Abstract
Low-intensity noisy galvanic vestibular stimulation (nGVS) is a promising non-invasive treatment for enhancing vestibular perceptual performance and postural control in patients with chronic vestibular hypofunction. However, this approach has so far been studied mainly under laboratory conditions. Evidence indicates that continuous application of nGVS in daily life is necessary for it to be effective. To address this need, we have developed a mobile nGVS stimulator and conducted a series of pilot studies to evaluate its safety, tolerability, functionality, and therapeutic effects. The device is a lightweight, compact, and portable AC stimulator featuring a user-friendly interface for the individualized adjustment of nGVS parameters. It includes an integrated motion sensor that automatically activates stimulation during body movement and deactivates it during inactivity, optimizing its practical use in real-world settings. The stimulator adheres to strict safety standards and, in initial long-term use, has exhibited only mild side effects (e.g., skin irritation and headaches), likely attributable to the current electrode placement, which requires further optimization. As expected, the device consistently elicits known vestibular sensorimotor reflex responses in healthy individuals. Importantly, further pilot studies in healthy participants demonstrate that the device can reliably replicate known facilitating effects on vestibular perception and postural control. Together, these findings suggest that this mobile stimulation device can facilitate the translation of nGVS into therapeutic everyday use.
Keywords: galvanic vestibular stimulation, stochastic resonance, bilateral vestibulopathy, remote therapy, electrical stimulation, balance disorders, motion sensor
1. Introduction
A permanent reduction of vestibular function can lead to symptoms such as postural instability or impaired gaze stabilization during head movements (1–3). These symptoms are particularly pronounced when affected individuals cannot compensate for the vestibular loss with other sensory inputs, such as while walking in the dark or on uneven surfaces (4, 5). As a result, this can significantly restrict everyday mobility, increase the risk of falls, and negatively affect quality of life (5–7). Chronic vestibular hypofunction occurs in well characterized cases of peripheral bilateral vestibulopathy (BVP) due to different aetiologies; it also becomes more common with aging due to the deterioration of peripheral vestibular structures (also called presbyvestibulopathy) (2, 8) and is sometimes associated with central neurodegenerative disorders, such as Parkinson’s disease (9).
Current therapeutic options for vestibular loss are largely limited to physical therapy, which aims to train the remaining intact sensory systems to compensate for the deficit. While this therapy is effective for most patients, it rarely leads to a sufficient improvement (10). Vibrotactile feedback is being explored to either enhance physical therapy (11) or provide continuous support for postural regulation in daily life (12). Besides, several alternative treatment approaches are being developed that aim to directly address vestibular hypofunction. One approach involves the use of a vestibular implant, which has shown promising effects in alleviating postural and other vestibular-related symptoms in selected patients (13). However, the benefits of such an invasive vestibular implant must be carefully balanced against the risks. A key limitation of such an implant is that it can only replace parts of the peripheral vestibular function, specifically the semicircular canals, but not the otolith organs (14, 15). Moreover, the precise placement of the implant is critical and involves several surgical risks, particularly the potential for permanent hearing loss.
Most patients with chronic vestibular hypofunction retain some residual vestibular function (1, 16). For these patients, a promising non-invasive alternative therapeutic approach involves enhancing this residual function through electrical low-intensity noise stimulation of the vestibular periphery, i.e., noisy galvanic vestibular stimulation (nGVS) (17, 18). A typical finding in patients with residual function is elevated detection thresholds for processing vestibular stimuli. Consequently, a relevant portion of naturally occurring vestibular stimuli remains subthreshold and therefore goes undetected (19). The idea behind the nGVS treatment approach is to lower these elevated thresholds through a phenomenon known as stochastic resonance, according to which the presence of a non-zero stochastic interference (i.e., noise) in a sensory system can amplify subthreshold stimuli and raise them above the detection threshold (20, 21). The weak noise in the vestibular periphery is achieved through galvanic vestibular stimulation (GVS) – an established technique for modulating vestibular receptors and afferents (22). In young healthy adults, nGVS lowers the vestibular perception threshold at intermediate, imperceptible noise levels (23–25) indicating that the signal-to-noise ratio in the vestibular system can be enhanced by weak external noise, even in this population. Beyond that, nGVS has been proven to facilitate a broad range of therapeutic effects in different clinical cohorts. In patients with BVP, it has been shown to enhance residual vestibular perceptual and sensorimotor functions while stabilizing balance during both static and dynamic postural tasks such as walking (26–30). Similar effects have been observed in elderly individuals and patients with various neurodegenerative diseases such as Parkinson’s disease (31–33) or Progressive Supranuclear Palsy (34).
The primary limitation of these experiments is their laboratory-based nature. The ecological validity of these treatment effects still needs to be evaluated in everyday applications with clinically relevant endpoints such as mobility, fall risk, and quality of life. Another significant factor is that most previous studies focused on short-term stimulation protocols below 1 h. While improvements in performance are frequently observed during stimulation, there is little evidence from previous studies to support long-lasting aftereffects of the stimulation (35, 36). Therefore, it is likely that continuous treatment with nGVS will be necessary to obtain sustained treatment effects. However, the feasibility and effectiveness of this approach in long-term use in everyday patient life remain unclear.
