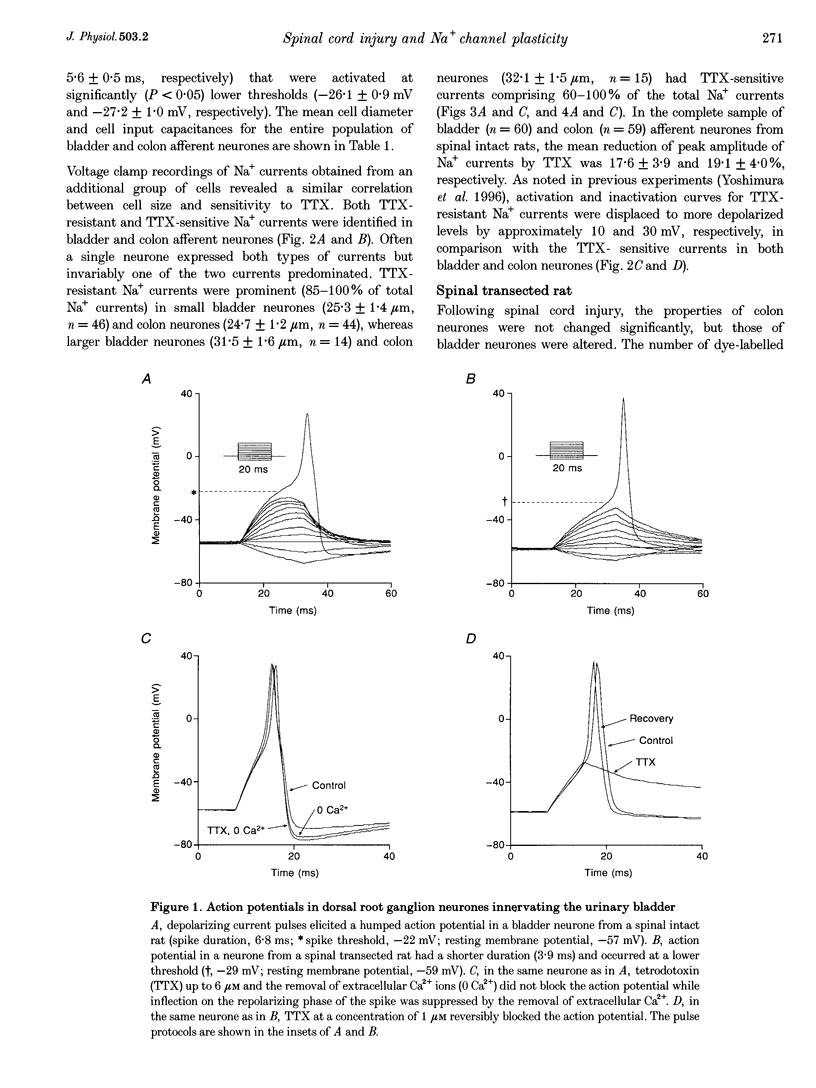
1. Whole-cell patch-clamp recordings in combination with axonal tracing techniques were used to investigate the effects of chronic spinal cord injury on the electrical properties of dorsal root ganglion neurones innervating the urinary bladder or colon of the adult rat. 2. In spinal intact animals, the majority (73-74%) of bladder and colon neurones which were small in size exhibited high-threshold humped spikes mediated by tetrodotoxin (TTX)-resistant Na+ channels, whereas large neurones had low-threshold narrow spikes mediated by TTX-sensitive Na+ channels. 3. In chronic spinal transected animals, 60% of bladder afferent neurones exhibited TTX-sensitive low-threshold spikes. The average diameter and input capacitance of the cells were significantly larger than those of cells obtained from spinal intact animals. 4. In bladder afferent neurones from chronic spinal transected rats, the density of TTX-resistant Na+ currents significantly decreased from 60.5 to 17.9 pA pF-1, whereas that of TTX-sensitive currents increased from 32.1 to 80.6 pA pF-1. 5. These changes in action potential and Na+ current characteristics were not detected in colon afferent neurones following spinal cord injury. 6. The results indicate that spinal cord injury increases bladder afferent neurone excitability by shifting the expression of Na+ channels from a high-threshold TTX-resistant type to a low-threshold TTX-sensitive type. This change in properties may occur in response to alterations in neurotrophic signals originating in the hypertrophied bladder.
