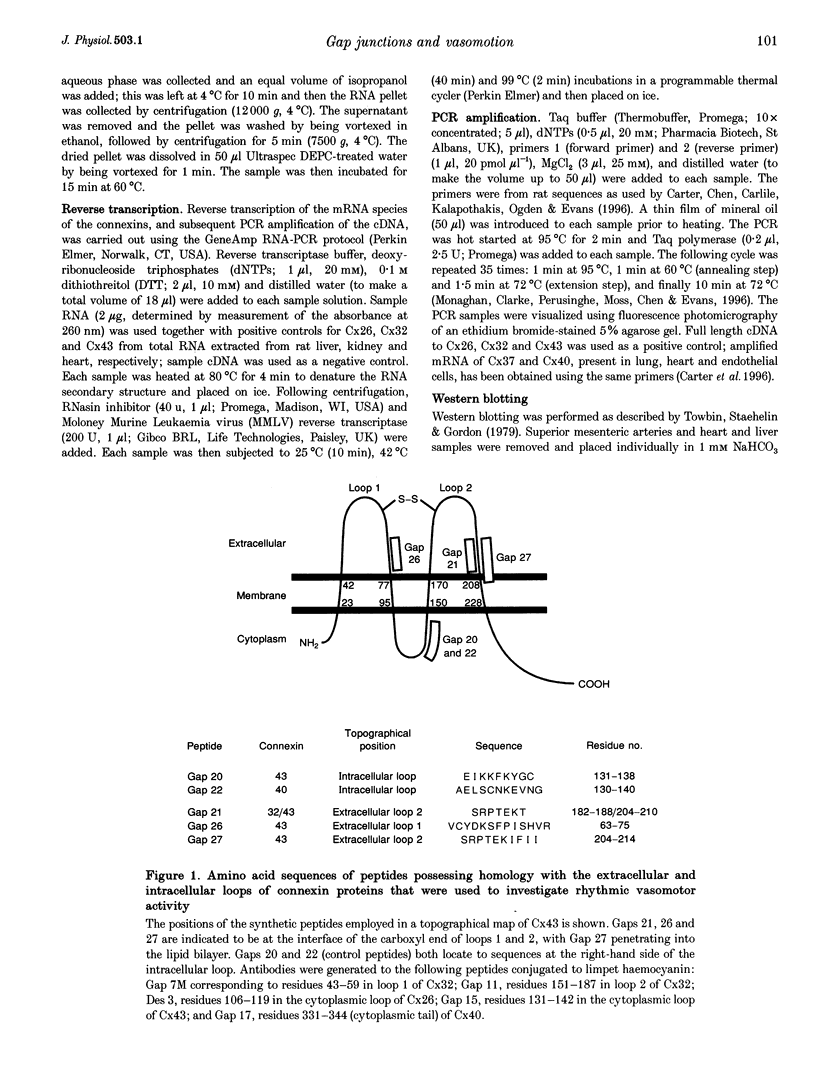
Abstract
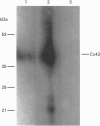
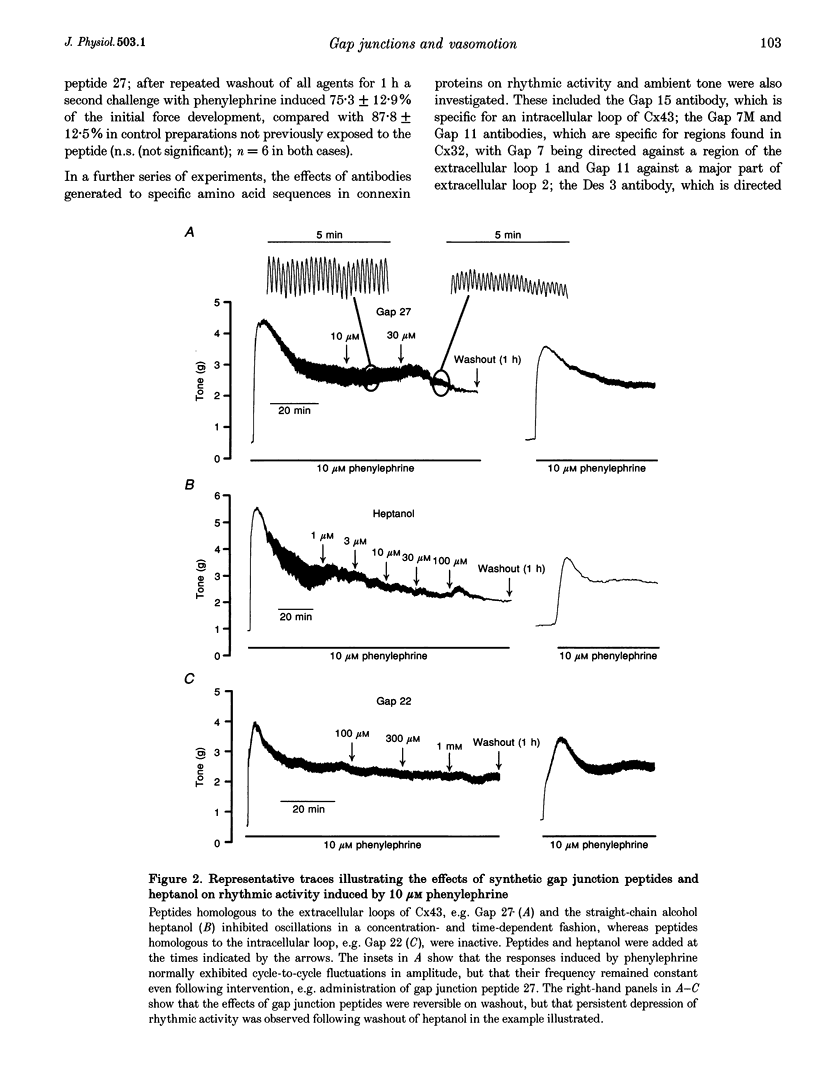
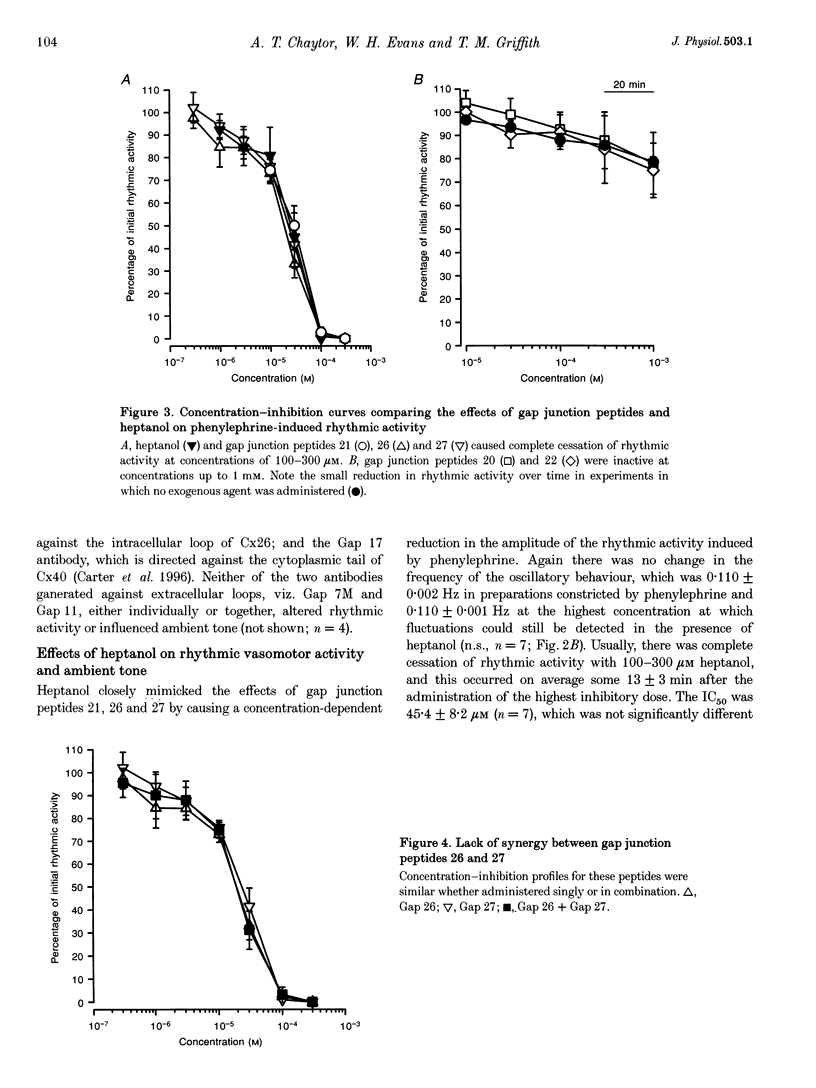
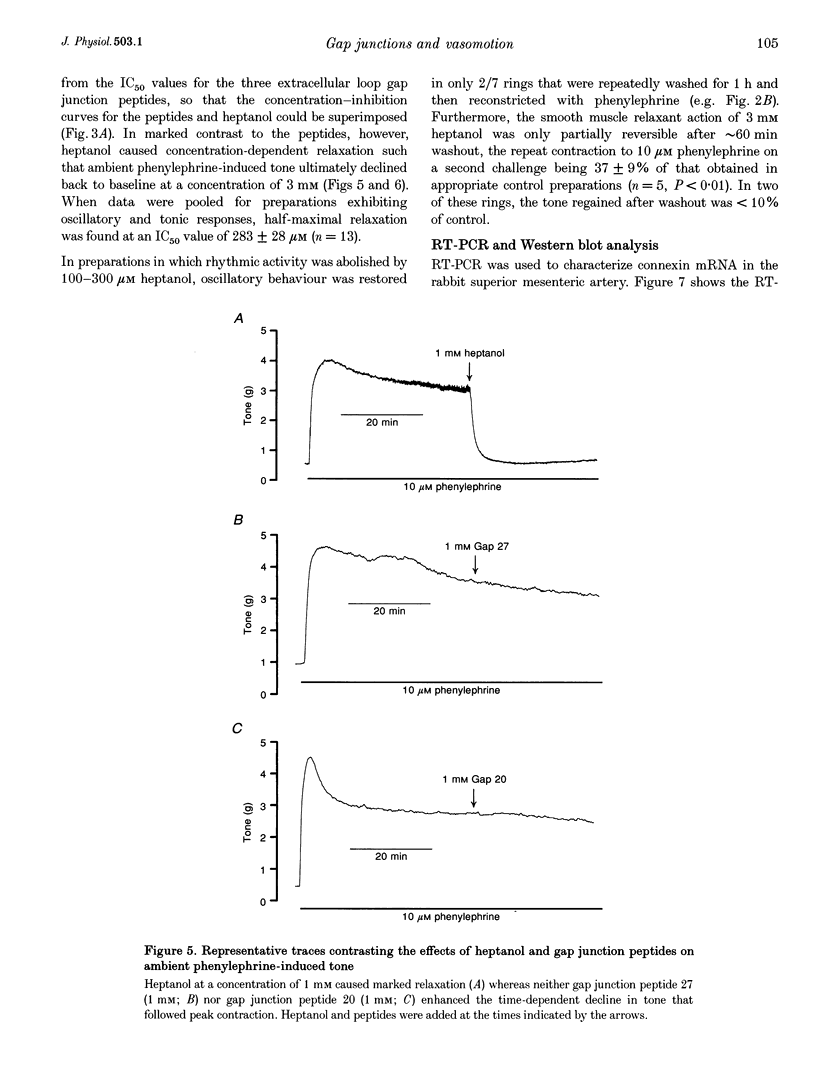
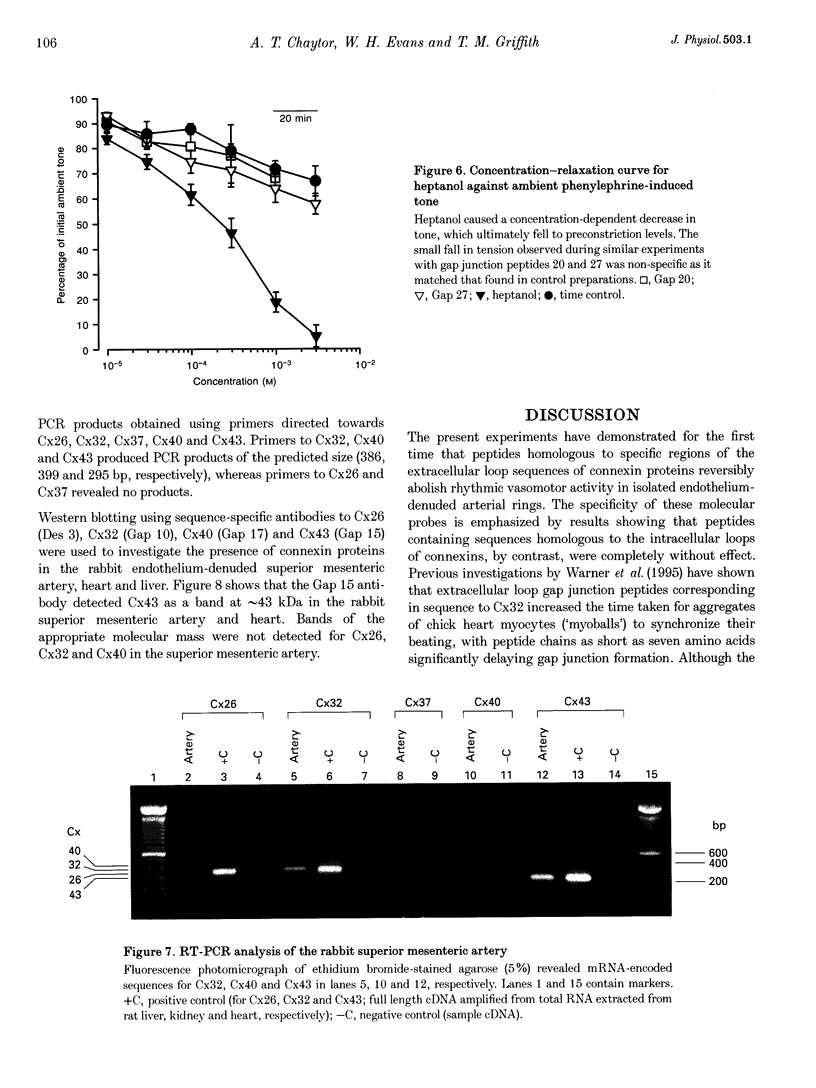
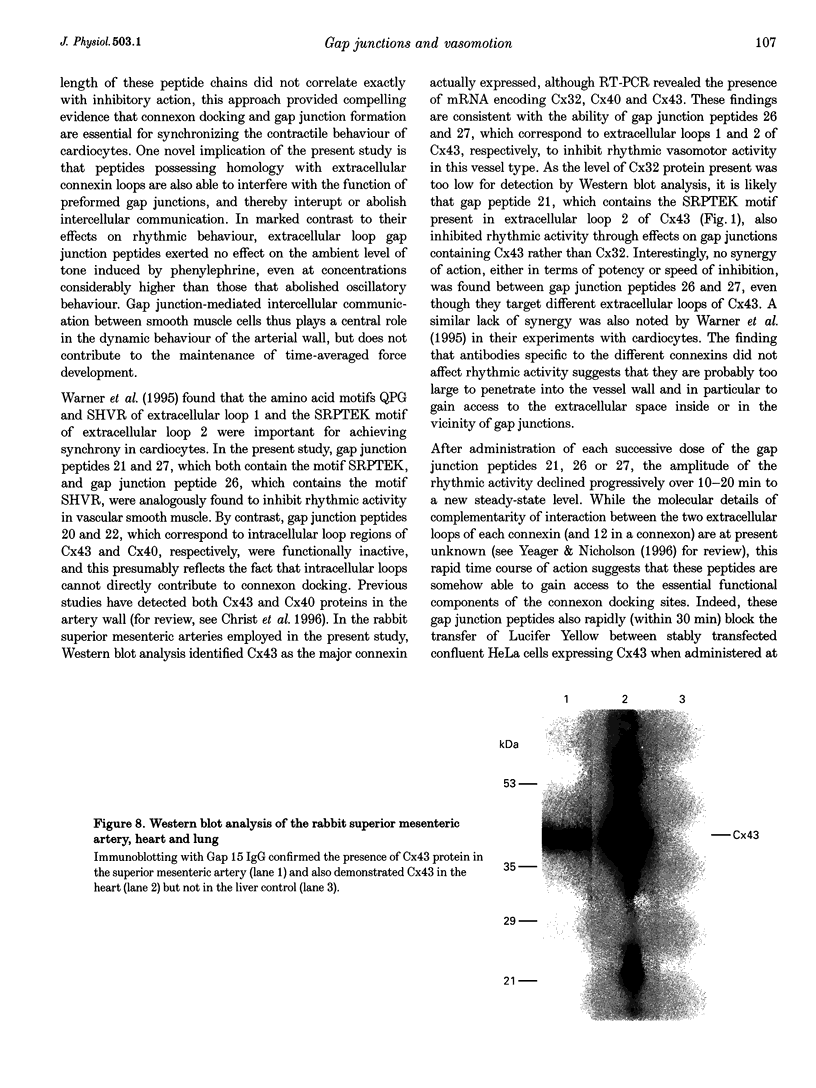
1. Phenylephrine (10 microM) evoked rises in tension in isolated rings of endothelium-denuded rabbit superior mesenteric artery. These increases consisted of a tonic component with superimposed rhythmic activity, the frequency of which generally remained constant over time but whose amplitude exhibited cycle-to-cycle variability. 2. The amplitude, but not the frequency, of the rhythmic activity was affected by a series of short peptides possessing sequence homology with extracellular loops 1 and 2 of connexin 43 (Cx43). Oscillatory behaviour was abolished at concentrations of 100-300 microM (IC50 of 20-30 microM), without change in average tone. No synergy was evident between peptides corresponding to the extracellular loops, and cytoplasmic loop peptides were biologically inactive. 3. The putative gap junction inhibitor heptanol mimicked the action of the extracellular loop peptides and abolished rhythmic activity at concentrations of 100-300 microM without effects on frequency. However, in marked contrast to the peptides, heptanol completely inhibited the contraction evoked by phenylephrine (IC50, 283 +/- 28 microM). 