Evaluating the effects of nGVS in everyday life over longer periods requires a mobile and everyday-suitable stimulation device. In response to this need, we developed a prototype wearable nGVS device in collaboration with an industry partner (neuroConn GmbH, Ilmenau, Germany). In the following, we will present the fundamental technical requirements for the stimulator and the design of the prototype. Subsequently, we will discuss initial findings regarding the tolerability of long-term stimulation with the prototype and the occurrence of side effects. After that, we will present initial results that assess the function of the device (i.e., vestibular nature of stimulation), particularly in terms of its ability to elicit vestibular sensorimotor responses at suprathreshold intensities. In the final section, we will present a series of experiments that attempt to replicate previously known facilitatory effects on vestibular perception and postural control using the mobile therapeutic device.
2. Stimulator design
We identified a set of requirements that the mobile stimulation device must meet to be suitable for use in patients. Since it is intended for all-day use, it must be comfortably wearable, portable, and energy-efficient to facilitate full-day usage. As nGVS therapy is only needed when the patient is actively moving, the stimulation device should be activated by head or body movement associated with stance and gait and automatically deactivate after a period of inactivity. Finally, the device should include a simple user interface, as the stimulation parameters for nGVS are known to vary between patients and therefore should be manually adjustable.
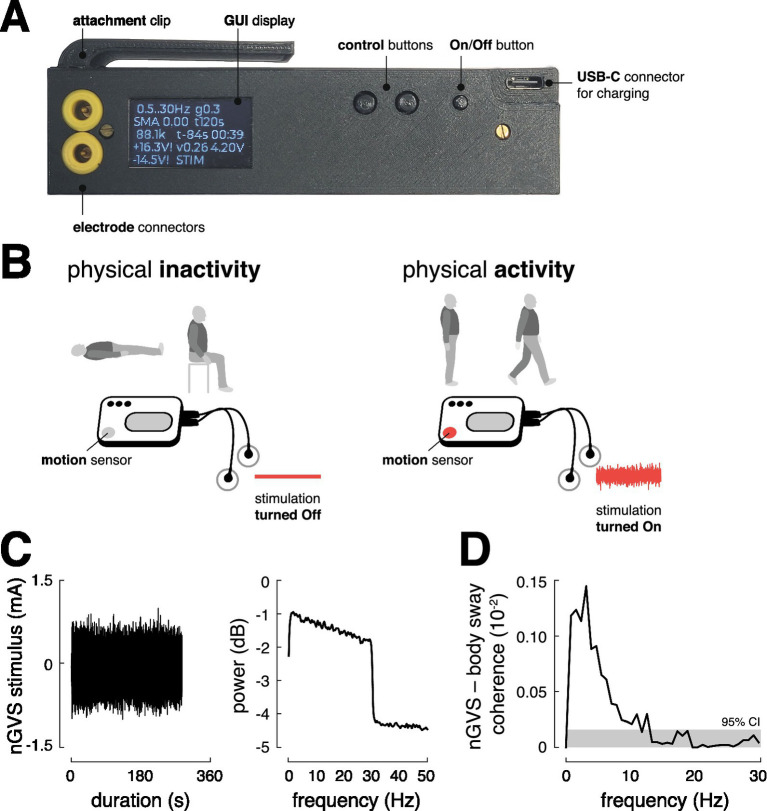
To meet these requirements, we designed a compact and lightweight prototype device (L x W x H: 122 × 32 × 30 mm, 100 g) that is robust enough to withstand being dropped and water-resistant to protect against moisture and water splashes (Figure 1A). It is powered by a rechargeable Li-Ion battery (3.7 V, 3100mAh) providing a battery life of at least 24 h, considering a typical current consumption (i.e., 65 mA). The device includes a triaxial accelerometer (MPU-6050, TDK InvenSense, Tokyo, Japan) that continuously monitors the patient’s movement and automatically switches the stimulation on at defined thresholds (movement intensity >0.135 g (37)) (Figure 1B). The stimulation is automatically switched off after an adjustable period (6 s – 30 min) during which the stimulation device remains still. The device includes a simple user interface (screen and adjustment buttons) through which the stimulation intensity, minimum stimulation duration, and other settings can be adjusted, and information about the current stimulation mode, operating voltage, impedance, etc., can be viewed. The AC source generates a zero-mean white noise signal (0.5–30 Hz) with 10 adjustable peak amplitudes ranging from ±0.1 mA to ±1.0 mA.
Figure 1.
Stimulator design and function. (A) Prototype of the stimulator with control buttons and a display for individual adjustment of the stimulation mode and an external charging option via USB-C connector. (B) The mobile stimulator contains an integrated motion sensor that can selectively turn the stimulation on and off depending on the user’s activity or inactivity. (C) Profile of the nGVS stimulus and corresponding power spectrum. (D) Significant coupling between the nGVS stimulus (±1 mA peak amplitude) and body sway, as assessed via magnitude-squared coherence (the gray area indicates the 95% confidence interval), suggests that the nGVS stimulus at suprathreshold intensity activates vestibular sensorimotor pathways as intended. GUI, graphical user interface; nGVS, noisy galvanic vestibular stimulation; CI, confidence intervals.
To ensure the device’s output during stimulation is equivalent to other white noise-generating devices, the mean output current was measured using a digital multimeter (SDM3065X, Siglent Technologies, Shenzhen, China), and the spectral properties of the passband, as well as the signal-to-noise ratio, were analyzed with a digital oscilloscope (DHO1072, Rigol Technologies, Suzhou, China). These analyses confirmed that the prototype’s stimulation output is consistent with that of established stimulation devices. For all investigations reported below, the stimulator was attached to the collar of the participants and stimulation was delivered via a pair of conductive-rubber electrodes (40 × 60 mm) attached over the left and right mastoid process behind the ears. The electrodes were held in place using an elastic headband. Electrode gel was applied before electrode placement to achieve uniform current density and minimize any irritation to the skin due to stimulation.
