Abstract
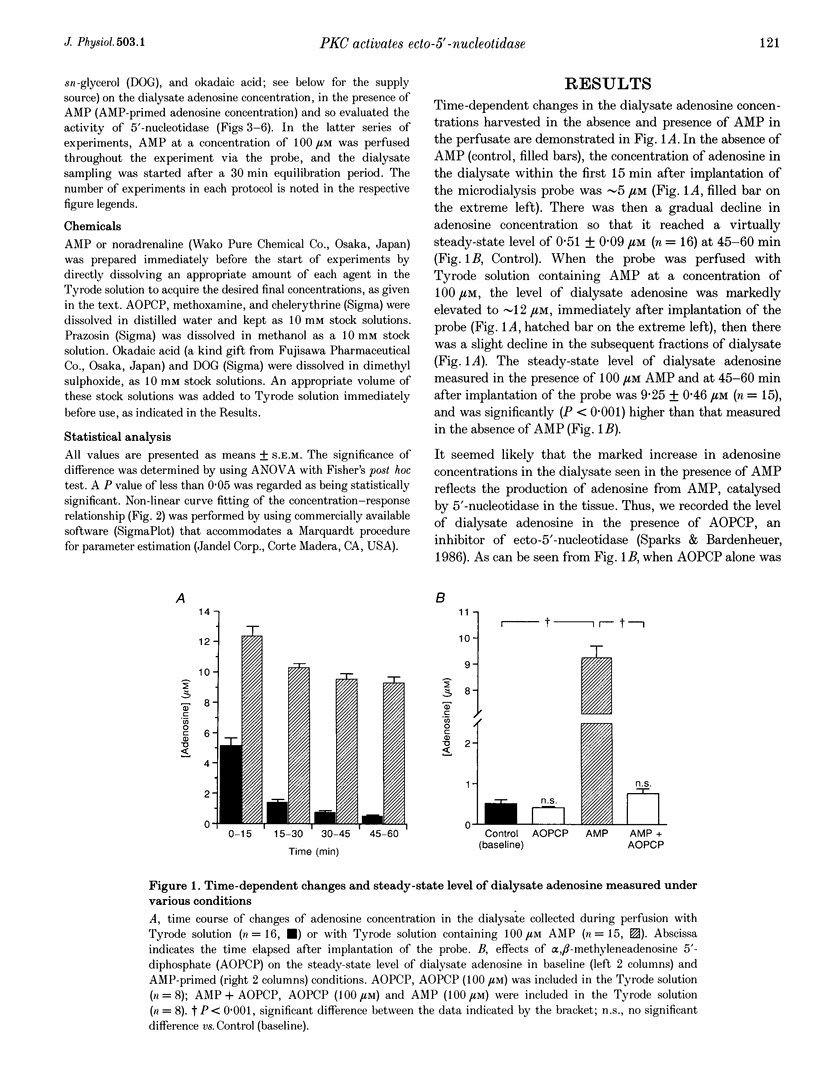
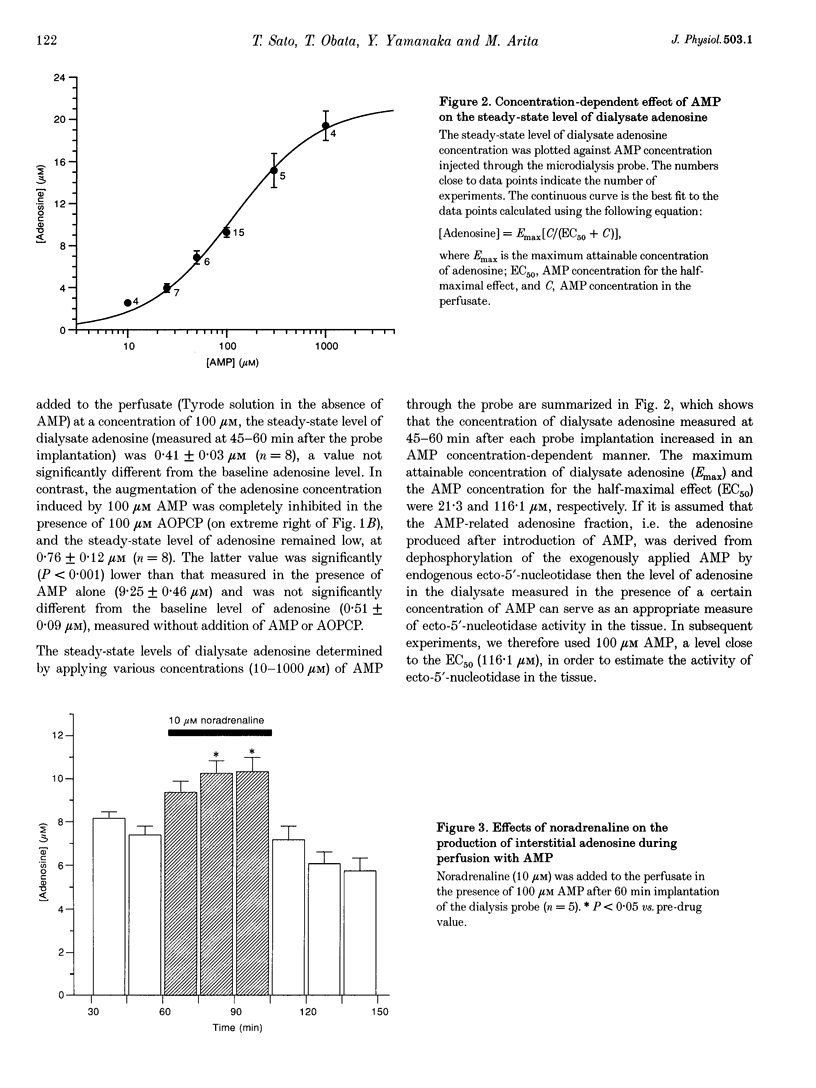
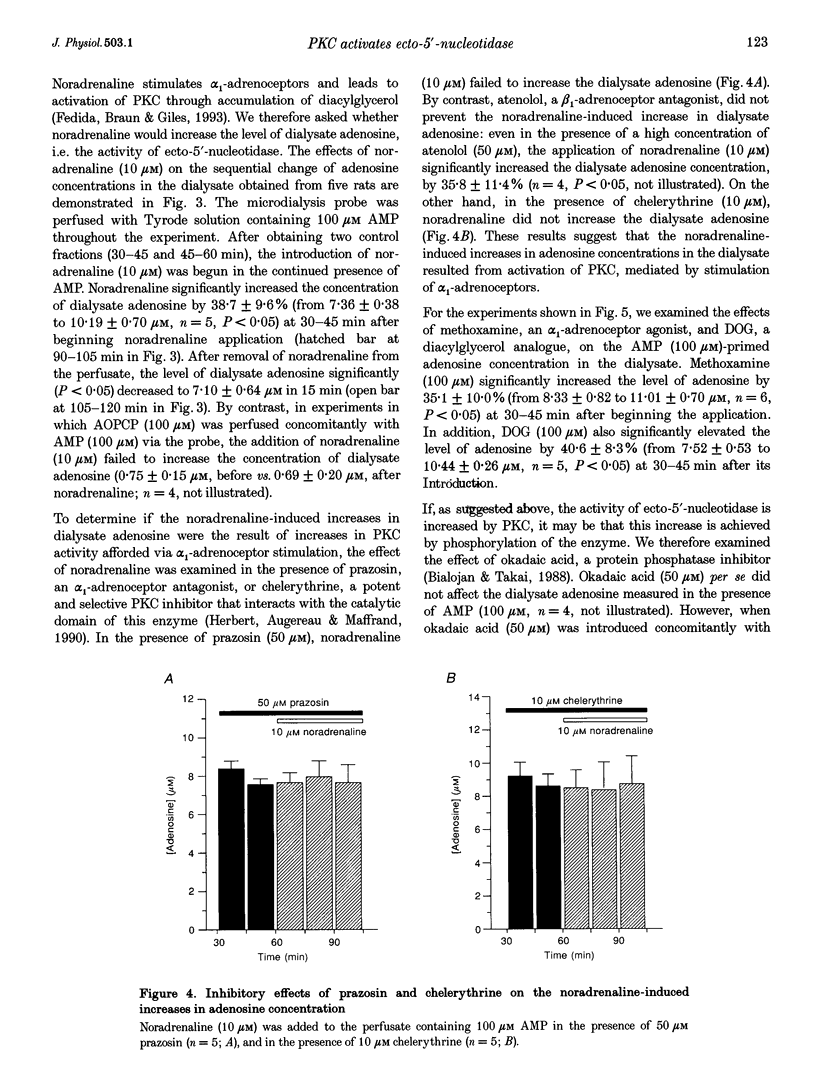
1. To determine whether protein kinase C (PKC)-mediated activation of ecto-5'-nucleotidase would increase interstitial adenosine concentrations in the rat heart in vivo, we made use of the microdialysis technique and a flexibly mounted probe, which was implanted in the left ventricular myocardium and perfused with Tyrode solution. 2. The baseline level of dialysate adenosine was 0.51 +/- 0.09 microM (n = 16). Perfusion of adenosine 5'-monophosphate (AMP, 100 microM) through the probe increased the dialysate adenosine concentration markedly to 9.25 +/- 0.46 microM (n = 15). alpha, beta-Methyleneadenosine 5'-diphosphate (AOPCP, 100 microM), an inhibitor of ecto-5'-nucleotidase, abolished the AMP-induced increase in dialysate adenosine, but did not affect the baseline level of adenosine. These observations suggest that the dialysate adenosine obtained during the perfusion with AMP, but not the baseline levels of adenosine, originated from the dephosphorylation of AMP by ecto-5'-nucleotidase. Thus, the level of adenosine measured during AMP perfusion gives an index of the activity of ecto-5'-nucleotidase in the tissue. 3. Noradrenaline (10 microM) increased the adenosine concentration measured in the presence of 100 microM AMP (i.e. the activity of ecto-5'-nucleotidase) by 38.7 +/- 9.6% (n = 5, P < 0.05), an increase which was inhibited by an antagonist of the alpha 1-adrenoceptor (prazosin, 50 microM) or of PKC (chelerythrine, 10 microM). Further application of either the alpha 1-adrenoceptor agonist methoxamine (100 microM) or the diacylglycerol analogue 1,2-dioctanoyl-sn-glycerol (DOG, 100 microM) also increased the adenosine concentration by 35.1 +/- 10.0% (n = 6, P < 0.05) or 40.6 +/- 8.3% (n = 5, P < 0.05), respectively. 