4. The presence of mRNA encoding Cx32, Cx40 and Cx43 was detected in the rabbit superior mesenteric artery by reverse transcriptase-polymerase chain reaction. Western blot analysis showed that Cx43 was the major connexin in the endothelium-denuded vessel wall. 5. We conclude that intercellular communication between vascular smooth muscle cells via gap junctions is essential for synchronized rhythmic activity in isolated arterial tissue, whereas tonic force development appears to be independent of cell-cell coupling. The molecular specificity of the peptide probes employed in the study suggests that the smooth muscle relaxant effects of heptanol may be non-specific and unrelated to inhibition of gap junctional communication.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler S. K., Poenie M., Tsien R. Y., Taylor P. Agonist-stimulated oscillations and cycling of intracellular free calcium in individual cultured muscle cells. J Biol Chem. 1988 Feb 5;263(4):1952–1959. [PubMed] [Google Scholar]
- Bastiaanse E. M., Jongsma H. J., van der Laarse A., Takens-Kwak B. R. Heptanol-induced decrease in cardiac gap junctional conductance is mediated by a decrease in the fluidity of membranous cholesterol-rich domains. J Membr Biol. 1993 Nov;136(2):135–145. doi: 10.1007/BF02505758. [DOI] [PubMed] [Google Scholar]