To ensure not only user comfort but also safety, the design of the mobile nGVS device incorporates several critical features that balance usability with safety measures. Internal safety features of the nGVS device include automatic shut-off, overload protection, and gradual adjustments in stimulation intensity to prevent abrupt changes during stimulation on- or offset (2 s interval fade in/ fade out). The device limits the operating voltage to ±17 V through firmware restricted pulse width modulation (PWM) control and hardware constraints to ±18 V using Zener diodes. Output power is further limited by a restricted pulse width for the step-up DC/DC converters, also reducing voltage under unscheduled load conditions. Additionally, the device employs AC coupling to prevent the application of DC currents, eliminating the possibility of charge accumulation during stimulation. To protect both the device and the patient, the system employs a relay that diverts unwanted currents potentially occurring during non-stimulation. Additionally, the battery automatically shuts off in case of under-voltage. Finally, the maximum duration of continuous stimulation, when the device is not being moved, is set to 30 min.
3. Safety and tolerability of stimulation
Based on these safety measures, we evaluated the safety and tolerability of a long-term application of the mobile nGVS device on 10 healthy individuals (5 females; mean age 28.7 ± 3.6 years). Participants were initially asked to complete a questionnaire assessing their current mental and physical condition (5-point Likert scale ranging from 0 (no symptoms/limitations) to 5 (strong symptoms/limitations)). Following this, participants wore the activated stimulator (at a subthreshold stimulation intensity set to 0.3 mA) for a period of 2 h. Participants were instructed to engage in their normal activities during the experiment, with some remaining stationary and others engaging in light physical activities (e.g., household tasks). After 1 h of stimulation, participants were asked to report their mental and physical condition (see Figure 2), with particular attention to any side effects and intensity [5-point Likert scale ranging from 0 (no symptoms/limitations) to 5 (strong symptoms/limitations)] commonly noted in previous studies on electrical stimulation (38). Ten minutes after the stimulation period, participants were again asked to report on their current mental and physical condition and regarding any noticeable symptoms following the stimulation.
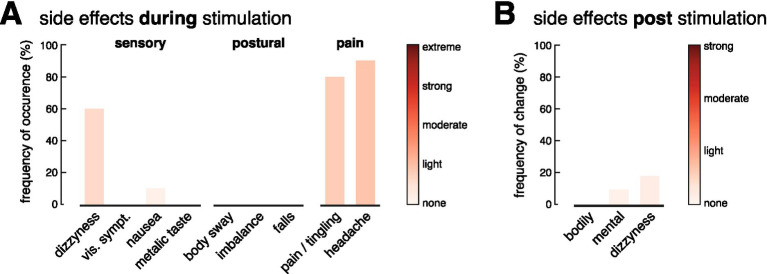
Figure 2.
Safety and tolerability of stimulation. Frequency and intensity of side effects experienced during (A) and 10 min after (B) a long-term stimulation (2 h) with the mobile stimulation device. During stimulation, 60% of participants reported mild dizziness that resolved immediately after, while 90% experienced mild headaches and 80% reported tingling at the electrode sites. Adjusting electrodes reduced discomfort in most cases. No serious side effects were reported after the stimulation.
During stimulation, more than half of the participants (60%) reported a mild sensation of dizziness, which disappeared immediately after the stimulation was turned off. No other sensory or postural effects were reported, except in one case, where mild nausea was reported, which, however, had already existed before the stimulation was started. Nearly all participants experienced a mild headache (90%) and/or a slight tingling sensation at the electrode placement sites on their skin (80%) (Figure 2). After adjusting the electrodes to restore optimal contact with the skin, this discomfort was diminished in most cases. None of the subjects reported pain at the stimulation site. The headache, often described as a pressing sensation near the temples, aligned with the position of the headband securing the electrodes. Six out of eight participants wearing the headband only as a control reported similar discomfort. After the stimulation ended and the electrodes were removed, no lingering symptoms were reported that had not been present prior to the stimulation.
4. Vestibular nature of stimulation
After evaluating the compatibility of long-term stimulation, we closely examined the core function of the mobile stimulation device. Specifically, we assessed whether it effectively stimulates vestibular pathways at suprathreshold intensity and elicits vestibular sensorimotor reflex responses as intended. We stimulated 6 healthy individuals (2 females; mean age 30.7 ± 4.4 years) with the maximum available noise intensity (peak amplitude of ±1 mA, 360 s stimulation duration). Simultaneously, we recorded body sway with an inertial measurement unit (IMU, Xsens, Movella Technologies, Enschede, The Netherlands) attached to the lower back (sacrum area) to assess vestibulospinal reflex responses. Since bipolar GVS stimulation primarily elicits a reflex response in the roll plane, the analysis focused on vestibulospinal responses in this plane (IMU-derived Euler angle in roll plane).
Correlation analysis in the frequency domain (magnitude-squared coherence) was performed to estimate average stimulation-induced variations in postural sway (39, 40). Coherence estimates with 95% confidence limits were calculated from the auto-spectra of the stimulation, body sway movement signals, as well as their cross-spectrum, using a finite fast Fourier transform with a block size of 1.28 s, providing a frequency resolution of 0.78 Hz (95% confidence limit for coherence estimates of 0.16 × 10−3). Coherence is a unitless measure between 1 (perfect linear relationship) and 0 (independence of signals).
