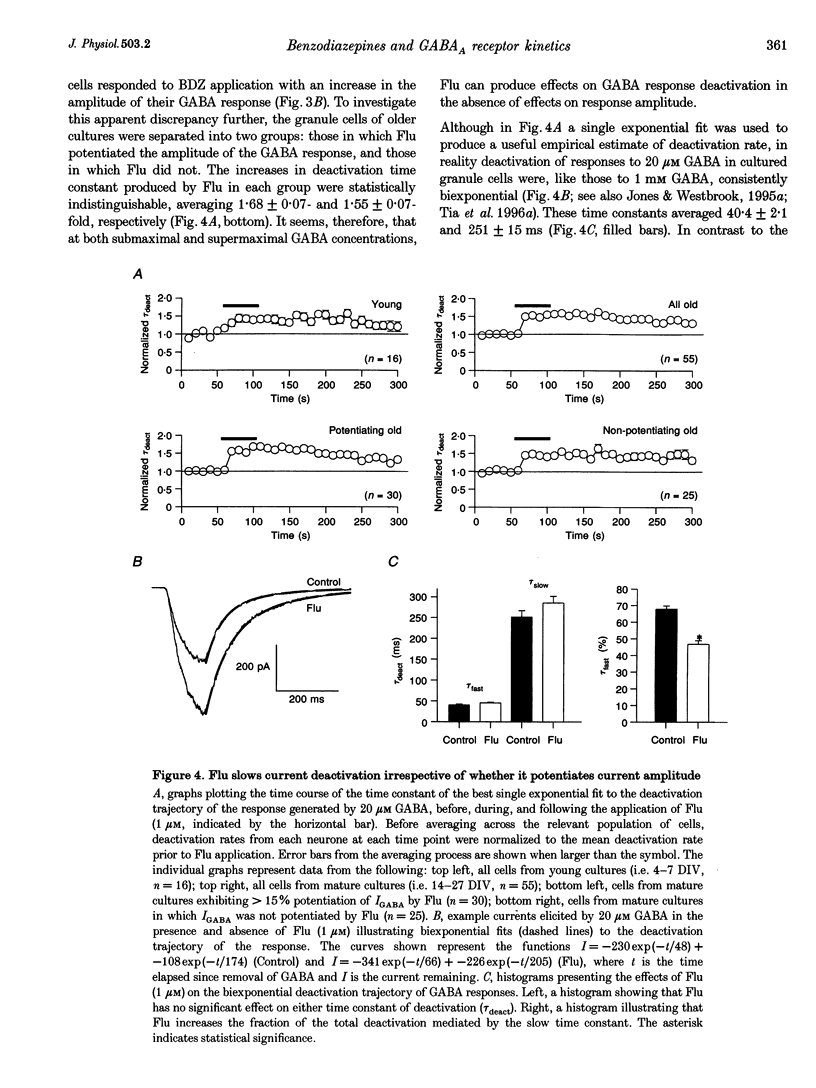
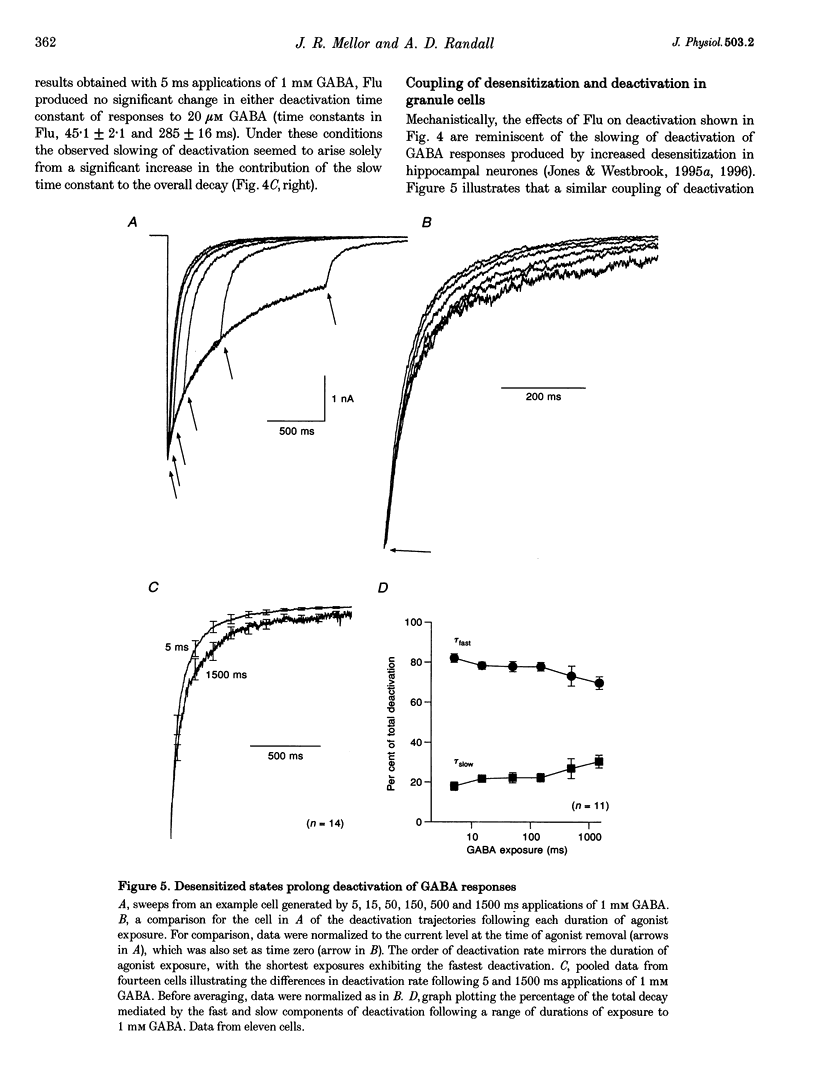
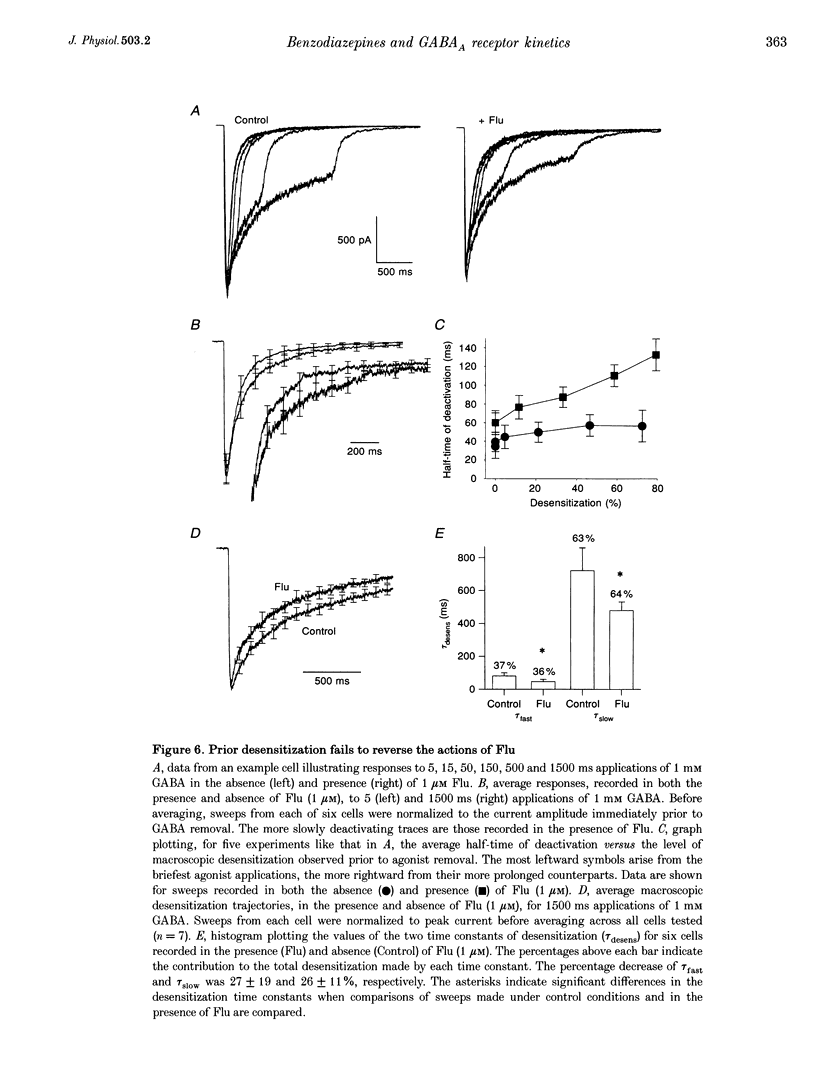
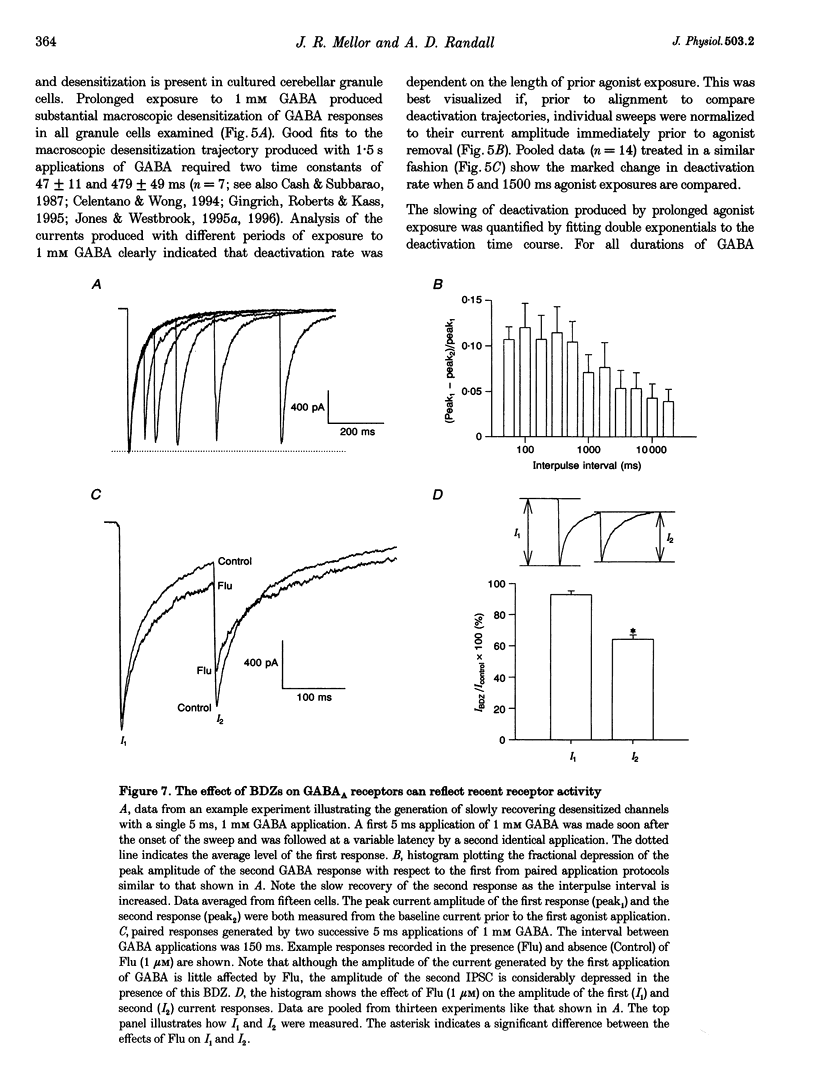
Abstract
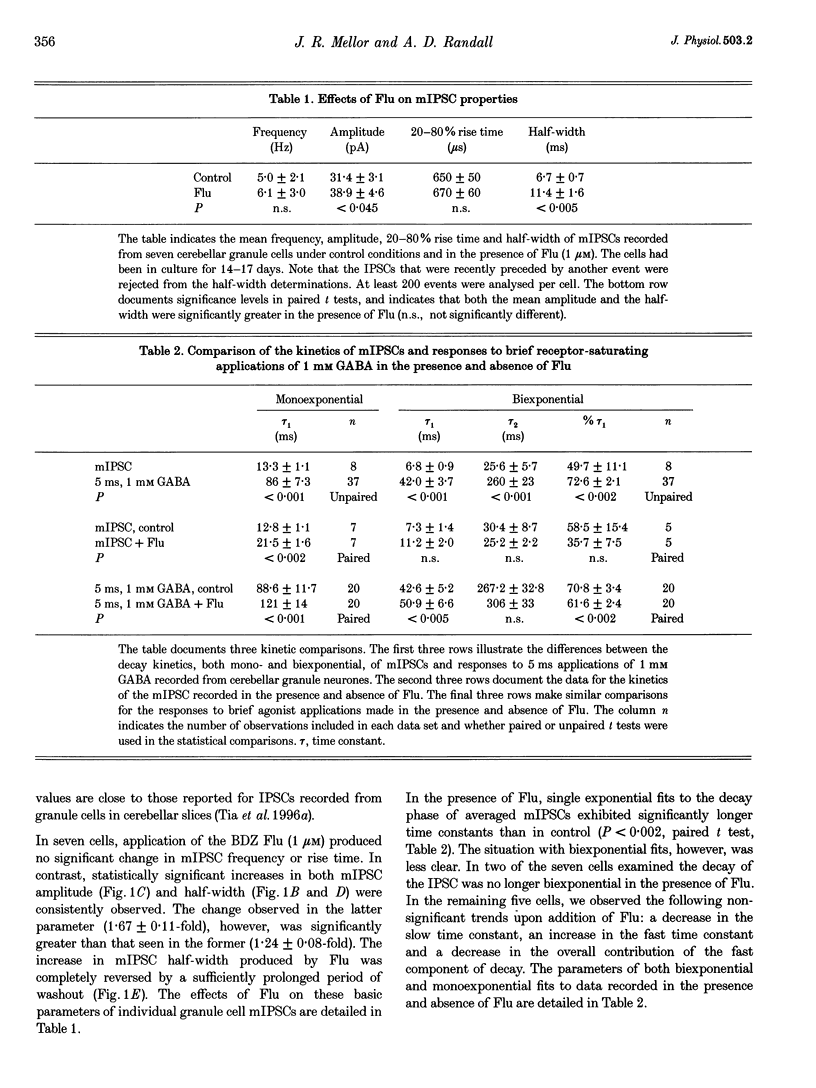
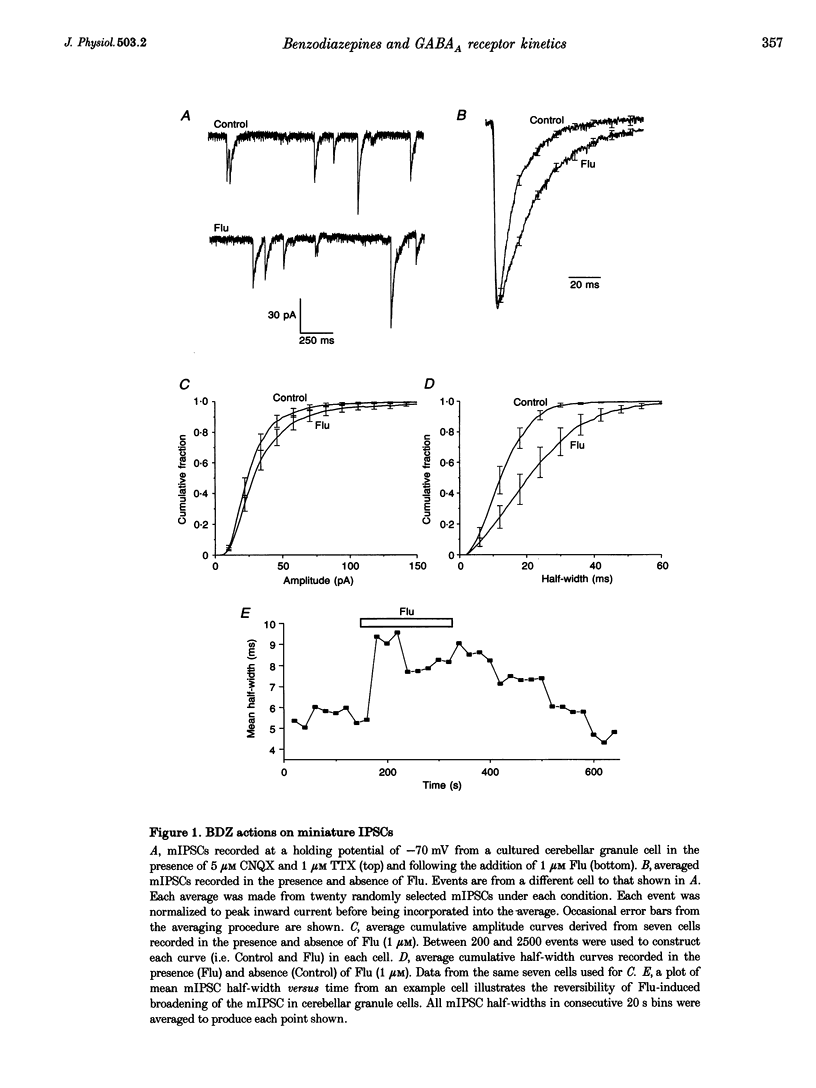
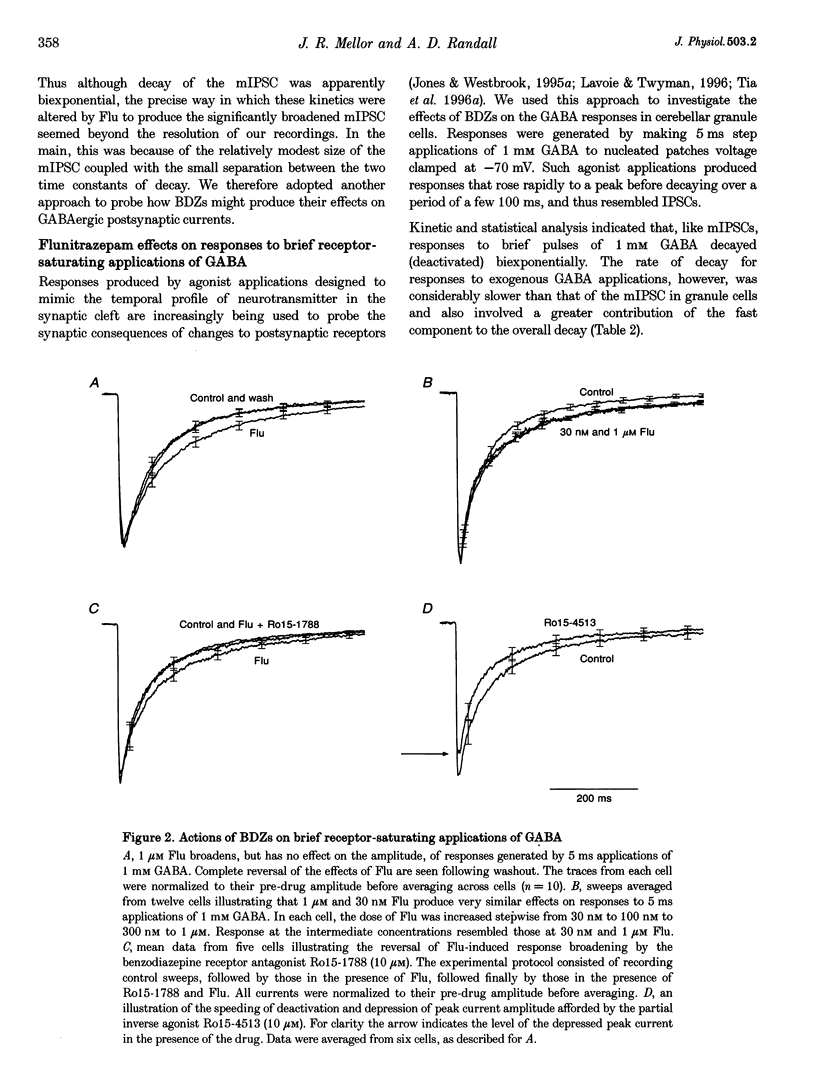
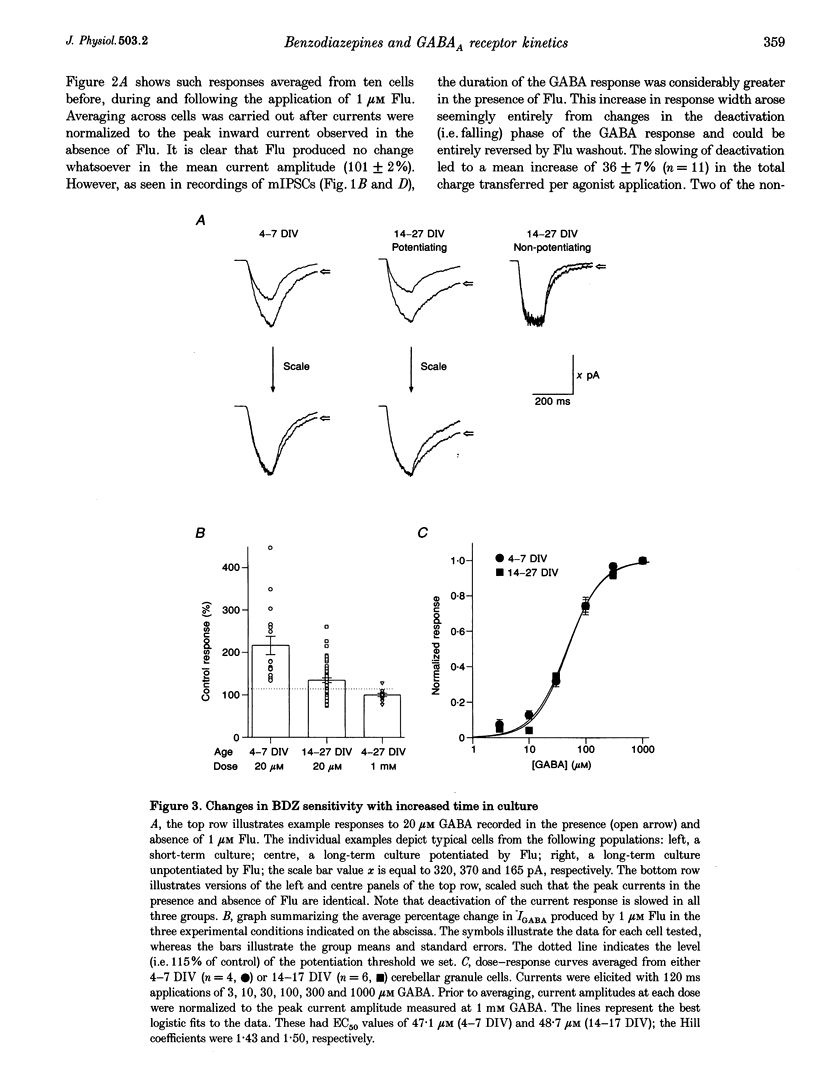
1. Miniature IPSCs recorded from cultured murine cerebellar granule cells increased in half-width and amplitude following application of the benzodiazepine (BDZ) Flunitrazepam (Flu, 1 microM). The increase in the half-width was much greater than that in the amplitude. 2. Five-millisecond applications of 1 mM GABA to nucleated outside-out patches elicited rapidly rising biexponentially decaying responses that resembled IPSCs. Flu had no effect on the amplitude of such responses, but consistently slowed their deactivation by approximately 50%. This effect was reversed by Flu washout or application of the BDZ antagonist Ro15-1788. The partial inverse agonist. Ro15-4513 speeded deactivation and depressed peak current amplitude by 23 +/- 12%. 