- Becker D. L., Evans W. H., Green C. R., Warner A. Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J Cell Sci. 1995 Apr;108(Pt 4):1455–1467. doi: 10.1242/jcs.108.4.1455. [DOI] [PubMed] [Google Scholar]
- Burt J. M. Block of intercellular communication: interaction of intracellular H+ and Ca2+. Am J Physiol. 1987 Oct;253(4 Pt 1):C607–C612. doi: 10.1152/ajpcell.1987.253.4.C607. [DOI] [PubMed] [Google Scholar]
- Burt J. M., Spray D. C. Volatile anesthetics block intercellular communication between neonatal rat myocardial cells. Circ Res. 1989 Sep;65(3):829–837. doi: 10.1161/01.res.65.3.829. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Chen X. Y., Carlile G., Kalapothakis E., Ogden D., Evans W. H. Porcine aortic endothelial gap junctions: identification and permeation by caged InsP3. J Cell Sci. 1996 Jul;109(Pt 7):1765–1773. doi: 10.1242/jcs.109.7.1765. [DOI] [PubMed] [Google Scholar]
- Christ G. J. Modulation of alpha 1-adrenergic contractility in isolated vascular tissues by heptanol: a functional demonstration of the potential importance of intercellular communication to vascular response generation. Life Sci. 1995;56(10):709–721. doi: 10.1016/0024-3205(95)00001-m. [DOI] [PubMed] [Google Scholar]
