Abstract
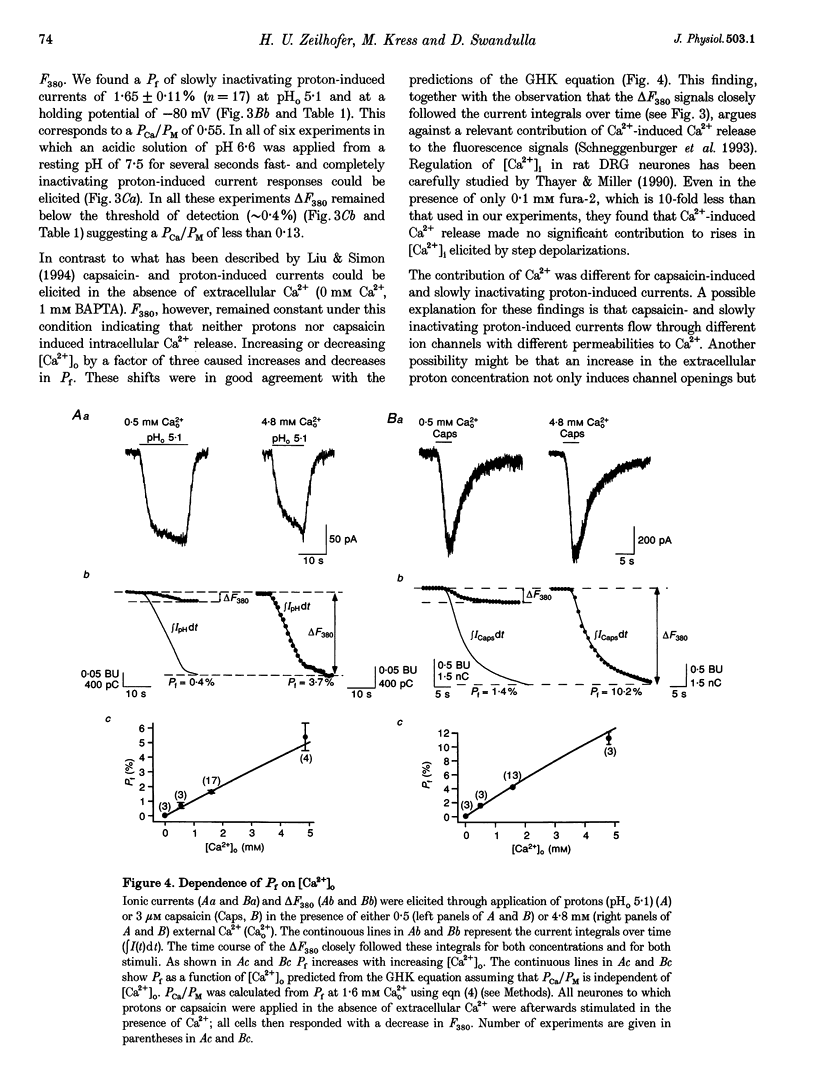
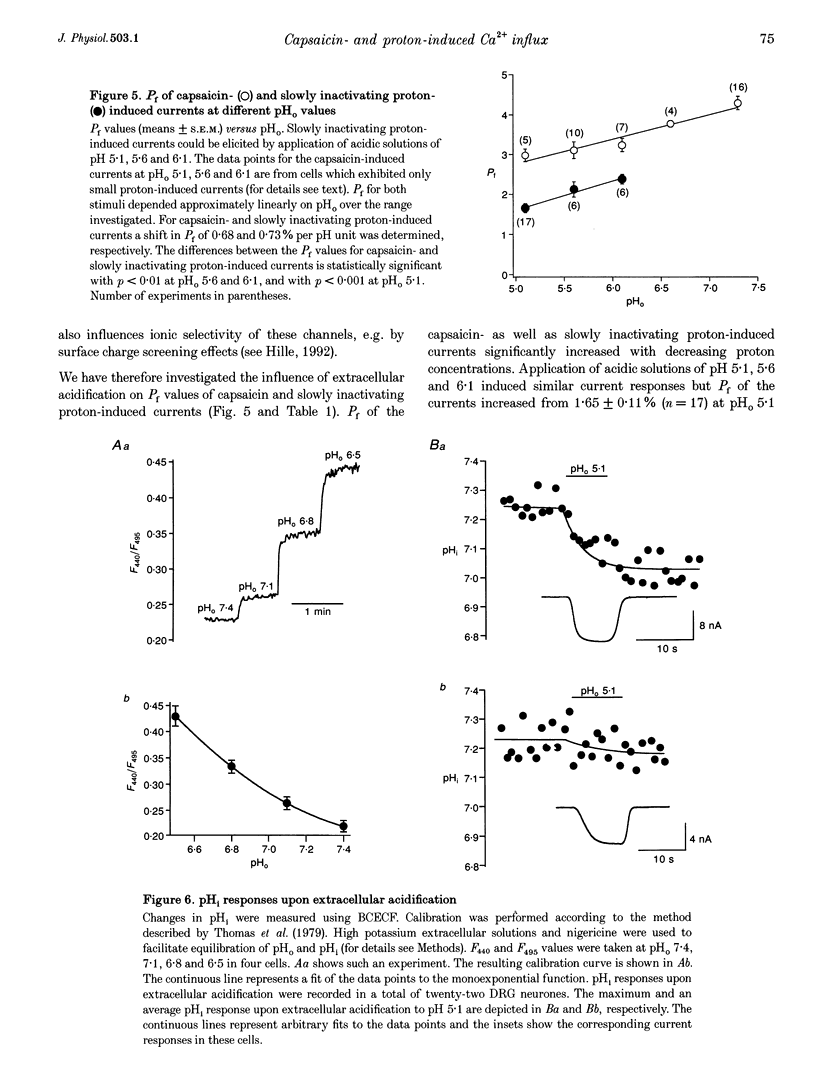
1. Capsaicin and protons cause excitation and sensitization of primary nociceptive afferents. In a subset of dorsal root ganglion (DRG) neurones, which probably represent nociceptive neurones, both capsaicin and protons induce slowly inactivating non-selective cation currents. Whole-cell as well as single channel currents activated by these two stimuli share many biophysical and physiological properties in these neurones. This has lead to the suggestion that protons and capsaicin might activate the same ion channels. 2. In this study we simultaneously measured fluorescence signals and whole-cell currents activated by capsaicin or protons in acutely isolated DRG neurones filled with a high concentration (1 mM) of the Ca2+ indicator dye fura-2. From these measurements the fractional contribution of Ca2+ (Pf; the portion of the whole-cell current carried by Ca2+) to capsaicin- and two types of proton-induced (fast and slowly inactivating) membrane currents was determined. 3. Capsaicin- and slowly inactivating proton-induced currents were accompanied by a change in fluorescence that was dependent on the presence of extracellular Ca2+. With 1.6 mM extracellular Ca2+ and at a holding potential of -80 mV Pf of capsaicin-induced currents (at pH 7.3) was 4.30 +/- 0.17% (mean +/- S.E.M.; no. of experiments, n = 16) and of slowly inactivating proton-induced currents (at pH 5.1) was 1.65 +/- 0.11% (n = 17). Pf of fast inactivating proton-induced currents was negligible. 4. Pf of capsaicin- and slowly inactivating proton-induced currents increased with increasing extracellular Ca2+ concentration (0.5-4.8 mM). 5. Pf of both current types decreased linearly with decreasing extracellular pH by about 0.7% per pH unit over the pH range investigated. When determined at the same extracellular pH Pf values were significantly different for the two current types at all pH values tested. 6. In summary, our results provide evidence that capsaicin and protons activate ion channels which are markedly permeable to Ca2+. The fractional contribution of Ca2+, however, was significantly different for capsaicin- and slowly inactivating proton-induced currents. This strongly suggests that the two stimuli activate different populations of ion channels and supports the possibility that Ca2+ influx through these channels may be important for Ca(2+)-dependent sensitization of primary nociceptive neurones.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer M. B., Simmons M. L., Murphy S., Gebhart G. F. Bradykinin and capsaicin stimulate cyclic GMP production in cultured rat dorsal root ganglion neurons via a nitrosyl intermediate. J Neurosci Res. 1993 Oct 15;36(3):280–289. doi: 10.1002/jnr.490360306. [DOI] [PubMed] [Google Scholar]