Full text
PDF

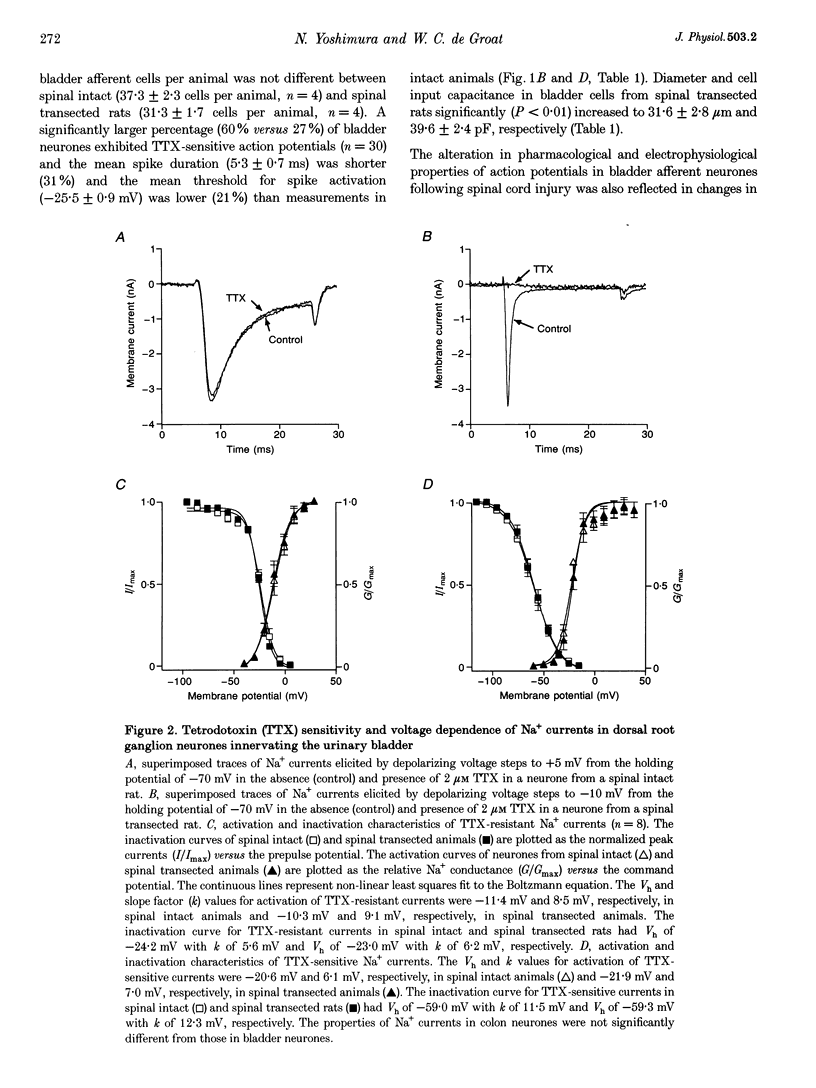


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguayo L. G., White G. Effects of nerve growth factor on TTX- and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992 Jan 20;570(1-2):61–67. doi: 10.1016/0006-8993(92)90564-p. [DOI] [PubMed] [Google Scholar]
- Akopian A. N., Sivilotti L., Wood J. N. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996 Jan 18;379(6562):257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
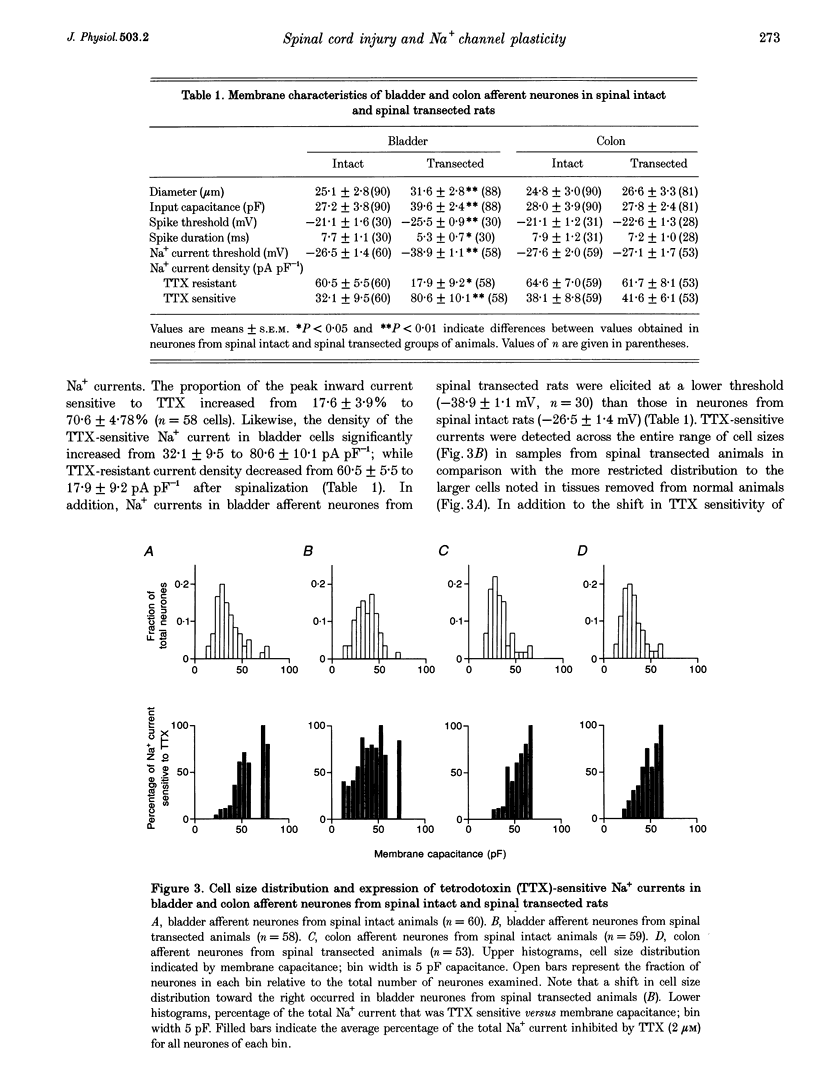
- Arbuckle J. B., Docherty R. J. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995 Feb 6;185(1):70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- De Groat W. C., Krier J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J Physiol. 1978 Mar;276:481–500. doi: 10.1113/jphysiol.1978.sp012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler C. J., Beck R. O., Gerrard S., Betts C. D., Fowler C. G. Intravesical capsaicin for treatment of detrusor hyperreflexia. J Neurol Neurosurg Psychiatry. 1994 Feb;57(2):169–173. doi: 10.1136/jnnp.57.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geirsson G., Fall M., Lindström S. The ice-water test--a simple and valuable supplement to routine cystometry. Br J Urol. 1993 Jun;71(6):681–685. doi: 10.1111/j.1464-410x.1993.tb16065.x. [DOI] [PubMed] [Google Scholar]
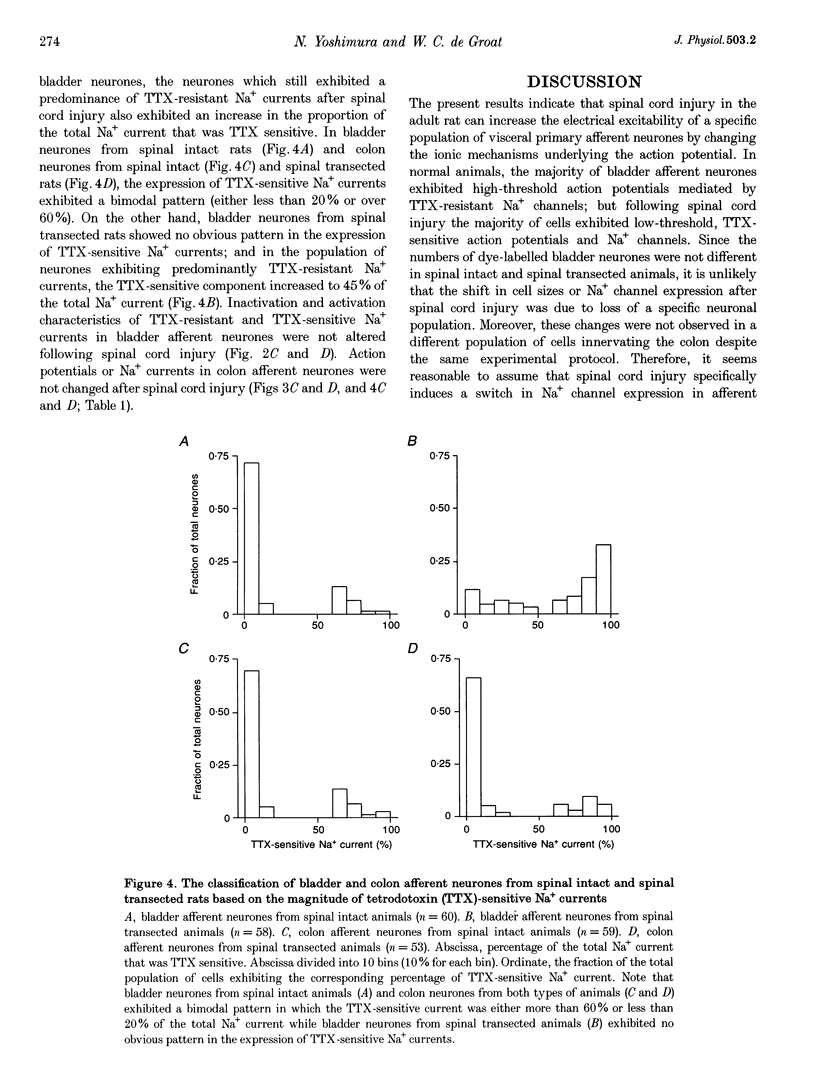