4. The presence of okadaic acid (50 microM), an inhibitor of protein phosphatase, enhanced the noradrenaline-induced increase in adenosine concentration by 112.4 +/- 35.9% (n = 4, P < 0.05), to a level significantly (P < 0.05) greater than the increase caused by noradrenaline alone (38.7 +/- 9.6%). 5. These data provide the first evidence that alpha 1-adrenoceptor stimulation and the subsequent activation of PKC can increase adenosine concentrations in interstitial spaces of ventricular muscle in vivo, through activation of endogenous ecto-5'-nucleotidase.
Full text
PDF

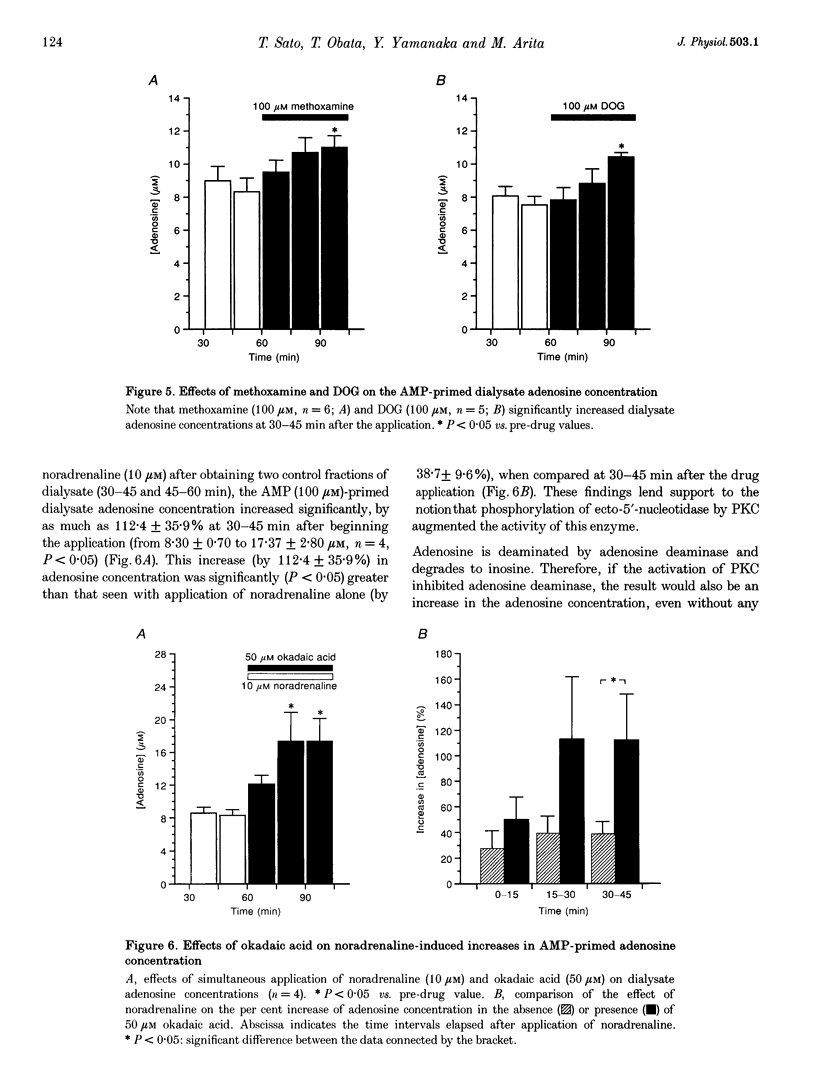



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A., Locke-Winter C., Rogers K. B., Mitchell M. B., Brew E. C., Cairns C. B., Bensard D. D., Harken A. H. Preconditioning against myocardial dysfunction after ischemia and reperfusion by an alpha 1-adrenergic mechanism. Circ Res. 1993 Oct;73(4):656–670. doi: 10.1161/01.res.73.4.656. [DOI] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ely S. W., Berne R. M. Protective effects of adenosine in myocardial ischemia. Circulation. 1992 Mar;85(3):893–904. doi: 10.1161/01.cir.85.3.893. [DOI] [PubMed] [Google Scholar]
- Fedida D., Braun A. P., Giles W. R. Alpha 1-adrenoceptors in myocardium: functional aspects and transmembrane signaling mechanisms. Physiol Rev. 1993 Apr;73(2):469–487. doi: 10.1152/physrev.1993.73.2.469. [DOI] [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Headrick J. P., Matherne G. P., Berne R. M. Myocardial adenosine formation during hypoxia: effects of ecto-5'-nucleotidase inhibition. J Mol Cell Cardiol. 1992 Mar;24(3):295–303. doi: 10.1016/0022-2828(92)93166-h. [DOI] [PubMed] [Google Scholar]
