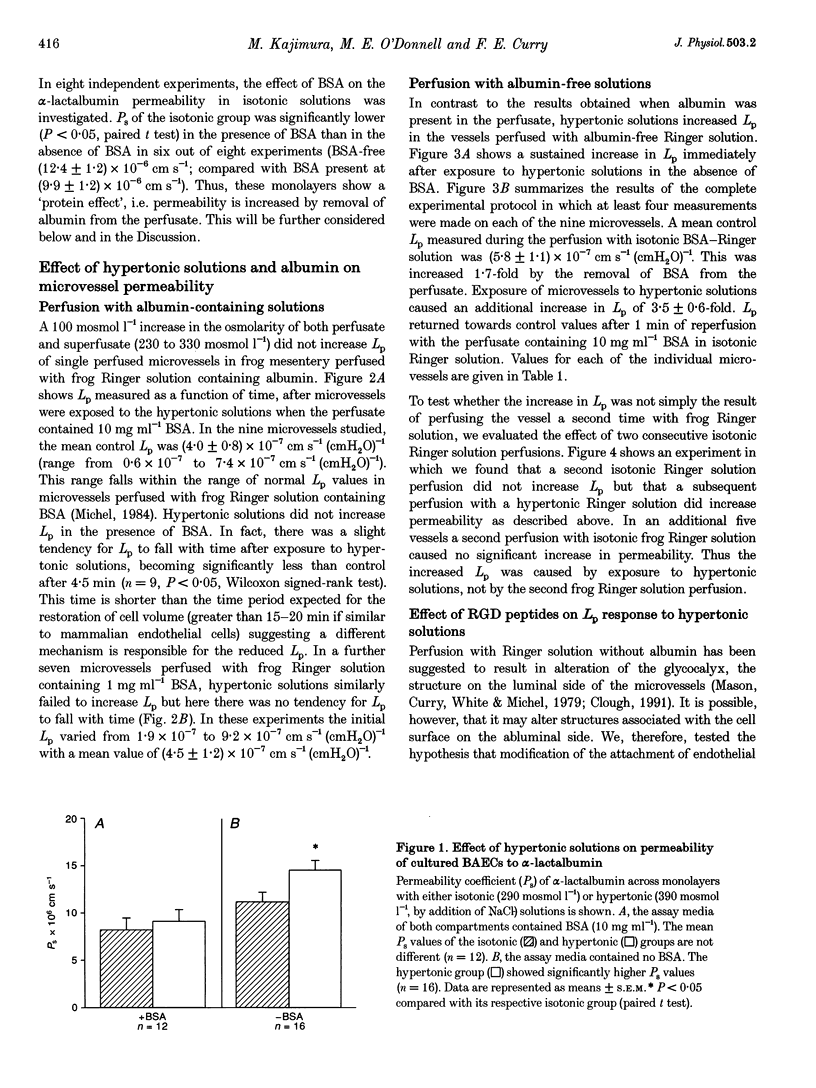
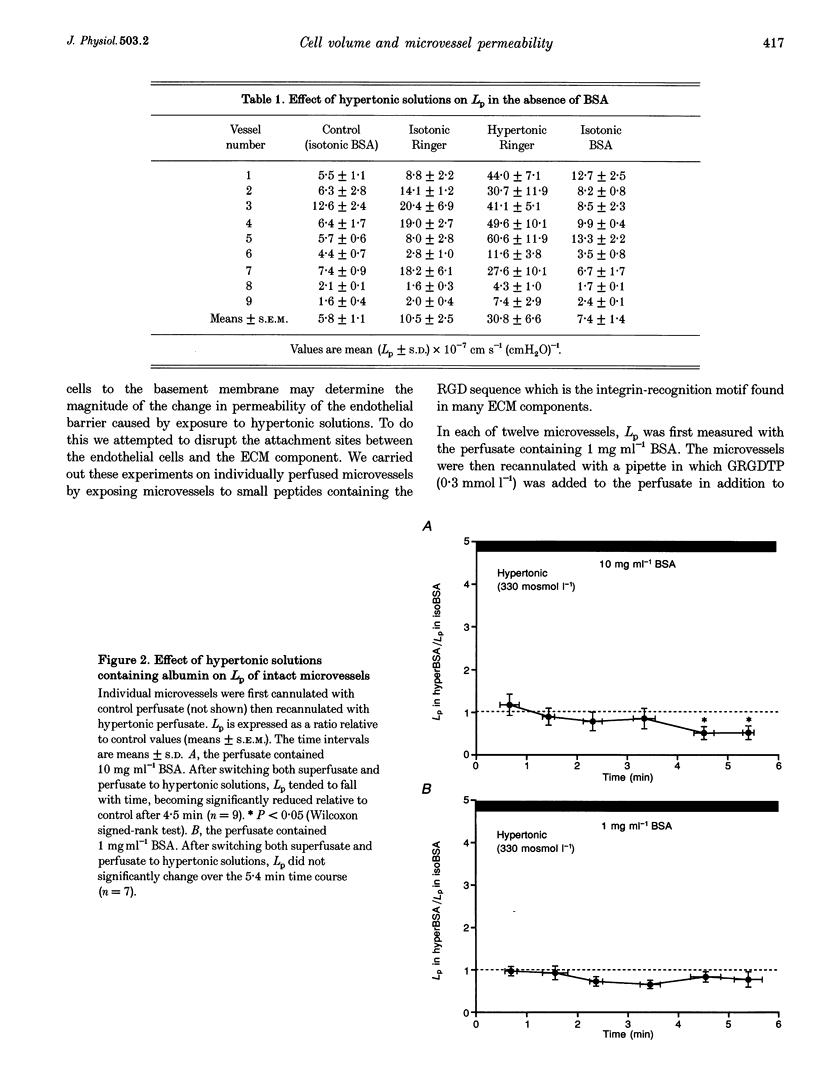
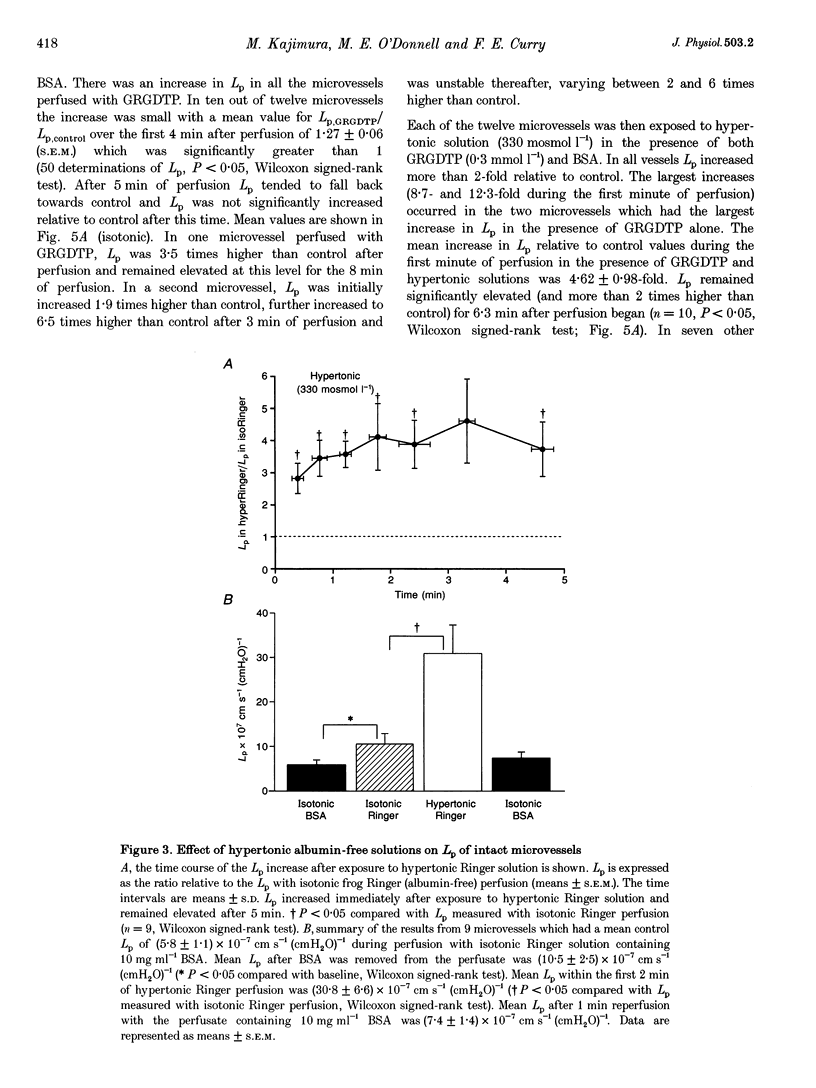
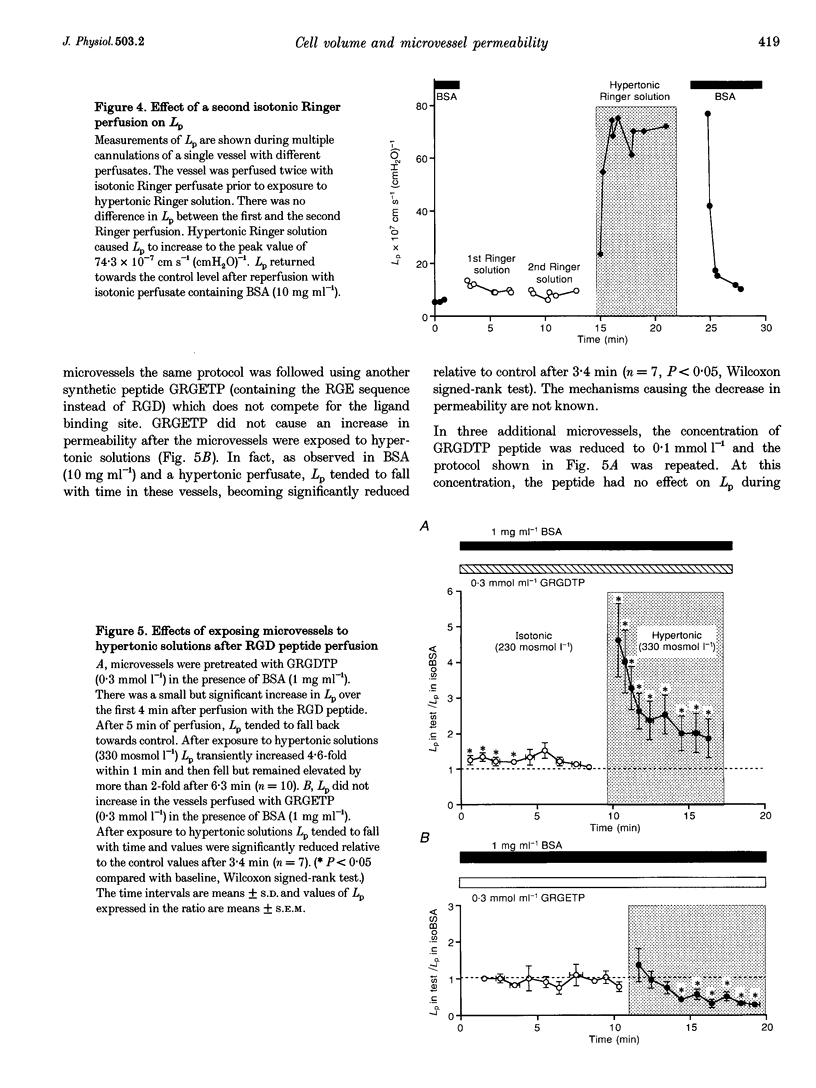
Abstract
1. We have tested the hypothesis that a reduction in endothelial cell volume increases microvessel permeability and that the degree of endothelial cell attachment to their basement membranes determines the magnitude of permeability changes caused by a reduction in endothelial cell volume. 2. A decrease in endothelial cell volume was imposed on both intact microvessels and cultured endothelial monolayers by raising osmolarity by 100 mosmol l-1. 3. We found that hypertonic solutions did not increase the hydraulic permeability (Lp) of individually perfused venular microvessels in frog mesentery when the perfusate contained albumin. Hypertonic solutions did increase Lp, however, after we perfused the microvessels with the peptide Gly-Arg-Gly Asp-Thr-Pro (GRGDTP; 0.3 mmol l-1), to disrupt integrin-dependent endothelial cell (EC) attachment to the extracellular matrix (ECM). 4. After albumin was removed from the perfusate, hypertonic solutions increased Lp of microvessels and the permeability of endothelial monolayers to alpha-lactalbumin. 5. Our findings indicate that endothelial cell integrin-ECM binding plays a role in transducing changes in cell volume and/or shape into changes in permeability. We hypothesize that removal of albumin from the vascular perfusate may compromise EC-ECM interactions via an integrin-dependent mechanism.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson R. H., Clough G. Plasma proteins modify the endothelial cell glycocalyx of frog mesenteric microvessels. J Physiol. 1992 Jan;445:473–486. doi: 10.1113/jphysiol.1992.sp018934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson R. H., Huxley V. H., Curry F. E. Single capillary permeability to proteins having similar size but different charge. Am J Physiol. 1988 Feb;254(2 Pt 2):H304–H312. doi: 10.1152/ajpheart.1988.254.2.H304. [DOI] [PubMed] [Google Scholar]