The analysis (Figure 1C) demonstrated that the stimulation elicits significant vestibulospinal responses in the frequency domain (with peak coherence around 3 Hz). These findings confirm that the mobile stimulator effectively activates established vestibular sensorimotor pathways at suprathreshold stimulation levels.
5. Faciliatory effects of stimulation
After confirming that the mobile stimulator activates vestibular pathways, we further investigated its potential therapeutic function. Specifically, whether subthreshold noise stimulation with the new device can reproduce facilitatory effects from the literature on vestibular perception thresholds and static postural control. Starting with perception, we investigated in 11 healthy young adults (5 females; mean age 29.0 ± 4 years) whether the stimulation effectively lowers the vestibular perceptual threshold, as previously reported (23–25, 28, 35). Using an established psychophysical paradigm, vestibular perception thresholds were determined as direction recognition thresholds (DRT) for head-centered roll-tilt motion (28). The DRTs were measured on a 6DOF motion platform (Moog 6DOF2000E, East Aurora, New York) two times with subjects either receiving zero-current sham stimulation (0 mA) or nGVS at a fixed intensity of 0.3 mA, in randomized order. During the investigation, non-vestibular cues were minimized using noise-canceling headphones and complete darkness (Figure 3A).
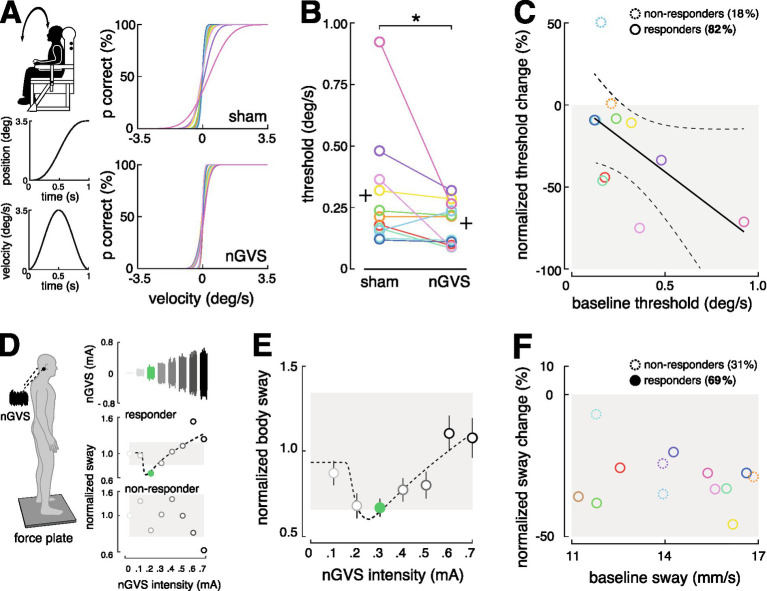
Figure 3.
Therapeutic effects of stimulation. (A) The effect of nGVS stimulation on vestibular perception in the roll plane was examined using a psychophysical paradigm on a motion platform. Individual psychometric curves for perceptual performance under zero-current sham vs. nGVS stimulation were calculated. (B) The nGVS stimulation led to an effective reduction in the perceptual threshold in 82% of the participants. (C) Participants with poorer baseline performance were more likely to benefit from the stimulation. (D) The effect of nGVS stimulation on postural control were assessed on a force plate. Participants who showed reduced body sway at intermediate nGVS intensities, and no effect or increased body sway at low or high intensities, were classified as responders (69%); the others were classified as non-responders (see exemplary participant outcomes). (E) Average modulation of body sway from all responding participants. (F) No correlation was observed between baseline performance and the response or non-response to the stimulation. nGVS, noisy galvanic vestibular stimulation.
Stimulation effectively reduced the perceptual threshold in 82% of participants (Wilcoxon signed-rank test, p = 0.032). Consistent with previous findings, individuals with a higher (i.e., worse) baseline perceptual threshold showed a greater likelihood of benefiting from stimulation (Spearman correlation coefficient R = 0.618; p = 0.049) (24, 28) (Figures 3B,C).
Effects on postural control were investigated in 13 healthy participants (5 females; mean age 28.6 ± 4.0 years) using a force platform (Kistler, 9261A, Kistler Group, Winterthur, Switzerland, 40 Hz), following a previously established experimental paradigm. Participants stood quietly on the platform with their eyes closed for 30 s in eight trials, while nGVS of varying intensities, from 0 mA (sham) to 0.7 mA, was applied in a randomized order. For each trial, the mean velocity of the center-of-pressure displacement was computed. The presence of a stabilizing effect on postural control was evaluated by assessing the change of body sway across the range of applied nGVS intensities, as previously described (32, 34, 41, 42) (Figure 3D). A positive response would be indicated by a reduction in body sway at medium nGVS intensities, no change at low or high intensities, or an increase at higher intensities, resulting in a characteristic bell-shaped response curve typical of SR effects.
Three experienced human evaluators rated whether these criteria were met for each participant. The evaluation showed that stimulation with the mobile stimulator led to a stabilization of postural control in 69% of participants (Figures 3D,E). In contrast to previous reports in patients, our healthy participants did not exhibit any correlation between baseline body sway and the response to nGVS stimulation (Figure 3F).
6. Discussion
Non-invasive low-intensity vestibular noise stimulation (i.e., nGVS) is a promising method to enhance vestibular function and improve postural stability in vestibular hypofunction. Recent research evidence indicates that for this treatment approach to be effective, it must be applied continuously in patients’ daily routines (35, 36, 43). To facilitate the translation of nGVS therapy into everyday real-world applications, we introduce a novel mobile, wearable nGVS device. The prototype device is equipped with comprehensive safety features – including automatic shut-off, overload protection, and gradual adjustments in stimulation intensity to prevent abrupt changes that could cause discomfort or injury. These mechanisms have been tested for safety and tolerability in human use.