3. The EC50 for GABA was between 45 and 50 microM. At submaximally effective agonist concentrations, Flu increased response amplitude and slowed response deactivation. Both effects were present in all cells taken from young cultures (4-7 days in vitro) but the latter was absent in 55% of the neurones obtained from older cultures (14-27 days in vitro). 4. With 120 ms applications of 20 microM GABA, responses activated monoexponentially (time constant, 39.8 +/- 2.8 ms) and deactivated biexponentially (time constants, 40.4 +/- 2.1 and 251 +/- 15 ms). Application of Flu slowed both activation and deactivation. The latter effect arose from an increased contribution of the slower component of decay. 5. Desensitization of responses to 1 mM GABA was biexponential, with time constants of 47 +/- 11 and 479 +/- 49 ms. Flu speeded desensitization by decreasing both fast and slow time constants. GABAA receptor desensitization consistently slowed subsequent deactivation. No significant relationship between the level of desensitization and the amount of slowing of deactivation produced by Flu was found. 6. Responses to paired 5 ms applications of 1 mM GABA indicated that the slowing of deactivation and the speeding of desensitization produced by Flu combine to generate a marked frequency dependence in the actions of this BDZ. Thus when compared with control responses, GABA-induced charge transfer was only enhanced by Flu during the first of two successive agonist applications.