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M., Rasio E. A. Hyperglycemia and microangiopathy in the eel. Diabetes. 1981 Apr;30(4):317–325. doi: 10.2337/diab.30.4.317. [DOI] [PubMed] [Google Scholar]
- Chen B. M., Grinnell A. D. Integrins and modulation of transmitter release from motor nerve terminals by stretch. Science. 1995 Sep 15;269(5230):1578–1580. doi: 10.1126/science.7667637. [DOI] [PubMed] [Google Scholar]
- Cheng Y. F., Kramer R. H. Human microvascular endothelial cells express integrin-related complexes that mediate adhesion to the extracellular matrix. J Cell Physiol. 1989 May;139(2):275–286. doi: 10.1002/jcp.1041390209. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A. Human endothelial cells synthesize and express an Arg-Gly-Asp-directed adhesion receptor involved in attachment to fibrinogen and von Willebrand factor. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6471–6475. doi: 10.1073/pnas.84.18.6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough G. Relationship between microvascular permeability and ultrastructure. Prog Biophys Mol Biol. 1991;55(1):47–69. doi: 10.1016/0079-6107(91)90011-g. [DOI] [PubMed] [Google Scholar]
- Conforti G., Dominguez-Jimenez C., Zanetti A., Gimbrone M. A., Jr, Cremona O., Marchisio P. C., Dejana E. Human endothelial cells express integrin receptors on the luminal aspect of their membrane. Blood. 1992 Jul 15;80(2):437–446. [PubMed] [Google Scholar]