- Bevan S., Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends Neurosci. 1994 Dec;17(12):509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bevan S., Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. J Physiol. 1991 Feb;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnashev N., Zhou Z., Neher E., Sakmann B. Fractional calcium currents through recombinant GluR channels of the NMDA, AMPA and kainate receptor subtypes. J Physiol. 1995 Jun 1;485(Pt 2):403–418. doi: 10.1113/jphysiol.1995.sp020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholewinski A., Burgess G. M., Bevan S. The role of calcium in capsaicin-induced desensitization in rat cultured dorsal root ganglion neurons. Neuroscience. 1993 Aug;55(4):1015–1023. doi: 10.1016/0306-4522(93)90315-7. [DOI] [PubMed] [Google Scholar]
- Culp W. J., Ochoa J., Cline M., Dotson R. Heat and mechanical hyperalgesia induced by capsaicin. Cross modality threshold modulation in human C nociceptors. Brain. 1989 Oct;112(Pt 5):1317–1331. doi: 10.1093/brain/112.5.1317. [DOI] [PubMed] [Google Scholar]
- Docherty R. J., Yeats J. C., Bevan S., Boddeke H. W. Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 1996 Apr;431(6):828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Frings S., Seifert R., Godde M., Kaupp U. B. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995 Jul;15(1):169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- García-Hirschfeld J., López-Briones L. G., Belmonte C., Valdeolmillos M. Intracellular free calcium responses to protons and capsaicin in cultured trigeminal neurons. Neuroscience. 1995 Jul;67(1):235–243. doi: 10.1016/0306-4522(95)00055-n. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harvey J. S., Davis C., James I. F., Burgess G. M. Activation of protein kinase C by the capsaicin analogue resiniferatoxin in sensory neurones. J Neurochem. 1995 Sep;65(3):1309–1317. doi: 10.1046/j.1471-4159.1995.65031309.x. [DOI] [PubMed] [Google Scholar]
- Holzer P. Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol Rev. 1991 Jun;43(2):143–201. [PubMed] [Google Scholar]
- Jacobus W. E., Taylor G. J., 4th, Hollis D. P., Nunnally R. L. Phosphorus nuclear magnetic resonance of perfused working rat hearts. Nature. 1977 Feb 24;265(5596):756–758. doi: 10.1038/265756a0. [DOI] [PubMed] [Google Scholar]
- Konnerth A., Lux H. D., Morad M. Proton-induced transformation of calcium channel in chick dorsal root ganglion cells. J Physiol. 1987 May;386:603–633. doi: 10.1113/jphysiol.1987.sp016553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovalchuk YuN, Krishtal O. A., Nowycky M. C. The proton-activated inward current of rat sensory neurons includes a calcium component. Neurosci Lett. 1990 Jul 31;115(2-3):237–242. doi: 10.1016/0304-3940(90)90461-h. [DOI] [PubMed] [Google Scholar]
- Lang E., Novak A., Reeh P. W., Handwerker H. O. Chemosensitivity of fine afferents from rat skin in vitro. J Neurophysiol. 1990 Apr;63(4):887–901. doi: 10.1152/jn.1990.63.4.887. [DOI] [PubMed] [Google Scholar]