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellantuono C., Reggi V., Tognoni G., Garattini S. Benzodiazepines: clinical pharmacology and therapeutic use. Drugs. 1980 Mar;19(3):195–219. doi: 10.2165/00003495-198019030-00004. [DOI] [PubMed] [Google Scholar]
- Cash D. J., Subbarao K. Channel opening of gamma-aminobutyric acid receptor from rat brain: molecular mechanisms of the receptor responses. Biochemistry. 1987 Dec 1;26(24):7562–7570. doi: 10.1021/bi00398a005. [DOI] [PubMed] [Google Scholar]
- Celentano J. J., Wong R. K. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994 Apr;66(4):1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. W., Farb D. H., Fischbach G. D. Chlordiazepoxide selectively augments GABA action in spinal cord cell cultures. Nature. 1977 Sep 22;269(5626):342–344. doi: 10.1038/269342a0. [DOI] [PubMed] [Google Scholar]
- Clements J. D. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996 May;19(5):163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Colquhoun D. Unravelling the paradox. Trends Pharmacol Sci. 1992 Dec;13(12):429–430. doi: 10.1016/0165-6147(92)90137-u. [DOI] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Davies C. H., Collingridge G. L. Regulation of EPSPs by the synaptic activation of GABAB autoreceptors in rat hippocampus. J Physiol. 