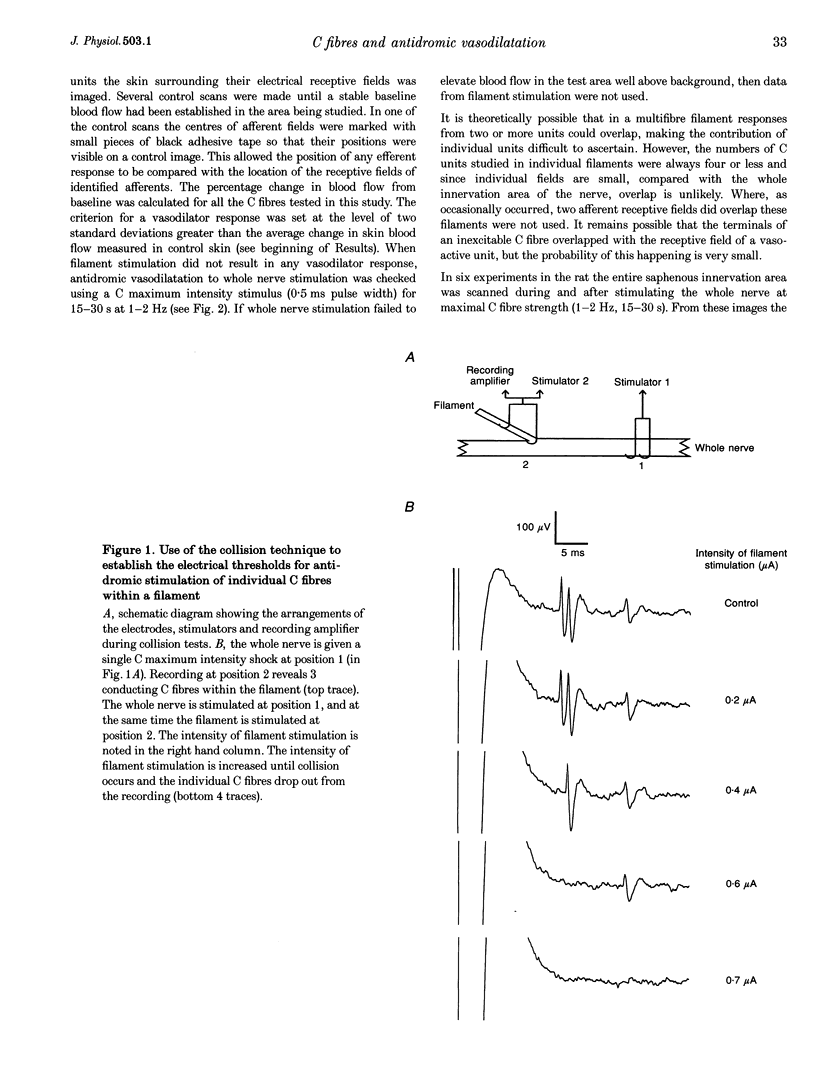
Abstract
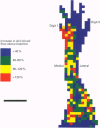
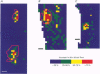
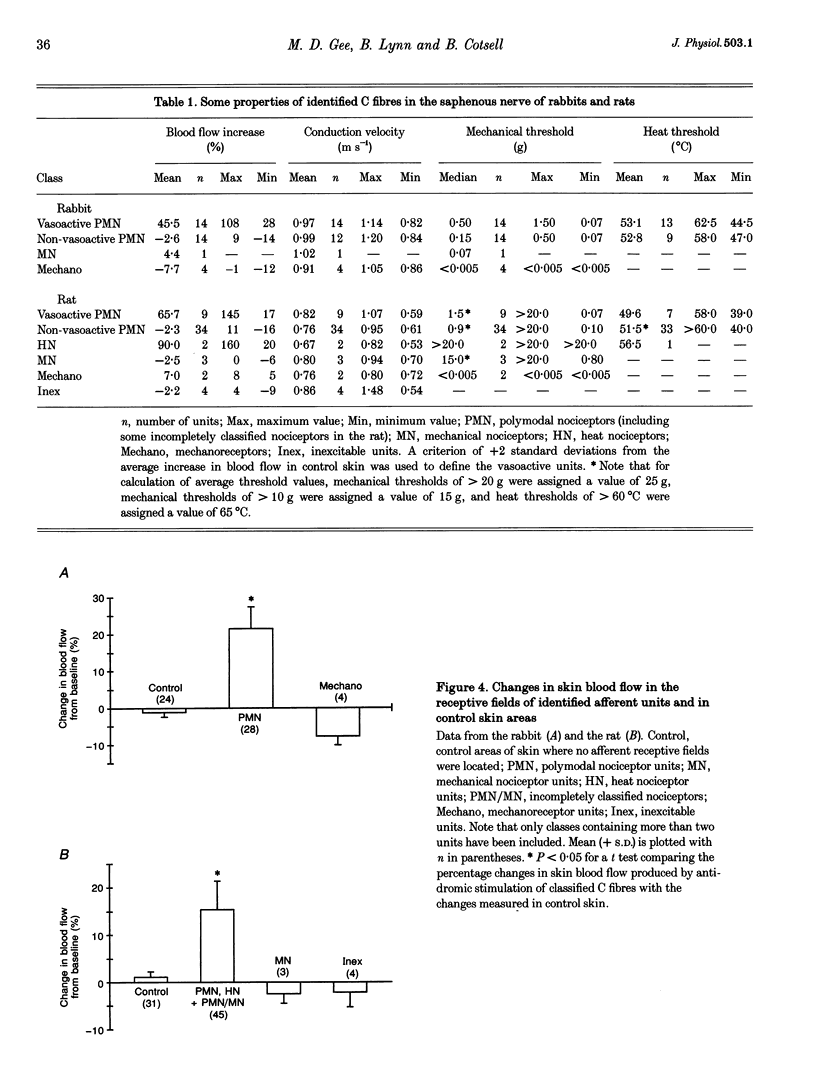
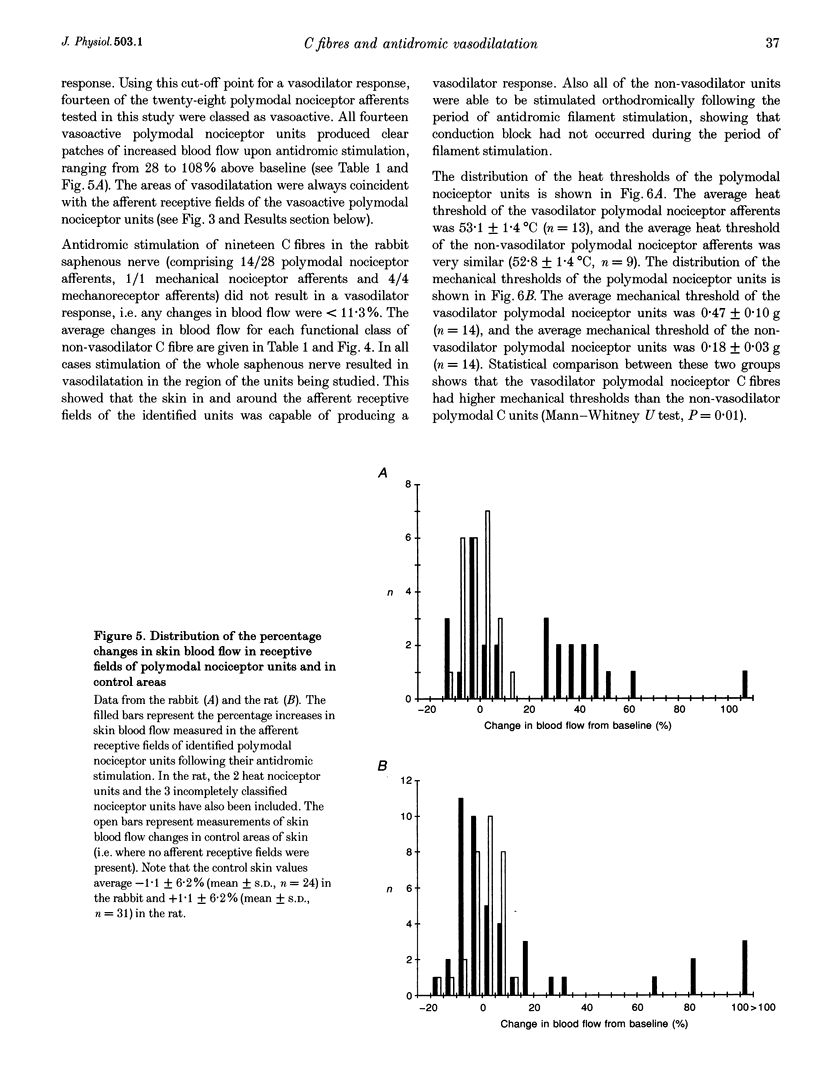
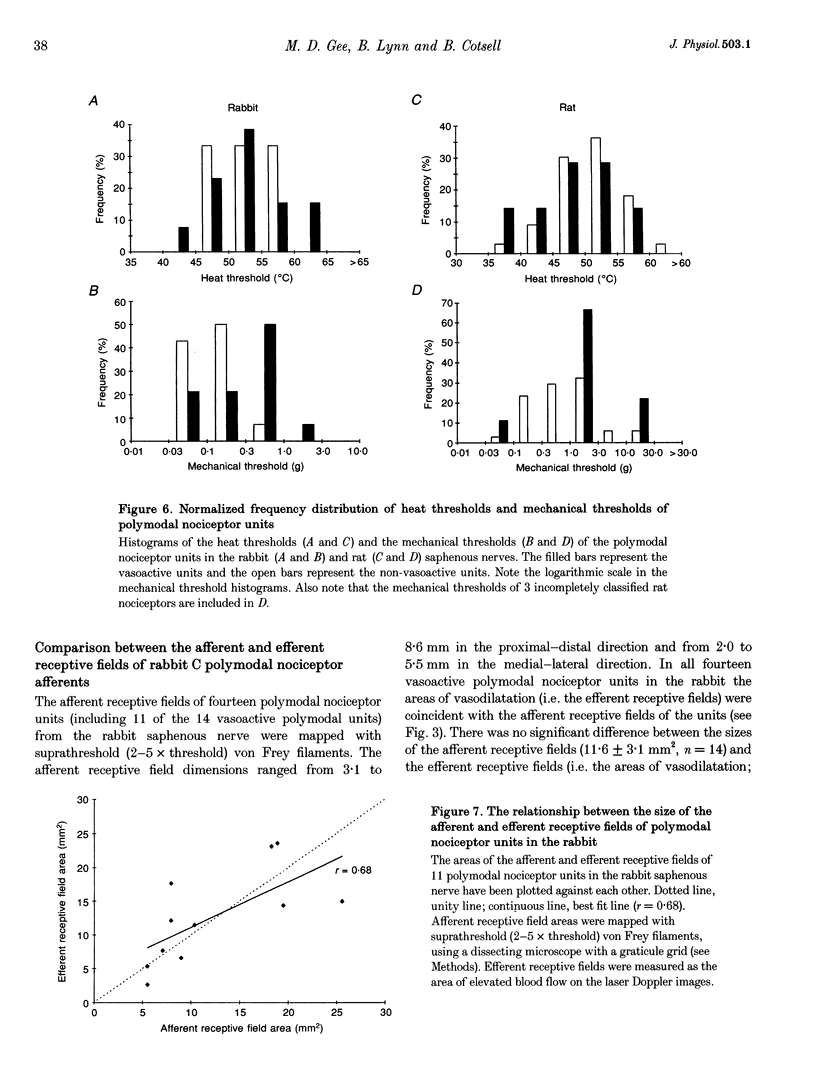
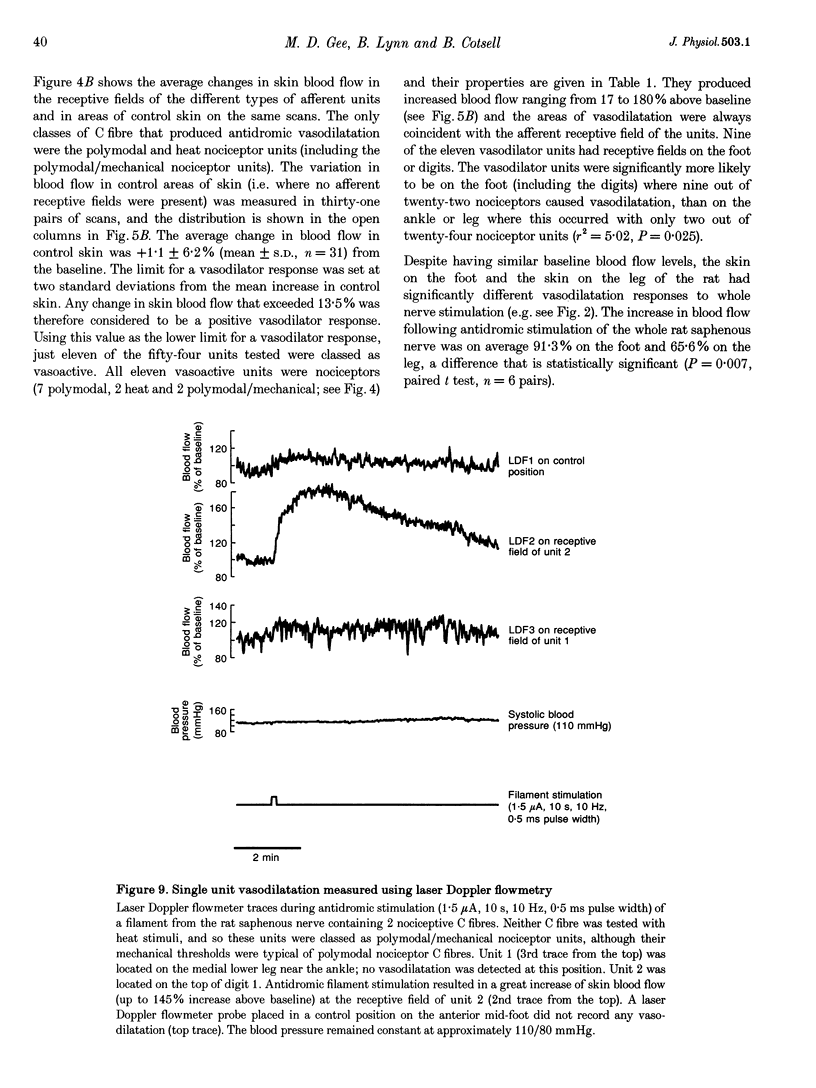
1. Skin blood flow was monitored during antidromic stimulation of identified cutaneous C fibres in fine filaments dissected from the saphenous nerve of anaesthetized rabbits and rats. The techniques used to monitor skin blood flow were laser Doppler perfusion imaging and laser Doppler flowmetry. 2. In the rabbit filaments a total of thirty-three C fibres were tested for their ability to produce antidromic vasodilatation. The only C fibres found to have vasodilator actions were of the polymodal nociceptor afferent class, and fourteen (50%) of the twenty-eight polymodal nociceptor units tested were vasoactive. The afferent receptive fields of polymodal nociceptor afferents were mapped carefully using suprathreshold mechanical stimuli, and there was a good correlation between afferent receptive field area and area of vasodilatation. 3. In the rat, eleven of the fifty-four C fibres antidromically stimulated had vasodilator actions. All eleven vasoactive C fibres were nociceptive and comprised seven polymodal nociceptor units, two heat nociceptor units and two incompletely classified nociceptor units. The area of increased blood flow was always coincident with the afferent field of the stimulated unit. 4. In the rat the vasodilator units were not evenly distributed over the saphenous nerve receptive field. Nine of the eleven vasoactive C fibres had receptive fields located on the foot or the digits, and only two were on the ankle or lower leg. Overall, the population of nociceptive C fibres was evenly distributed over the saphenous nerve receptive field. 