- Curry F. E., Michel C. C., Mason J. C. Osmotic reflextion coefficients of capillary walls to low molecular weight hydrophilic solutes measured in single perfused capillaries of the frog mesentery. J Physiol. 1976 Oct;261(2):319–336. doi: 10.1113/jphysiol.1976.sp011561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Cell volume regulation in frog urinary bladder. Fed Proc. 1985 Jun;44(9):2520–2525. [PubMed] [Google Scholar]
- DeSimone D. W. Adhesion and matrix in vertebrate development. Curr Opin Cell Biol. 1994 Oct;6(5):747–751. doi: 10.1016/0955-0674(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Dejana E., Colella S., Conforti G., Abbadini M., Gaboli M., Marchisio P. C. Fibronectin and vitronectin regulate the organization of their respective Arg-Gly-Asp adhesion receptors in cultured human endothelial cells. J Cell Biol. 1988 Sep;107(3):1215–1223. doi: 10.1083/jcb.107.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eveloff J. L., Warnock D. G. Activation of ion transport systems during cell volume regulation. Am J Physiol. 1987 Jan;252(1 Pt 2):F1–10. doi: 10.1152/ajprenal.1987.252.1.F1. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Granger J. P., Brace R. A., Parker R. E., Taylor A. E. Analysis of the permeability characteristics of cat intestinal capillaries. Circ Res. 1979 Mar;44(3):335–344. doi: 10.1161/01.res.44.3.335. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A., Sarkadi B., Gelfand E. W. Responses of lymphocytes to anisotonic media: volume-regulating behavior. Am J Physiol. 1984 Mar;246(3 Pt 1):C204–C215. doi: 10.1152/ajpcell.1984.246.3.C204. [DOI] [PubMed] [Google Scholar]
- He P., Curry F. E. Albumin modulation of capillary permeability: role of endothelial cell [Ca2+]i. Am J Physiol. 1993 Jul;265(1 Pt 2):H74–H82. doi: 10.1152/ajpheart.1993.265.1.H74. [DOI] [PubMed] [Google Scholar]