1996 Oct 15;496(Pt 2):451–470. doi: 10.1113/jphysiol.1996.sp021698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koninck Y., Mody I. Noise analysis of miniature IPSCs in adult rat brain slices: properties and modulation of synaptic GABAA receptor channels. J Neurophysiol. 1994 Apr;71(4):1318–1335. doi: 10.1152/jn.1994.71.4.1318. [DOI] [PubMed] [Google Scholar]
- Eghbali M., Curmi J. P., Birnir B., Gage P. W. Hippocampal GABA(A) channel conductance increased by diazepam. Nature. 1997 Jul 3;388(6637):71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- Farrant M., Gibbs T. T., Farb D. H. Molecular and cellular mechanisms of GABA/benzodiazepine-receptor regulation: electrophysiological and biochemical studies. Neurochem Res. 1990 Feb;15(2):175–191. doi: 10.1007/BF00972208. [DOI] [PubMed] [Google Scholar]
- Frerking M., Borges S., Wilson M. Variation in GABA mini amplitude is the consequence of variation in transmitter concentration. Neuron. 1995 Oct;15(4):885–895. doi: 10.1016/0896-6273(95)90179-5. [DOI] [PubMed] [Google Scholar]
- Gao B., Fritschy J. M. Cerebellar granule cells in vitro recapitulate the in vivo pattern of GABAA-receptor subunit expression. Brain Res Dev Brain Res. 1995 Aug 28;88(1):1–16. doi: 10.1016/0165-3806(95)00062-i. [DOI] [PubMed] [Google Scholar]
- Gingrich K. J., Roberts W. A., Kass R. S. Dependence of the GABAA receptor gating kinetics on the alpha-subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995 Dec 1;489(Pt 2):529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Korpi E. R., McKernan R. M., Pelz R., Nusser Z., Mäkelä R., Mellor J. R., Pollard S., Bahn S., Stephenson F. A. Ligand-gated ion channel subunit partnerships: GABAA receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997 Feb 15;17(4):1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. V., Westbrook G. L. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995 Jul;15(1):181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Westbrook G. L. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996 Mar;19(3):96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- King G. L., Knox J. J., Dingledine R. Reduction of inhibition by a benzodiazepine antagonist, Ro15-1788, in the rat hippocampal slice. Neuroscience. 1985 Jun;15(2):371–378. doi: 10.1016/0306-4522(85)90219-2. [DOI] [PubMed] [Google Scholar]