- Christ G. J., Spray D. C., el-Sabban M., Moore L. K., Brink P. R. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996 Oct;79(4):631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- Darrow B. J., Laing J. G., Lampe P. D., Saffitz J. E., Beyer E. C. Expression of multiple connexins in cultured neonatal rat ventricular myocytes. Circ Res. 1995 Mar;76(3):381–387. doi: 10.1161/01.res.76.3.381. [DOI] [PubMed] [Google Scholar]
- Deutsch D. E., Williams J. A., Yule D. I. Halothane and octanol block Ca2+ oscillations in pancreatic acini by multiple mechanisms. Am J Physiol. 1995 Nov;269(5 Pt 1):G779–G788. doi: 10.1152/ajpgi.1995.269.5.G779. [DOI] [PubMed] [Google Scholar]
- Donahue H. J., Guilak F., Vander Molen M. A., McLeod K. J., Rubin C. T., Grande D. A., Brink P. R. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap junctions. J Bone Miner Res. 1995 Sep;10(9):1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- Evans W. H., Carlile G., Rahman S., Török K. Gap junction communication channel: peptides and anti-peptide antibodies as structural probes. Biochem Soc Trans. 1992 Nov;20(4):856–861. doi: 10.1042/bst0200856. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H. Complexity of chaotic vasomotion is insensitive to flow and pressure but can be regulated by external control. Am J Physiol. 