- Huxley V. H., Curry F. E. Albumin modulation of capillary permeability: test of an adsorption mechanism. Am J Physiol. 1985 Feb;248(2 Pt 2):H264–H273. doi: 10.1152/ajpheart.1985.248.2.H264. [DOI] [PubMed] [Google Scholar]
- Lampugnani M. G., Resnati M., Dejana E., Marchisio P. C. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991 Feb;112(3):479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., Michel C. C. The effects of proteins upon the filtration coefficient of individually perfused frog mesenteric capillaries. Microvasc Res. 1977 Mar;13(2):185–202. doi: 10.1016/0026-2862(77)90084-x. [DOI] [PubMed] [Google Scholar]
- Mason J. C., Curry F. E., White I. F., Michel C. C. The ultrastructure of frog mesenteric capillaries of known filtration coefficient. Q J Exp Physiol Cogn Med Sci. 1979 Jul;64(3):217–224. doi: 10.1113/expphysiol.1979.sp002474. [DOI] [PubMed] [Google Scholar]
- Michel C. C., Mason J. C., Curry F. E., Tooke J. E., Hunter P. J. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974 Oct;59(4):283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- Neal C. R., Michel C. C. Transcellular gaps in microvascular walls of frog and rat when permeability is increased by perfusion with the ionophore A23187. J Physiol. 1995 Oct 15;488(Pt 2):427–437. doi: 10.1113/jphysiol.1995.sp020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M. E. Role of Na-K-Cl cotransport in vascular endothelial cell volume regulation. Am J Physiol. 1993 May;264(5 Pt 1):C1316–C1326. doi: 10.1152/ajpcell.1993.264.5.C1316. [DOI] [PubMed] [Google Scholar]
- PAPPENHEIMER J. R., RENKIN E. M., BORRERO L. M. Filtration, diffusion and molecular sieving through peripheral capillary membranes; a contribution to the pore theory of capillary permeability. Am J Physiol. 1951 Oct;167(1):13–46. doi: 10.1152/ajplegacy.1951.167.1.13. [DOI] [PubMed] [Google Scholar]