5. In both the rabbit and the rat, a subclass of polymodal nociceptor afferents form the majority of the vasoactive units and will make the main contribution to axon reflex flare and other neurogenic inflammatory responses involving vasodilatation. The vasoactive polymodal nociceptor units tend to have relatively low mechanical sensitivity, although they have typical heat thresholds. In the rat heat nociceptor units also have vasodilator actions. However, such heat nociceptor units form a minor functional class of afferent C fibre in the rat saphenous nerve, and are not found in the rabbit saphenous nerve. 6. The findings from this study in the rabbit and the rat are compared with the situation in pig skin. The close relationship between afferent receptive field area and spread of flare across species is noted, and the way these measures increase with body size is discussed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asahina A., Hosoi J., Grabbe S., Granstein R. D. Modulation of Langerhans cell function by epidermal nerves. J Allergy Clin Immunol. 1995 Dec;96(6 Pt 2):1178–1182. doi: 10.1016/s0091-6749(95)70203-2. [DOI] [PubMed] [Google Scholar]
- Baumann T. K., Simone D. A., Shain C. N., LaMotte R. H. Neurogenic hyperalgesia: the search for the primary cutaneous afferent fibers that contribute to capsaicin-induced pain and hyperalgesia. J Neurophysiol. 1991 Jul;66(1):212–227. doi: 10.1152/jn.1991.66.1.212. [DOI] [PubMed] [Google Scholar]