- Geirsson G., Fall M., Sullivan L. Clinical and urodynamic effects of intravesical capsaicin treatment in patients with chronic traumatic spinal detrusor hyperreflexia. J Urol. 1995 Nov;154(5):1825–1829. [PubMed] [Google Scholar]
- Jeftinija S. The role of tetrodotoxin-resistant sodium channels of small primary afferent fibers. Brain Res. 1994 Mar 7;639(1):125–134. doi: 10.1016/0006-8993(94)91772-8. [DOI] [PubMed] [Google Scholar]
- Keast J. R., De Groat W. C. Segmental distribution and peptide content of primary afferent neurons innervating the urogenital organs and colon of male rats. J Comp Neurol. 1992 May 22;319(4):615–623. doi: 10.1002/cne.903190411. [DOI] [PubMed] [Google Scholar]
- Keast J. R., de Groat W. C. Immunohistochemical characterization of pelvic neurons which project to the bladder, colon, or penis in rats. J Comp Neurol. 1989 Oct 15;288(3):387–400. doi: 10.1002/cne.902880303. [DOI] [PubMed] [Google Scholar]
- Kruse M. N., Belton A. L., de Groat W. C. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. Am J Physiol. 1993 Jun;264(6 Pt 2):R1157–R1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Kruse M. N., Bray L. A., de Groat W. C. Influence of spinal cord injury on the morphology of bladder afferent and efferent neurons. J Auton Nerv Syst. 1995 Sep 5;54(3):215–224. doi: 10.1016/0165-1838(95)00011-l. [DOI] [PubMed] [Google Scholar]