In a series of experiments, we evaluated the device’s function to activate the vestibular periphery and to elicit facilitatory effects on the vestibular perceptual and balance control level. Our mobile stimulator uses an AC power source for energy efficiency. Unlike stationary DC stimulators, the noise signal generated by the new stimulator has a slightly higher lower frequency cut-off at around 0.5 Hz (compared to commonly reported 0.02 Hz (43–46)). Irrespective of this difference, our investigations demonstrate that the mobile stimulator can reliably elicit stable vestibular reflex responses (39, 40) and reproduce faciliatory effects on vestibular perception and postural control, comparable to those observed in previous studies with stationary DC stimulators (23–26, 35, 42, 47). Taken together, these findings indicate that the new device performs its intended function effectively.
nGVS therapy is required only when the patient is active and moving, not during periods of rest or sleep. To accommodate this, the mobile nGVS device is equipped with a motion sensor that automatically activates or deactivates stimulation based on the user’s activity level. This feature not only improves the device’s energy efficiency but also provides additional advantages. Previous studies have demonstrated that a motion sensor placed on the head can not only differentiate between active and inactive phases but also provide detailed insights into daily mobility patterns, such as sitting, standing, walking, stair climbing, and even specific gait characteristics (48–50). In the future, such information could offer valuable feedback on the therapy’s effectiveness in improving mobility and reducing fall risk. The mobile nGVS device further features a user-friendly interface that allows easy adjustment of stimulation parameters for individual use and could be operated effortlessly by participants in our initial studies.
Although our mobile nGVS stimulator is compact and portable, it is currently limited using large conventional electrodes, which are unsuitable for daily use. In initial evaluation studies, we used electrode gel and secured the electrodes behind the ear with an elastic headband to improve conductivity. However, electrode displacement from head movements and pressure from the headband likely contribute to the commonly reported discomfort and long-term side effects, such as skin irritation and tension headaches. Similar side effects have been previously reported in other studies involving prolonged application nGVS (51–54), while no such symptoms were described during short-term stimulation (38, 53, 55).
For the device’s success in clinical use, these side effects must be addressed through further innovations in stimulation electrode design. Previous results indicate that the therapeutic effects of nGVS are likely enhanced by more focal stimulation (i.e., smaller electrodes) (56, 57), which would allow to effectively reduce the skin-electrode contact area. To prevent tension headaches, downsized electrodes must be further securely placed behind the ear. Stable skin contact needs to be ensured without the use of a headband or similar accessory, as demonstrated for instance by bone-conducting headphones that can be worn comfortably for extended periods without side effects (58). Furthermore, different electrode materials (54, 59, 60) and, if necessary, conductive media should be evaluated for both functionality – including their ability to maintain low impedance, durability, and consistent performance over extended use – and user factors such as comfort, reusability, and adaptability to bone contours (60–64). In addition to optimizing the device’s electrodes, studies in clinical cohorts are necessary to assess its long-term viability and effectiveness of the mobile nGVS device.
In conclusion, this study presents a new mobile nGVS device that adheres to strict safety standards and successfully replicates the facilitatory effects known from stationary nGVS devices. Further optimization of the stimulation electrodes is essential to ensure practicality and tolerability for everyday use. Once these challenges are addressed, the device will allow the treatment approach to be integrated into daily life for the first time, enabling a more precise evaluation of its therapeutic effects on clinically relevant outcomes, such as mobility, gait, and fall risk. Overall, the introduction of the mobile nGVS prototype device represents an important first step toward establishing a broadly available therapeutic tool for patients with chronic vestibular hypofunction and related disorders.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the German Federal Ministry of Education and Research (BMBF 13GW0490B).