- Bayliss W. M. On the origin from the spinal cord of the vaso-dilator fibres of the hind-limb, and on the nature of these fibres. J Physiol. 1901 Feb 28;26(3-4):173–209. doi: 10.1113/jphysiol.1901.sp000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharali L. A., Lisney S. J. Reinnervation of skin by polymodal nociceptors in rats. Prog Brain Res. 1988;74:247–251. doi: 10.1016/s0079-6123(08)63020-4. [DOI] [PubMed] [Google Scholar]
- Bharali L. A., Lisney S. J. The relationship between unmyelinated afferent type and neurogenic plasma extravasation in normal and reinnervated rat skin. Neuroscience. 1992;47(3):703–712. doi: 10.1016/0306-4522(92)90178-5. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Jernbeck J., Stains W., Kjartansson J., Haegerstrand A., Hökfelt T., Brodin E., Cuello A. C., Brown J. C. Calcitonin gene-related peptide-like immunoreactivity in nerve fibers in the human skin. Relation to fibers containing substance P-, somatostatin- and vasocactive intestinalpolypeptide-like immunoreactivity. Histochemistry. 1989;91(1):35–38. doi: 10.1007/BF00501907. [DOI] [PubMed] [Google Scholar]
- Fleischer E., Handwerker H. O., Joukhadar S. Unmyelinated nociceptive units in two skin areas of the rat. Brain Res. 1983 May 9;267(1):81–92. doi: 10.1016/0006-8993(83)91041-7. [DOI] [PubMed] [Google Scholar]
- García-Caballero T., Gallego R., Rosón E., Fraga M., Beiras A. Calcitonin gene-related peptide (CGRP) immunoreactivity in the neuroendocrine Merkel cells and nerve fibres of pig and human skin. Histochemistry. 1989;92(2):127–132. doi: 10.1007/BF00490231. [DOI] [PubMed] [Google Scholar]
