Abstract
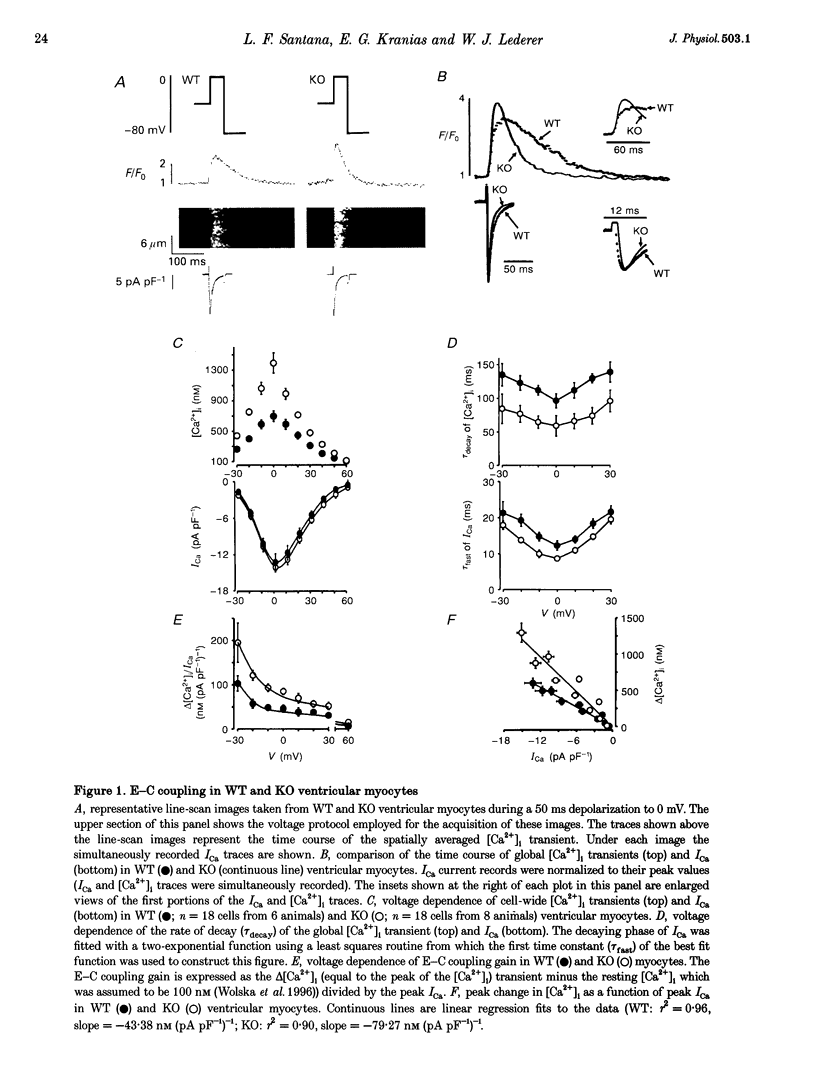
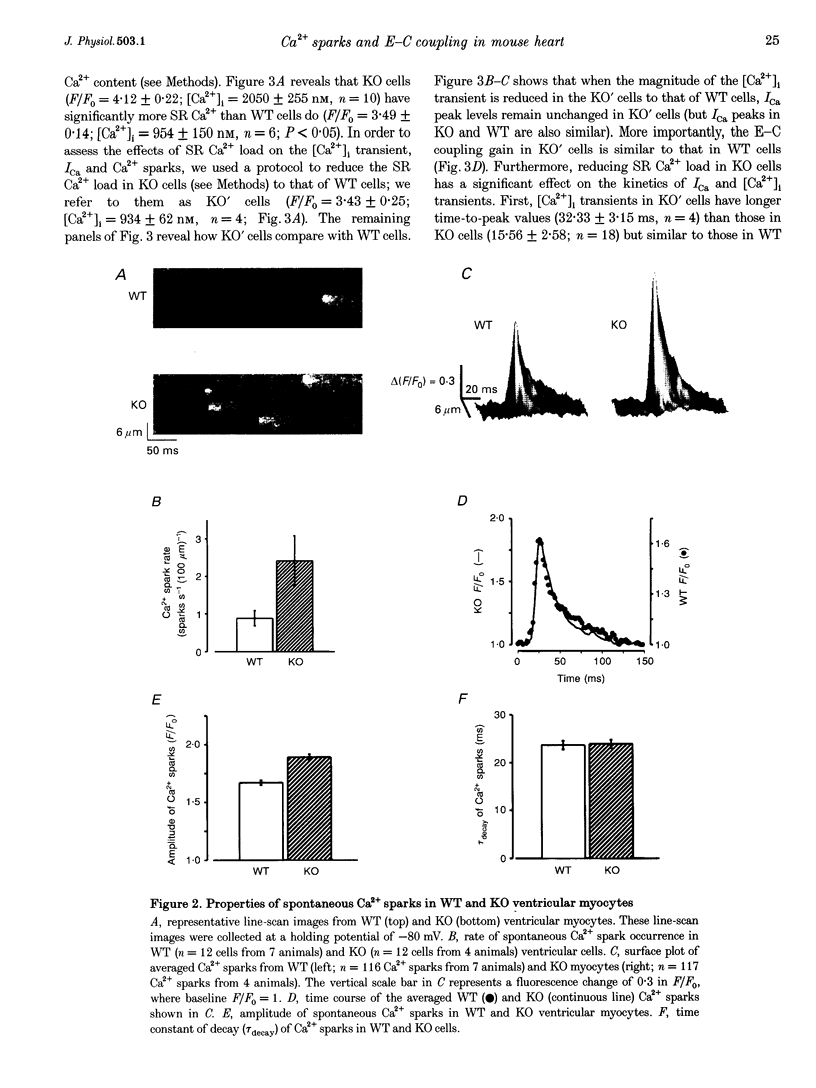
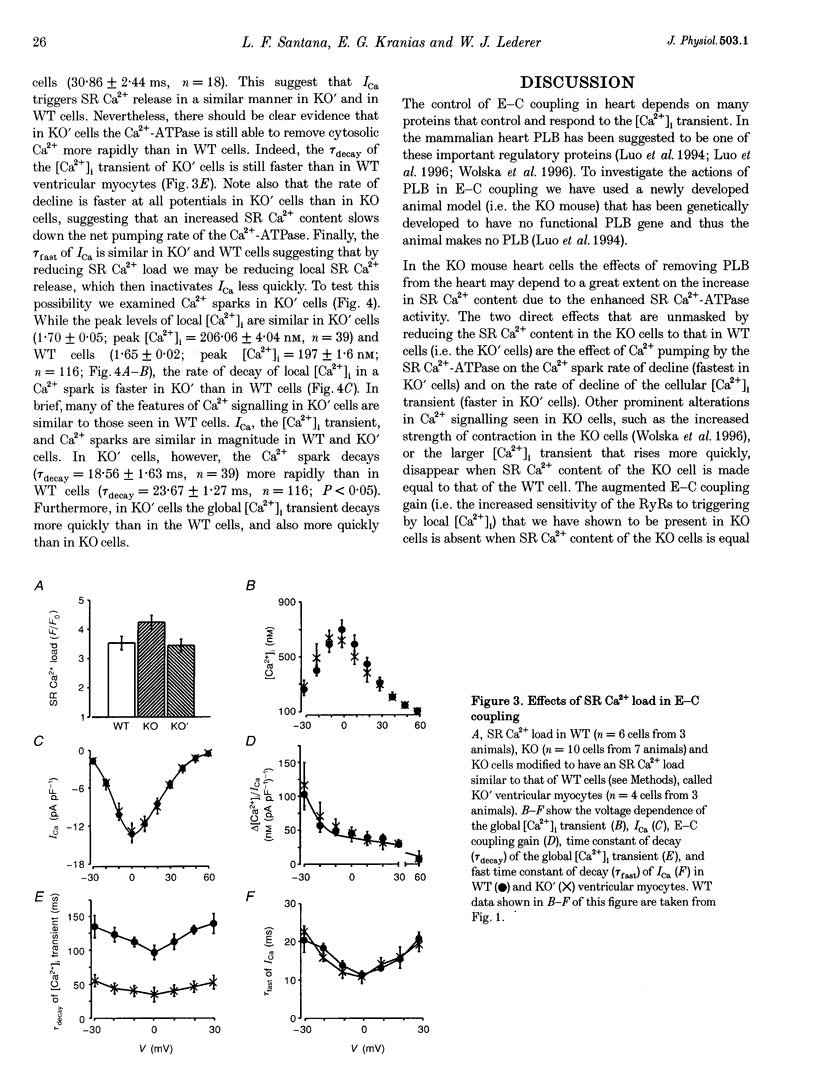
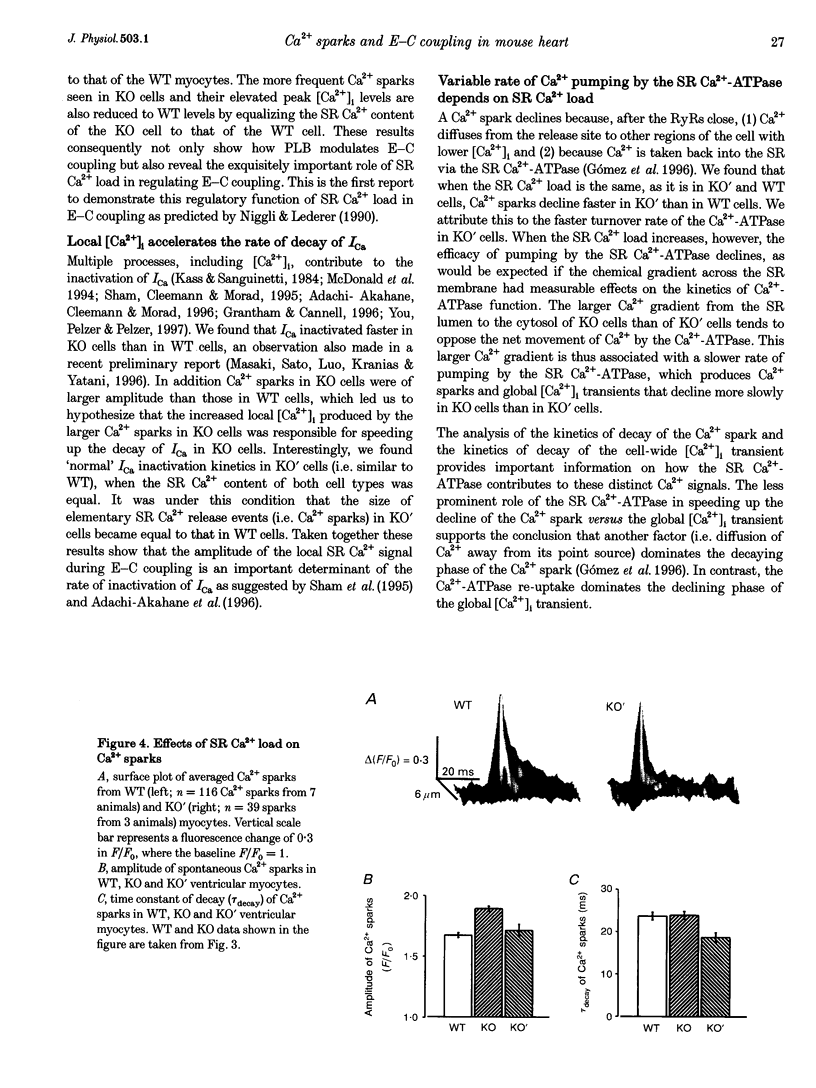
1. We examined [Ca2+]i and L-type Ca2+ channel current (ICa) in single cardiac myocytes to determine how the intracellular protein phospholamban (PLB) influences excitation-contraction (E-C) coupling in heart. Wild type (WT) and PLB-deficient (KO) mice were used. Cells were patch clamped in whole-cell mode while [Ca2+]i was imaged simultaneously using the Ca2+ indicator fluo-3 and a confocal microscope. 2. Although ICa was similar in magnitude, the decay of ICa was faster in KO than in WT cells and the [Ca2+]i transient was larger and decayed faster. Furthermore, the E-C coupling 'gain' (measured as delta[Ca2+]i/ICa) was larger in KO cells than in WT cells. 3. Spontaneous Ca2+ sparks were three times more frequent and larger in KO cells than in WT myocytes but, surprisingly, the time constants of decay were similar. 4. SR Ca2+ content was significantly greater in KO than in WT cells. When the SR Ca2+ content in KO cells was reduced to that in WT cells, Ca2+ sparks in these 'modified' (KO') cells decayed faster. E-C coupling gain, [Ca2+]i transient amplitude and the kinetics of decay of ICa were similar in KO' and WT cells. 5. We conclude that SR Ca2+ content influences (1) ICa, (2) the amplitude and kinetics of Ca2+ sparks and [Ca2+]i transients, (3) the sensitivity of the RyRs to triggering by [Ca2+]i, (4) the amount of Ca2+ released, (5) the magnitude of the E-C coupling 'gain' function, and (6) the rate of Ca2+ re-uptake by the SR Ca(2+)-ATPase. In KO cells, the larger [Ca2+]i transients and Ca2+ sparks speed up ICa inactivation. Finally, we conclude that PLB plays an important regulatory role in E-C coupling by modulating SR Ca(2+)-ATPase activity, which establishes the SR Ca2+ content and consequently influences the characteristics of local and global Ca2+ signalling.