- Herbert J. M., Augereau J. M., Gleye J., Maffrand J. P. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1990 Nov 15;172(3):993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Hori M., Kitakaze M. Adenosine, the heart, and coronary circulation. Hypertension. 1991 Nov;18(5):565–574. doi: 10.1161/01.hyp.18.5.565. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Hori M., Kamada T. Role of adenosine and its interaction with alpha adrenoceptor activity in ischaemic and reperfusion injury of the myocardium. Cardiovasc Res. 1993 Jan;27(1):18–27. doi: 10.1093/cvr/27.1.18. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Hori M., Morioka T., Minamino T., Takashima S., Okazaki Y., Node K., Komamura K., Iwakura K., Itoh T. Alpha 1-adrenoceptor activation increases ecto-5'-nucleotidase activity and adenosine release in rat cardiomyocytes by activating protein kinase C. Circulation. 1995 Apr 15;91(8):2226–2234. doi: 10.1161/01.cir.91.8.2226. [DOI] [PubMed] [Google Scholar]
- Kitakaze M., Hori M., Morioka T., Minamino T., Takashima S., Sato H., Shinozaki Y., Chujo M., Mori H., Inoue M. Alpha 1-adrenoceptor activation mediates the infarct size-limiting effect of ischemic preconditioning through augmentation of 5'-nucleotidase activity. J Clin Invest. 1994 May;93(5):2197–2205. doi: 10.1172/JCI117216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasley R. D., Mentzer R. M., Jr Adenosine improves recovery of postischemic myocardial function via an adenosine A1 receptor mechanism. Am J Physiol. 1992 Nov;263(5 Pt 2):H1460–H1465. doi: 10.1152/ajpheart.1992.263.5.H1460. [DOI] [PubMed] [Google Scholar]
- Lasley R. D., Rhee J. W., Van Wylen D. G., Mentzer R. M., Jr Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J Mol Cell Cardiol. 1990 Jan;22(1):39–47. doi: 10.1016/0022-2828(90)90970-d. [DOI] [PubMed] [Google Scholar]