- Garret C., Carruette A., Fardin V., Moussaoui S., Peyronel J. F., Blanchard J. C., Laduron P. M. Pharmacological properties of a potent and selective nonpeptide substance P antagonist. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):10208–10212. doi: 10.1073/pnas.88.22.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos A. P. Functional properties of primary afferent units probably related to pain mechanisms in primate glabrous skin. J Neurophysiol. 1976 Jan;39(1):71–83. doi: 10.1152/jn.1976.39.1.71. [DOI] [PubMed] [Google Scholar]
- Hosoi J., Murphy G. F., Egan C. L., Lerner E. A., Grabbe S., Asahina A., Granstein R. D. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993 May 13;363(6425):159–163. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br J Pharmacol Chemother. 1967 Sep;31(1):138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jancsó N., Jancsó-Gábor A., Szolcsányi J. The role of sensory nerve endings in neurogenic inflammation induced in human skin and in the eye and paw of the rat. Br J Pharmacol Chemother. 1968 May;33(1):32–41. doi: 10.1111/j.1476-5381.1968.tb00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W., Lisney S. J. Small diameter myelinated afferents produce vasodilatation but not plasma extravasation in rat skin. J Physiol. 1989 Aug;415:477–486. doi: 10.1113/jphysiol.1989.sp017732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenins P. Identification of the unmyelinated sensory nerves which evoke plasma extravasation in response to antidromic stimulation. Neurosci Lett. 1981 Sep 1;25(2):137–141. doi: 10.1016/0304-3940(81)90321-9. [DOI] [PubMed] [Google Scholar]