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi-Akahane S., Cleemann L., Morad M. Cross-signaling between L-type Ca2+ channels and ryanodine receptors in rat ventricular myocytes. J Gen Physiol. 1996 Nov;108(5):435–454. doi: 10.1085/jgp.108.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke C. W., Egan T. M., Wier W. G. Processes that remove calcium from the cytoplasm during excitation-contraction coupling in intact rat heart cells. J Physiol. 1994 Feb 1;474(3):447–462. doi: 10.1113/jphysiol.1994.sp020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassani J. W., Yuan W., Bers D. M. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995 May;268(5 Pt 1):C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Berlin J. R., Konishi M. Ca2+ transients in cardiac myocytes measured with high and low affinity Ca2+ indicators. Biophys J. 1993 Oct;65(4):1632–1647. doi: 10.1016/S0006-3495(93)81211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J. 1994 Nov;67(5):1942–1956. doi: 10.1016/S0006-3495(94)80677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Cheng H., Lederer W. J. The control of calcium release in heart muscle. Science. 1995 May 19;268(5213):1045–1049. doi: 10.1126/science.7754384. [DOI] [PubMed] [Google Scholar]
- Cheng H., Cannell M. B., Lederer W. J. Propagation of excitation-contraction coupling into ventricular myocytes. Pflugers Arch. 1994 Oct;428(3-4):415–417. doi: 10.1007/BF00724526. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer M. R., Lederer W. J., Cannell M. B. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996 Jan;270(1 Pt 1):C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- Cheng H., Lederer W. J., Cannell M. B. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993 Oct 29;262(5134):740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Chu G., Luo W., Slack J. P., Tilgmann C., Sweet W. E., Spindler M., Saupe K. W., Boivin G. P., Moravec C. S., Matlib M. A. Compensatory mechanisms associated with the hyperdynamic function of phospholamban-deficient mouse hearts. Circ Res. 1996 Dec;79(6):1064–1076. doi: 10.1161/01.res.79.6.1064. [DOI] [PubMed] [Google Scholar]
- Crespo L. M., Grantham C. J., Cannell M. B. Kinetics, stoichiometry and role of the Na-Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990 Jun 14;345(6276):618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol. 1983 Jul;245(1):C1–14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- Grantham C. J., Cannell M. B. Ca2+ influx during the cardiac action potential in guinea pig ventricular myocytes. Circ Res. 1996 Aug;79(2):194–200. doi: 10.1161/01.res.79.2.194. [DOI] [PubMed] [Google Scholar]
- Gómez A. M., Cheng H., Lederer W. J., Bers D. M. Ca2+ diffusion and sarcoplasmic reticulum transport both contribute to [Ca2+]i decline during Ca2+ sparks in rat ventricular myocytes. J Physiol. 1996 Oct 15;496(Pt 2):575–581. doi: 10.1113/jphysiol.1996.sp021708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks M. J., Shigekawa M., Katz A. M. Mechanism by which cyclic adenosine 3':5'-monophosphate-dependent protein kinase stimulates calcium transport in cardiac sarcoplasmic reticulum. Circ Res. 1979 Mar;44(3):384–391. doi: 10.1161/01.res.44.3.384. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Sanguinetti M. C. Inactivation of calcium channel current in the calf cardiac Purkinje fiber. Evidence for voltage- and calcium-mediated mechanisms. J Gen Physiol. 