1995 Aug;269(2 Pt 2):H656–H668. doi: 10.1152/ajpheart.1995.269.2.H656. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H. EDRF suppresses chaotic pressure oscillations in isolated resistance artery without influencing intrinsic complexity. Am J Physiol. 1994 May;266(5 Pt 2):H1786–H1800. doi: 10.1152/ajpheart.1994.266.5.H1786. [DOI] [PubMed] [Google Scholar]
- Kimura H., Oyamada Y., Ohshika H., Mori M., Oyamada M. Reversible inhibition of gap junctional intercellular communication, synchronous contraction, and synchronism of intracellular Ca2+ fluctuation in cultured neonatal rat cardiac myocytes by heptanol. Exp Cell Res. 1995 Oct;220(2):348–356. doi: 10.1006/excr.1995.1325. [DOI] [PubMed] [Google Scholar]
- Kumar N. M., Gilula N. B. The gap junction communication channel. Cell. 1996 Feb 9;84(3):381–388. doi: 10.1016/s0092-8674(00)81282-9. [DOI] [PubMed] [Google Scholar]
- Li H., Liu T. F., Lazrak A., Peracchia C., Goldberg G. S., Lampe P. D., Johnson R. G. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J Cell Biol. 1996 Aug;134(4):1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohler F. S., Heath J. E. Oscillating heat flow from rabbit's pinna. Am J Physiol. 1988 Sep;255(3 Pt 2):R464–R469. doi: 10.1152/ajpregu.1988.255.3.R464. [DOI] [PubMed] [Google Scholar]