- Liu G. S., Thornton J., Van Winkle D. M., Stanley A. W., Olsson R. A., Downey J. M. Protection against infarction afforded by preconditioning is mediated by A1 adenosine receptors in rabbit heart. Circulation. 1991 Jul;84(1):350–356. doi: 10.1161/01.cir.84.1.350. [DOI] [PubMed] [Google Scholar]
- Liu Y., Ytrehus K., Downey J. M. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J Mol Cell Cardiol. 1994 May;26(5):661–668. doi: 10.1006/jmcc.1994.1078. [DOI] [PubMed] [Google Scholar]
- Obata T., Hosokawa H., Yamanaka Y. In vivo monitoring of norepinephrine and .OH generation on myocardial ischemic injury by dialysis technique. Am J Physiol. 1994 Mar;266(3 Pt 2):H903–H908. doi: 10.1152/ajpheart.1994.266.3.H903. [DOI] [PubMed] [Google Scholar]
- Schulz R., Rose J., Post H., Heusch G. Involvement of endogenous adenosine in ischaemic preconditioning in swine. Pflugers Arch. 1995 Jun;430(2):273–282. doi: 10.1007/BF00374659. [DOI] [PubMed] [Google Scholar]
- Sparks H. V., Jr, Bardenheuer H. Regulation of adenosine formation by the heart. Circ Res. 1986 Feb;58(2):193–201. doi: 10.1161/01.res.58.2.193. [DOI] [PubMed] [Google Scholar]
- Speechly-Dick M. E., Mocanu M. M., Yellon D. M. Protein kinase C. Its role in ischemic preconditioning in the rat. Circ Res. 1994 Sep;75(3):586–590. doi: 10.1161/01.res.75.3.586. [DOI] [PubMed] [Google Scholar]
- Strasser R. H., Braun-Dullaeus R., Walendzik H., Marquetant R. Alpha 1-receptor-independent activation of protein kinase C in acute myocardial ischemia. Mechanisms for sensitization of the adenylyl cyclase system. Circ Res. 1992 Jun;70(6):1304–1312. doi: 10.1161/01.res.70.6.1304. [DOI] [PubMed] [Google Scholar]