- Kolston J., Lisney S. J. A study of vasodilator responses evoked by antidromic stimulation of A delta afferent nerve fibers supplying normal and reinnervated rat skin. Microvasc Res. 1993 Sep;46(2):143–157. doi: 10.1006/mvre.1993.1043. [DOI] [PubMed] [Google Scholar]
- Kress M., Koltzenburg M., Reeh P. W., Handwerker H. O. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibers in vivo and in vitro. J Neurophysiol. 1992 Aug;68(2):581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- Lewin G. R., Lisney S. J., Mendell L. M. Neonatal Anti-NGF Treatment Reduces the Adelta- and C-Fibre Evoked Vasodilator Responses in Rat Skin: Evidence That Nociceptor Afferents Mediate Antidromic Vasodilatation. Eur J Neurosci. 1992;4(12):1213–1218. doi: 10.1111/j.1460-9568.1992.tb00146.x. [DOI] [PubMed] [Google Scholar]
- Lisney S. J. Functional aspects of the regeneration of unmyelinated axons in the rat saphenous nerve. J Neurol Sci. 1987 Sep;80(2-3):289–298. doi: 10.1016/0022-510x(87)90163-8. [DOI] [PubMed] [Google Scholar]
- Lynn B., Carpenter S. E. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982 Apr 22;238(1):29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- Lynn B., Cotsell B. Blood flow increases in the skin of the anaesthetized rat that follow antidromic sensory nerve stimulation and strong mechanical stimulation. Neurosci Lett. 1992 Mar 30;137(2):249–252. doi: 10.1016/0304-3940(92)90415-4. [DOI] [PubMed] [Google Scholar]