1984 Nov;84(5):705–726. doi: 10.1085/jgp.84.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby M. S., Sagara Y., Gaa S., Inesi G., Lederer W. J., Rogers T. B. Thapsigargin inhibits contraction and Ca2+ transient in cardiac cells by specific inhibition of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem. 1992 Jun 25;267(18):12545–12551. [PubMed] [Google Scholar]
- Kiss E., Ball N. A., Kranias E. G., Walsh R. A. Differential changes in cardiac phospholamban and sarcoplasmic reticular Ca(2+)-ATPase protein levels. Effects on Ca2+ transport and mechanics in compensated pressure-overload hypertrophy and congestive heart failure. Circ Res. 1995 Oct;77(4):759–764. doi: 10.1161/01.res.77.4.759. [DOI] [PubMed] [Google Scholar]
- Leblanc N., Hume J. R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990 Apr 20;248(4953):372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Spitzer K. W., Kohmoto O., Bridge J. H. Depolarization-induced Ca entry via Na-Ca exchange triggers SR release in guinea pig cardiac myocytes. Am J Physiol. 1994 Apr;266(4 Pt 2):H1422–H1433. doi: 10.1152/ajpheart.1994.266.4.H1422. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Jones L. R., Hathaway D. R., Henry B. G., Watanabe A. M. beta-Adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983 Jan 10;258(1):464–471. [PubMed] [Google Scholar]
- Lipp P., Niggli E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J Physiol. 1994 Feb 1;474(3):439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W., Grupp I. L., Harrer J., Ponniah S., Grupp G., Duffy J. J., Doetschman T., Kranias E. G. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994 Sep;75(3):401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- Luo W., Wolska B. M., Grupp I. L., Harrer J. M., Haghighi K., Ferguson D. G., Slack J. P., Grupp G., Doetschman T., Solaro R. J. Phospholamban gene dosage effects in the mammalian heart. Circ Res. 1996 May;78(5):839–847. doi: 10.1161/01.res.78.5.839. [DOI] [PubMed] [Google Scholar]