- Sullivan J. M., Alpers J. B. In vitro regulation of rat heart 5'-nucleotidase by adenine nucleotides and magnesium. J Biol Chem. 1971 May 10;246(9):3057–3063. [PubMed] [Google Scholar]
- Thornton J. D., Liu G. S., Olsson R. A., Downey J. M. Intravenous pretreatment with A1-selective adenosine analogues protects the heart against infarction. Circulation. 1992 Feb;85(2):659–665. doi: 10.1161/01.cir.85.2.659. [DOI] [PubMed] [Google Scholar]
- Toombs C. F., McGee S., Johnston W. E., Vinten-Johansen J. Myocardial protective effects of adenosine. Infarct size reduction with pretreatment and continued receptor stimulation during ischemia. Circulation. 1992 Sep;86(3):986–994. doi: 10.1161/01.cir.86.3.986. [DOI] [PubMed] [Google Scholar]
- Truong V. L., Collinson A. R., Lowenstein J. M. 5'-Nucleotidases in rat heart. Evidence for the occurrence of two soluble enzymes with different substrate specificities. Biochem J. 1988 Jul 1;253(1):117–121. doi: 10.1042/bj2530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wylen D. G., Schmit T. J., Lasley R. D., Gingell R. L., Mentzer R. M., Jr Cardiac microdialysis in isolated rat hearts: interstitial purine metabolites during ischemia. Am J Physiol. 1992 Jun;262(6 Pt 2):H1934–H1938. doi: 10.1152/ajpheart.1992.262.6.H1934. [DOI] [PubMed] [Google Scholar]
- Van Wylen D. G., Willis J., Sodhi J., Weiss R. J., Lasley R. D., Mentzer R. M., Jr Cardiac microdialysis to estimate interstitial adenosine and coronary blood flow. Am J Physiol. 1990 Jun;258(6 Pt 2):H1642–H1649. doi: 10.1152/ajpheart.1990.258.6.H1642. [DOI] [PubMed] [Google Scholar]
- Zhu Q. Y., Headrick J. P., Berne R. M. Transmural distribution of extracellular purines in isolated guinea pig heart. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):657–660. doi: 10.1073/pnas.88.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]