- Lynn B., Faulstroh K., Pierau F. K. The classification and properties of nociceptive afferent units from the skin of the anaesthetized pig. Eur J Neurosci. 1995 Mar 1;7(3):431–437. doi: 10.1111/j.1460-9568.1995.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Lynn B., Schütterle S., Pierau F. K. The vasodilator component of neurogenic inflammation is caused by a special subclass of heat-sensitive nociceptors in the skin of the pig. J Physiol. 1996 Jul 15;494(Pt 2):587–593. doi: 10.1113/jphysiol.1996.sp021516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn B., Shakhanbeh J. Neurogenic inflammation in the skin of the rabbit. Agents Actions. 1988 Dec;25(3-4):228–230. doi: 10.1007/BF01965019. [DOI] [PubMed] [Google Scholar]
- Lynn B. The heat sensitization of polymodal nociceptors in the rabbit and its independence of the local blood flow. J Physiol. 1979 Feb;287:493–507. doi: 10.1113/jphysiol.1979.sp012672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C., Woolf C. J., Fitzgerald M., Lindsay R. M., Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32(2):493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Schmelz M., Forster C., Ringkamp M., Torebjörk E., Handwerker H. Novel classes of responsive and unresponsive C nociceptors in human skin. J Neurosci. 1995 Jan;15(1 Pt 1):333–341. doi: 10.1523/JNEUROSCI.15-01-00333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea V. K., Perl E. R. Sensory receptors with unmyelinated (C) fibers innervating the skin of the rabbit's ear. J Neurophysiol. 1985 Sep;54(3):491–501. doi: 10.1152/jn.1985.54.3.491. [DOI] [PubMed] [Google Scholar]
- Szolcsányi J. Capsaicin-sensitive sensory nerve terminals with local and systemic efferent functions: facts and scopes of an unorthodox neuroregulatory mechanism. Prog Brain Res. 1996;113:343–359. doi: 10.1016/s0079-6123(08)61097-3. [DOI] [PubMed] [Google Scholar]