- Qiao R. L., Yan W., Lum H., Malik A. B. Arg-Gly-Asp peptide increases endothelial hydraulic conductivity: comparison with thrombin response. Am J Physiol. 1995 Jul;269(1 Pt 1):C110–C117. doi: 10.1152/ajpcell.1995.269.1.C110. [DOI] [PubMed] [Google Scholar]
- Rapoport S. I., Fredericks W. R., Ohno K., Pettigrew K. D. Quantitative aspects of reversible osmotic opening of the blood-brain barrier. Am J Physiol. 1980 May;238(5):R421–R431. doi: 10.1152/ajpregu.1980.238.5.R421. [DOI] [PubMed] [Google Scholar]
- Rasio E. A., Bendayan M., Goresky C. A. The effect of hyperosmolality on the permeability and structure of the capillaries of the isolated rete mirabile of the eel. Circ Res. 1981 Sep;49(3):661–676. doi: 10.1161/01.res.49.3.661. [DOI] [PubMed] [Google Scholar]
- Shepard J. M., Goderie S. K., Brzyski N., Del Vecchio P. J., Malik A. B., Kimelberg H. K. Effects of alterations in endothelial cell volume on transendothelial albumin permeability. J Cell Physiol. 1987 Nov;133(2):389–394. doi: 10.1002/jcp.1041330226. [DOI] [PubMed] [Google Scholar]
- Tsukada H., Ying X., Fu C., Ishikawa S., McKeown-Longo P., Albelda S., Bhattacharya S., Bray B. A., Bhattacharya J. Ligation of endothelial alpha v beta 3 integrin increases capillary hydraulic conductivity of rat lung. Circ Res. 1995 Oct;77(4):651–659. doi: 10.1161/01.res.77.4.651. [DOI] [PubMed] [Google Scholar]
- Ussing H. H. Volume regulation of frog skin epithelium. Acta Physiol Scand. 1982 Mar;114(3):363–369. doi: 10.1111/j.1748-1716.1982.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Wolf M. B., Watson P. D. Measurement of osmotic reflection coefficient for small molecules in cat hindlimbs. Am J Physiol. 1989 Jan;256(1 Pt 2):H282–H290. doi: 10.1152/ajpheart.1989.256.1.H282. [DOI] [PubMed] [Google Scholar]