- Monaghan P., Clarke C., Perusinghe N. P., Moss D. W., Chen X. Y., Evans W. H. Gap junction distribution and connexin expression in human breast. Exp Cell Res. 1996 Feb 25;223(1):29–38. doi: 10.1006/excr.1996.0055. [DOI] [PubMed] [Google Scholar]
- Parthimos D., Edwards D. H., Griffith T. M. Comparison of chaotic and sinusoidal vasomotion in the regulation of microvascular flow. Cardiovasc Res. 1996 Mar;31(3):388–399. [PubMed] [Google Scholar]
- Perkins G., Goodenough D., Sosinsky G. Three-dimensional structure of the gap junction connexon. Biophys J. 1997 Feb;72(2 Pt 1):533–544. doi: 10.1016/s0006-3495(97)78693-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S., Evans W. H. Topography of connexin32 in rat liver gap junctions. Evidence for an intramolecular disulphide linkage connecting the two extracellular peptide loops. J Cell Sci. 1991 Nov;100(Pt 3):567–578. doi: 10.1242/jcs.100.3.567. [DOI] [PubMed] [Google Scholar]
- Sneyd J., Keizer J., Sanderson M. J. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J. 1995 Nov;9(14):1463–1472. doi: 10.1096/fasebj.9.14.7589988. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venance L., Piomelli D., Glowinski J., Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature. 1995 Aug 17;376(6541):590–594. doi: 10.1038/376590a0. [DOI] [PubMed] [Google Scholar]