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The Ethics Committee of the Medical Faculty of the Ludwig-Maximilians-University approved the study protocol (20–1137), which was conducted in accordance with the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SK: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. JD: Formal analysis, Investigation, Methodology, Software, Writing – review & editing. SB: Formal analysis, Investigation, Methodology, Resources, Software, Writing – review & editing. KJ: Conceptualization, Funding acquisition, Project administration, Supervision, Validation, Writing – review & editing. MW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Zingler VC, Cnyrim C, Jahn K, Weintz E, Fernbacher J, Frenzel C, et al. Causative factors and epidemiology of bilateral Vestibulopathy in 255 patients. Ann Neurol. (2007) 61:524–32. doi: 10.1002/ana.21105, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Sprenger A, Wojak JF, Jandl NM, Helmchen C. Postural control in bilateral vestibular failure: its relation to visual, proprioceptive, vestibular, and cognitive input. Front Neurol. (2017) 8:444. doi: 10.3389/fneur.2017.00444, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guinand N, Pijnenburg M, Janssen M, Kingma H. Visual acuity while walking and Oscillopsia severity in healthy subjects and patients with unilateral and bilateral vestibular function loss. Arch Otolaryngol Head Neck Surg. (2012) 138:301–6. doi: 10.1001/archoto.2012.4, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Strupp M, Dlugaiczyk J, Ertl-Wagner BB, Rujescu D, Westhofen M, Dieterich M. Vestibular Disorders. Dtsch Arztebl Int. (2020) 117:300–10. doi: 10.3238/arztebl.2020.0300, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wuehr M, Decker J, Schenkel F, Jahn K, Schniepp R. Impact on daily mobility and risk of falling in bilateral Vestibulopathy. J Neurol. (2022) 269:5746–54. doi: 10.1007/s00415-022-11043-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinand N, Boselie F, Guyot JP, Kingma H. Quality of life of patients with bilateral Vestibulopathy. Ann Otol Rhinol Laryngol. (2012) 121:471–7. doi: 10.1177/000348941212100708 [DOI] [PubMed] [Google Scholar]
- 7.Schlick C, Schniepp R, Loidl V, Wuehr M, Hesselbarth K, Jahn K. Falls and fear of falling in Vertigo and balance disorders: a controlled cross-sectional study. J Vestib Res. (2016) 25:241–51. doi: 10.3233/VES-150564 [DOI] [PubMed] [Google Scholar]
- 8.Agrawal Y, Van de Berg R, Wuyts F, Walther L, Magnusson M, Oh E, et al. Presbyvestibulopathy: diagnostic criteria consensus document of the classification committee of the Barany society. J Vestib Res. (2019) 29:161–70. doi: 10.3233/ves-190672, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith PF. Vestibular functions and Parkinson's disease. Front Neurol. (2018) 9:1085. doi: 10.3389/fneur.2018.01085, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitney SL, Alghadir AH, Anwer S. Recent evidence about the effectiveness of vestibular rehabilitation. Curr Treat Options Neurol. (2016) 18:13. doi: 10.1007/s11940-016-0395-4 [DOI] [PubMed] [Google Scholar]
- 11.Basta D, Rossi-Izquierdo M, Wonneberger K, Brugnera C, Bittar RSM, Greters ME, et al. Individualized Vibrotactile Neurofeedback training in patients with chronic bilateral Vestibulopathy. Brain Sci. (2023) 13:1219. doi: 10.3390/brainsci13081219, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kingma H, Felipe L, Gerards MC, Gerits P, Guinand N, Perez-Fornos A, et al. Vibrotactile feedback improves balance and mobility in patients with severe bilateral vestibular loss. J Neurol. (2019) 266:19–26. doi: 10.1007/s00415-018-9133-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chow MR, Ayiotis AI, Schoo DP, Gimmon Y, Lane KE, Morris BJ, et al. Posture, gait, quality of life, and hearing with a vestibular implant. N Engl J Med. (2021) 384:521–32. doi: 10.1056/NEJMoa2020457, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boutabla A, Cavuscens S, Ranieri M, Cretallaz C, Kingma H, van de Berg R, et al. Simultaneous activation of multiple vestibular pathways upon electrical stimulation of Semicircular Canal afferents. J Neurol. (2020) 267:273–84. doi: 10.1007/s00415-020-10120-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Berg R, Ramos A, van Rompaey V, Bisdorff A, Perez-Fornos A, Rubinstein JT, et al. The vestibular implant: opinion statement on implantation criteria for research. J Vestib Res. (2020) 30:213–23. doi: 10.3233/VES-200701, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Stiphout L, Pleshkov M, Lucieer F, Dobbels B, Mavrodiev V, Guinand N, et al. Patterns of vestibular impairment in bilateral Vestibulopathy and its relation to etiology. Front Neurol. (2022) 13:856472. doi: 10.3389/fneur.2022.856472, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuehr M, Decker J, Schniepp R. Noisy galvanic vestibular stimulation: an emerging treatment option for bilateral Vestibulopathy. J Neurol. (2017) 264:81–6. doi: 10.1007/s00415-017-8481-4, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Lajoie K, Marigold DS, Valdes BA, Menon C. The potential of Noisy galvanic vestibular stimulation for optimizing and assisting human performance. Neuropsychologia. (2021) 152:107751. doi: 10.1016/j.neuropsychologia.2021.107751, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Priesol AJ, Valko Y, Merfeld DM, Lewis RF. Motion perception in patients with idiopathic bilateral vestibular Hypofunction. Otolaryngol Head Neck Surg. (2014) 150:1040–2. doi: 10.1177/0194599814526557, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Collins J, Chow CC, Imhoff TT. Stochastic resonance without tuning. Nature. (1995) 376:236–8. doi: 10.1038/376236a0 [DOI] [PubMed] [Google Scholar]