- Krespan B., Springfield S. A., Haas H., Geller H. M. Electrophysiological studies on benzodiazepine antagonists. Brain Res. 1984 Mar 19;295(2):265–274. doi: 10.1016/0006-8993(84)90975-2. [DOI] [PubMed] [Google Scholar]
- Lin X., Bulleit R. F. Cell intrinsic mechanisms regulate mouse cerebellar granule neuron differentiation. Neurosci Lett. 1996 Dec 13;220(2):81–84. doi: 10.1016/s0304-3940(96)13214-6. [DOI] [PubMed] [Google Scholar]
- MacDonald R. L., Twyman R. E. Kinetic properties and regulation of GABAA receptor channels. Ion Channels. 1992;3:315–343. doi: 10.1007/978-1-4615-3328-3_10. [DOI] [PubMed] [Google Scholar]
- Macdonald R., Barker J. L. Benzodiazepines specifically modulate GABA-mediated postsynaptic inhibition in cultured mammalian neurones. Nature. 1978 Feb 9;271(5645):563–564. doi: 10.1038/271563a0. [DOI] [PubMed] [Google Scholar]
- Maconochie D. J., Zempel J. M., Steinbach J. H. How quickly can GABAA receptors open? Neuron. 1994 Jan;12(1):61–71. doi: 10.1016/0896-6273(94)90152-x. [DOI] [PubMed] [Google Scholar]
- Mody I., De Koninck Y., Otis T. S., Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994 Dec;17(12):517–525. doi: 10.1016/0166-2236(94)90155-4. [DOI] [PubMed] [Google Scholar]