- Quasthoff S., Grosskreutz J., Schröder J. M., Schneider U., Grafe P. Calcium potentials and tetrodotoxin-resistant sodium potentials in unmyelinated C fibres of biopsied human sural nerve. Neuroscience. 1995 Dec;69(3):955–965. doi: 10.1016/0306-4522(95)00307-5. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L., Delgado S. G., Fish L. M., Koch B. D., Jakeman L. B., Stewart G. R., Sze P., Hunter J. C., Eglen R. M., Herman R. C. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996 Mar 15;271(11):5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Steers W. D., Ciambotti J., Erdman S., de Groat W. C. Morphological plasticity in efferent pathways to the urinary bladder of the rat following urethral obstruction. J Neurosci. 1990 Jun;10(6):1943–1951. doi: 10.1523/JNEUROSCI.10-06-01943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazulla S., Studholme K. M. Glycinergic interplexiform cells make synaptic contact with amacrine cell bodies in goldfish retina. J Comp Neurol. 1991 Aug 1;310(1):1–10. doi: 10.1002/cne.903100103. [DOI] [PubMed] [Google Scholar]
- Yoshimura N., White G., Weight F. F., de Groat W. C. Different types of Na+ and A-type K+ currents in dorsal root ganglion neurones innervating the rat urinary bladder. J Physiol. 1996 Jul 1;494(Pt 1):1–16. doi: 10.1113/jphysiol.1996.sp021471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura N., White G., Weight F. F., de Groat W. C. Patch-clamp recordings from subpopulations of autonomic and afferent neurons identified by axonal tracing techniques. J Auton Nerv Syst. 1994 Sep;49(1):85–92. doi: 10.1016/0165-1838(94)90024-8. [DOI] [PubMed] [Google Scholar]