- 21.McDonnell MD, Ward LM. The benefits of noise in neural systems: bridging theory and experiment. Nat Rev Neurosci. (2011) 12:415–25. doi: 10.1038/nrn3061 [DOI] [PubMed] [Google Scholar]
- 22.Dlugaiczyk J, Wuehr M, Straka H. Electrical stimulation of vestibular Endorgans In: Fritzsch B, editor. The senses: A comprehensive reference. 2nd ed. Oxford: Elsevier; (2020). 635–71. [Google Scholar]
- 23.Galvan-Garza RC, Clark TK, Mulavara AP, Oman CM. Exhibition of stochastic resonance in vestibular tilt motion perception. Brain Stimul. (2018) 11:716–22. doi: 10.1016/j.brs.2018.03.017, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Keywan A, Jahn K, Wuehr M. Noisy galvanic vestibular stimulation primarily affects otolith-mediated motion perception. Neuroscience. (2019) 399:161–6. doi: 10.1016/j.neuroscience.2018.12.031 [DOI] [PubMed] [Google Scholar]
- 25.Keywan A, Wuehr M, Pradhan C, Jahn K. Noisy galvanic stimulation improves roll-tilt vestibular perception in healthy subjects. Front Neurol. (2018) 9:83. doi: 10.3389/fneur.2018.00083, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwasaki S, Yamamoto Y, Togo F, Kinoshita M, Yoshifuji Y, Fujimoto C, et al. Noisy vestibular stimulation improves body balance in bilateral Vestibulopathy. Neurology. (2014) 82:969–75. doi: 10.1212/WNL.0000000000000215, PMID: [DOI] [PubMed] [Google Scholar]
- 27.Wuehr M, Nusser E, Decker J, Krafczyk S, Straube A, Brandt T, et al. Noisy vestibular stimulation improves dynamic walking stability in bilateral Vestibulopathy. Neurology. (2016) 86:2196–202. doi: 10.1212/WNL.0000000000002748 [DOI] [PubMed] [Google Scholar]
- 28.Wuehr M, Eder J, Keywan A, Jahn K. Noisy galvanic vestibular stimulation improves vestibular perception in bilateral Vestibulopathy. J Neurol. (2023) 270:938–43. doi: 10.1007/s00415-022-11438-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eder J, Kellerer S, Amberger T, Keywan A, Dlugaiczyk J, Wuehr M, et al. Combining vestibular rehabilitation with Noisy galvanic vestibular stimulation for treatment of bilateral Vestibulopathy. J Neurol. (2022) 269:5731–7. doi: 10.1007/s00415-022-11033-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McLaren R, Smith PF, Taylor RL, Ravindran S, Rashid U, Taylor D. Efficacy of Ngvs to improve postural stability in people with bilateral Vestibulopathy: a systematic review and Meta-analysis. Front Neurosci. (2022) 16:16. doi: 10.3389/fnins.2022.1010239, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Samoudi G, Jivegard M, Mulavara AP, Bergquist F. Effects of stochastic vestibular galvanic stimulation and Ldopa on balance and motor symptoms in patients with Parkinson's disease. Brain Stimul. (2015) 8:474–80. doi: 10.1016/j.brs.2014.11.019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wuehr M, Schmidmeier F, Katzdobler S, Fietzek UM, Levin J, Zwergal A. Effects of low-intensity vestibular noise stimulation on postural instability in patients with Parkinson's disease. J Parkinsons Dis. (2022) 12:1611–8. doi: 10.3233/JPD-213127, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Peto D, Schmidmeier F, Katzdobler S, Fietzek UM, Levin J, Wuehr M, et al. No evidence for effects of low-intensity vestibular noise stimulation on mild-to-moderate gait impairments in patients with Parkinson's disease. J Neurol. (2024) 271:5489–97. doi: 10.1007/s00415-024-12504-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuehr M, Peto D, Fietzek UM, Katzdobler S, Nubling G, Zaganjori M, et al. Low-intensity vestibular noise stimulation improves postural symptoms in progressive Supranuclear palsy. J Neurol. (2024) 271:4577–86. doi: 10.1007/s00415-024-12419-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Keywan A, Badarna H, Jahn K, Wuehr M. No evidence for after-effects of Noisy galvanic vestibular stimulation on motion perception. Sci Rep. (2020) 10:2545. doi: 10.1038/s41598-020-59374-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nooristani M, Maheu M, Houde M-S, Bacon B-A, Champoux F. Questioning the lasting effect of galvanic vestibular stimulation on postural control. PLoS One. (2019) 14:619. doi: 10.1371/journal.pone.0224619, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lugade V, Fortune E, Morrow M, Kaufman K. Validity of using tri-axial accelerometers to measure human movement - part I: posture and movement detection. Med Eng Phys. (2014) 36:169–76. doi: 10.1016/j.medengphy.2013.06.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson D, Zubko O, Sakel M. Safety of repeated sessions of galvanic vestibular stimulation following stroke: a single-case study. Brain Inj. (2009) 23:841–5. doi: 10.1080/02699050903232541, PMID: [DOI] [PubMed] [Google Scholar]
- 39.Dakin CJ, Son GM, Inglis JT, Blouin JS. Frequency response of human vestibular reflexes characterized by stochastic stimuli. J Physiol. (2007) 583:1117–27. doi: 10.1113/jphysiol.2007.133264, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dietrich H, Heidger F, Schniepp R, MacNeilage PR, Glasauer S, Wuehr M. Head motion predictability explains activity-dependent suppression of vestibular balance control. Sci Rep. (2020) 10:668. doi: 10.1038/s41598-019-57400-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asslander L, Giboin LS, Gruber M, Schniepp R, Wuehr M. No evidence for stochastic resonance effects on standing balance when applying Noisy galvanic vestibular stimulation in young healthy adults. Sci Rep. (2021) 11:12327. doi: 10.1038/s41598-021-91808-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wuehr M, Eder J, Kellerer S, Amberger T, Jahn K. Mechanisms underlying treatment effects of vestibular noise stimulation on postural instability in patients with bilateral Vestibulopathy. J Neurol. (2023) 271:1408–15. doi: 10.1007/s00415-023-12085-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujimoto C, Egami N, Kawahara T, Uemura Y, Yamamoto Y, Yamasoba T, et al. Noisy galvanic vestibular stimulation sustainably improves posture in bilateral Vestibulopathy. Front Neurol. (2018) 9:900. doi: 10.3389/fneur.2018.00900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto C, Kawahara T, Kinoshita M, Ichijo K, Oka M, Kamogashira T, et al. Minimally important differences for subjective improvement in postural stability in patients with bilateral Vestibulopathy. Neurosci Lett. (2021) 747:135706. doi: 10.1016/j.neulet.2021.135706, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Iwasaki S, Fujimoto C, Egami N, Kinoshita M, Togo F, Yamamoto Y, et al. Noisy vestibular stimulation increases gait speed in Normals and in bilateral Vestibulopathy. Brain Stimul. (2018) 11:709–15. doi: 10.1016/j.brs.2018.03.005, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Fujimoto C, Yamamoto Y, Kamogashira T, Kinoshita M, Egami N, Uemura Y, et al. Noisy galvanic vestibular stimulation induces a sustained improvement in body balance in elderly adults. Sci Rep. (2016) 6:37575. doi: 10.1038/srep37575, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mulavara AP, Fiedler MJ, Kofman IS, Wood SJ, Serrador JM, Peters B, et al. Improving balance function using vestibular stochastic resonance: optimizing stimulus characteristics. Exp Brain Res. (2011) 210:303–12. doi: 10.1007/s00221-011-2633-z, PMID: [DOI] [PubMed] [Google Scholar]
- 48.Boborzi L, Decker J, Rezaei R, Schniepp R, Wuehr M. Human activity recognition in a free-living environment using an ear-worn motion sensor. Sensors. (2024) 24:2665. doi: 10.3390/s24092665, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seifer A-K, Dorschky E, Küderle A, Moradi H, Hannemann R, Eskofier BM. Eargait: estimation of temporal gait parameters from hearing aid integrated inertial sensors. Sensors. (2023) 23:565. doi: 10.3390/s23146565, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Decker J, Boborzi L, Schniepp R, Jahn K, Wuehr M. Mobile spatiotemporal gait segmentation using an ear-worn motion sensor and deep learning. Sensors (Basel). (2024) 24:6442. doi: 10.20944/preprints202408.1616.v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ruet A, Jokic C, Denise P, Leroy F, Azouvi P. Does galvanic vestibular stimulation reduce spatial neglect? A negative study. Ann Phys Rehabil Med. (2014) 57:570–7. doi: 10.1016/j.rehab.2014.09.009, PMID: [DOI] [PubMed] [Google Scholar]
- 52.Utz KS, Korluss K, Schmidt L, Rosenthal A, Oppenlander K, Keller I, et al. Minor adverse effects of galvanic vestibular stimulation in persons with stroke and healthy individuals. Brain Inj. (2011) 25:1058–69. doi: 10.3109/02699052.2011.607789 [DOI] [PubMed] [Google Scholar]
- 53.Matsugi A, Nagino K, Shiozaki T, Okada Y, Mori N, Nakamura J, et al. No impact of stochastic galvanic vestibular stimulation on arterial pressure and heart rate variability in the elderly population. Front Hum Neurosci. (2021) 15:646127. doi: 10.3389/fnhum.2021.646127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Obst MA, Heldmann M, Alicart H, Tittgemeyer M, Munte TF. Effect of short-term transcutaneous Vagus nerve stimulation (Tvns) on brain processing of food cues: an electrophysiological study. Front Hum Neurosci. (2020) 14:206. doi: 10.3389/fnhum.2020.00206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lotfi Y, Farahani A, Azimiyan M, Moossavi A, Bakhshi E. Comparison of efficacy of vestibular rehabilitation and Noisy galvanic vestibular stimulation to improve dizziness and balance in patients with multiple sclerosis. J Vestib Res. (2021) 31:541–51. doi: 10.3233/VES-201609, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Truong DQ, Guillen A, Nooristani M, Maheu M, Champoux F, Datta A. Impact of galvanic vestibular stimulation electrode current density on brain current flow patterns: does electrode size matter? PLoS One. (2023) 18:e0273883. doi: 10.1371/journal.pone.0273883, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nooristani M, Maheu M, Bacon BA, Champoux F. The importance of Ngvs current density for postural control enhancement. Brain Stimul. (2019) 12:1592–4. doi: 10.1016/j.brs.2019.07.022 [DOI] [PubMed] [Google Scholar]
- 58.Montuwy A, Cahour B, Dommes A. Using sensory wearable devices to navigate the City: effectiveness and user experience in older pedestrians. Multimodal Technol Interact. (2019) 3:17. doi: 10.3390/mti3010017 [DOI] [Google Scholar]
- 59.Badran BW, Yu AB, Adair D, Mappin G, DeVries WH, Jenkins DD, et al. Laboratory Administration of Transcutaneous Auricular Vagus Nerve Stimulation (Tavns): technique, targeting, and considerations. J Vis Exp. (2020):143. doi: 10.3791/58984, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Euler L, Guo L, Persson N-K. A review of textile-based electrodes developed for electrostimulation. Text Res J. (2021) 92:1300–20. doi: 10.1177/00405175211051949 [DOI] [Google Scholar]
- 61.RaviChandran N, Teo MY, Aw K, McDaid A. Design of Transcutaneous Stimulation Electrodes for wearable Neuroprostheses. IEEE Trans Neural Syst Rehabil Eng. (2020) 28:1651–60. doi: 10.1109/TNSRE.2020.2994900, PMID: [DOI] [PubMed] [Google Scholar]
- 62.Minhas P, Bansal V, Patel J, Ho JS, Diaz J, Datta A, et al. Electrodes for high-definition transcutaneous dc stimulation for applications in drug delivery and electrotherapy. Including Tdcs J Neurosci Methods. (2010) 190:188–97. doi: 10.1016/j.jneumeth.2010.05.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson M. Transcutaneous electrical nerve stimulation: mechanisms. Clin Appl Evid Rev Pain. (2007) 1:7–11. doi: 10.1177/204946370700100103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). (2013) 120:821–7. doi: 10.1007/s00702-012-0908-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.