- López-López J. R., Shacklock P. S., Balke C. W., Wier W. G. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science. 1995 May 19;268(5213):1042–1045. doi: 10.1126/science.7754383. [DOI] [PubMed] [Google Scholar]
- Niggli E., Lederer W. J. Voltage-independent calcium release in heart muscle. Science. 1990 Oct 26;250(4980):565–568. doi: 10.1126/science.2173135. [DOI] [PubMed] [Google Scholar]
- Powell T., Noma A., Shioya T., Kozlowski R. Z. Turnover rate of the cardiac Na(+)-Ca2+ exchanger in guinea-pig ventricular myocytes. J Physiol. 1993 Dec;472:45–53. doi: 10.1113/jphysiol.1993.sp019935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham J. S., Cleemann L., Morad M. Functional coupling of Ca2+ channels and ryanodine receptors in cardiac myocytes. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):121–125. doi: 10.1073/pnas.92.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia H. H., Kaplan J. H., Ellis-Davies G. C., Lederer W. J. Rapid adaptation of cardiac ryanodine receptors: modulation by Mg2+ and phosphorylation. Science. 1995 Mar 31;267(5206):1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Lindemann J. P., Jones L. R. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to beta-adrenergic stimulation. J Biol Chem. 1989 Jul 5;264(19):11468–11474. [PubMed] [Google Scholar]
- You Y., Pelzer D. J., Pelzer S. Modulation of L-type Ca2+ current by fast and slow Ca2+ buffering in guinea pig ventricular cardiomyocytes. Biophys J. 1997 Jan;72(1):175–187. doi: 10.1016/S0006-3495(97)78656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]