- Warner A., Clements D. K., Parikh S., Evans W. H., DeHaan R. L. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol. 1995 Nov 1;488(Pt 3):721–728. doi: 10.1113/jphysiol.1995.sp021003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner A. Gap junctions in development--a perspective. Semin Cell Biol. 1992 Feb;3(1):81–91. doi: 10.1016/s1043-4682(10)80009-1. [DOI] [PubMed] [Google Scholar]
- Watts S. W., Tsai M. L., Loch-Caruso R., Webb R. C. Gap junctional communication and vascular smooth muscle reactivity: use of tetraethylammonium chloride. J Vasc Res. 1994 Nov-Dec;31(6):307–313. doi: 10.1159/000159057. [DOI] [PubMed] [Google Scholar]
- Watts S. W., Webb R. C. Vascular gap junctional communication is increased in mineralocorticoid-salt hypertension. Hypertension. 1996 Nov;28(5):888–893. doi: 10.1161/01.hyp.28.5.888. [DOI] [PubMed] [Google Scholar]
- Yeager M., Nicholson B. J. Structure of gap junction intercellular channels. Curr Opin Struct Biol. 1996 Apr;6(2):183–192. doi: 10.1016/s0959-440x(96)80073-x. [DOI] [PubMed] [Google Scholar]
- Ying X., Minamiya Y., Fu C., Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circ Res. 1996 Oct;79(4):898–908. doi: 10.1161/01.res.79.4.898. [DOI] [PubMed] [Google Scholar]