- Liu L., Simon S. A. A rapid capsaicin-activated current in rat trigeminal ganglion neurons. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):738–741. doi: 10.1073/pnas.91.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. The use of fura-2 for estimating Ca buffers and Ca fluxes. Neuropharmacology. 1995 Nov;34(11):1423–1442. doi: 10.1016/0028-3908(95)00144-u. [DOI] [PubMed] [Google Scholar]
- Petersen M., LaMotte R. H. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993 Jul;54(1):37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- Petsche U., Fleischer E., Lembeck F., Handwerker H. O. The effect of capsaicin application to a peripheral nerve on impulse conduction in functionally identified afferent nerve fibres. Brain Res. 1983 Apr 18;265(2):233–240. doi: 10.1016/0006-8993(83)90337-2. [DOI] [PubMed] [Google Scholar]
- Santicioli P., Del Bianco E., Figini M., Bevan S., Maggi C. A. Effect of capsazepine on the release of calcitonin gene-related peptide-like immunoreactivity (CGRP-LI) induced by low pH, capsaicin and potassium in rat soleus muscle. Br J Pharmacol. 1993 Oct;110(2):609–612. doi: 10.1111/j.1476-5381.1993.tb13854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simone D. A., Ochoa J. Early and late effects of prolonged topical capsaicin on cutaneous sensibility and neurogenic vasodilatation in humans. Pain. 1991 Dec;47(3):285–294. doi: 10.1016/0304-3959(91)90217-L. [DOI] [PubMed] [Google Scholar]
- Spruston N., Jonas P., Sakmann B. Dendritic glutamate receptor channels in rat hippocampal CA3 and CA1 pyramidal neurons. J Physiol. 1995 Jan 15;482(Pt 2):325–352. doi: 10.1113/jphysiol.1995.sp020521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen K. H., Reeh P. W., Anton F., Handwerker H. O. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992 Jan;12(1):86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szolcsányi J. Selective responsiveness of polymodal nociceptors of the rabbit ear to capsaicin, bradykinin and ultra-violet irradiation. J Physiol. 1987 Jul;388:9–23. doi: 10.1113/jphysiol.1987.sp016598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Vizzard M. A., Erdman S. L., de Groat W. C. Increased expression of neuronal nitric oxide synthase in dorsal root ganglion neurons after systemic capsaicin administration. Neuroscience. 1995 Jul;67(1):1–5. doi: 10.1016/0306-4522(95)00137-8. [DOI] [PubMed] [Google Scholar]
- White R. L., Doeller J. E., Verselis V. K., Wittenberg B. A. Gap junctional conductance between pairs of ventricular myocytes is modulated synergistically by H+ and Ca++. J Gen Physiol. 1990 Jun;95(6):1061–1075. doi: 10.1085/jgp.95.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer H. U., Swandulla D., Reeh P. W., Kress M. Ca2+ permeability of the sustained proton-induced cation current in adult rat dorsal root ganglion neurons. J Neurophysiol. 1996 Nov;76(5):2834–2840. doi: 10.1152/jn.1996.76.5.2834. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Neher E. Calcium permeability of nicotinic acetylcholine receptor channels in bovine adrenal chromaffin cells. Pflugers Arch. 1993 Dec;425(5-6):511–517. doi: 10.1007/BF00374879. [DOI] [PubMed] [Google Scholar]