- Otis T. S., Mody I. Modulation of decay kinetics and frequency of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents in hippocampal neurons. Neuroscience. 1992 Jul;49(1):13–32. doi: 10.1016/0306-4522(92)90073-b. [DOI] [PubMed] [Google Scholar]
- Puia G., Costa E., Vicini S. Functional diversity of GABA-activated Cl- currents in Purkinje versus granule neurons in rat cerebellar slices. Neuron. 1994 Jan;12(1):117–126. doi: 10.1016/0896-6273(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers C. J., Twyman R. E., Macdonald R. L. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994 Feb 15;475(1):69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson H. H., Harris R. A. Neurobiology of alcohol abuse. Trends Pharmacol Sci. 1992 May;13(5):206–211. doi: 10.1016/0165-6147(92)90065-e. [DOI] [PubMed] [Google Scholar]
- Segal M., Barker J. L. Rat hippocampal neurons in culture: voltage-clamp analysis of inhibitory synaptic connections. J Neurophysiol. 1984 Sep;52(3):469–487. doi: 10.1152/jn.1984.52.3.469. [DOI] [PubMed] [Google Scholar]
- Study R. E., Barker J. L. Diazepam and (--)-pentobarbital: fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7180–7184. doi: 10.1073/pnas.78.11.7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S., Wang J. F., Kotchabhakdi N., Vicini S. Developmental changes of inhibitory synaptic currents in cerebellar granule neurons: role of GABA(A) receptor alpha 6 subunit. J Neurosci. 1996 Jun 1;16(11):3630–3640. doi: 10.1523/JNEUROSCI.16-11-03630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S., Alho H., Costa E., Mienville J. M., Santi M. R., Vaccarino F. M. Modulation of gamma-aminobutyric acid-mediated inhibitory synaptic currents in dissociated cortical cell cultures. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9269–9273. doi: 10.1073/pnas.83.23.9269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S., Mienville J. M., Costa E. Actions of benzodiazepine and beta-carboline derivatives on gamma-aminobutyric acid-activated Cl- channels recorded from membrane patches of neonatal rat cortical neurons in culture. J Pharmacol Exp Ther. 1987 Dec;243(3):1195–1201. [PubMed] [Google Scholar]
- Wisden W., Laurie D. J., Monyer H., Seeburg P. H. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992 Mar;12(3):1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Weiner J. L., Carlen P. L. Potentiation of gamma-aminobutyric acid type A receptor-mediated synaptic currents by pentobarbital and diazepam in immature hippocampal CA1 neurons. J Pharmacol Exp Ther. 1993 Sep;266(3):1227–1235. [PubMed] [Google Scholar]


