Abstract
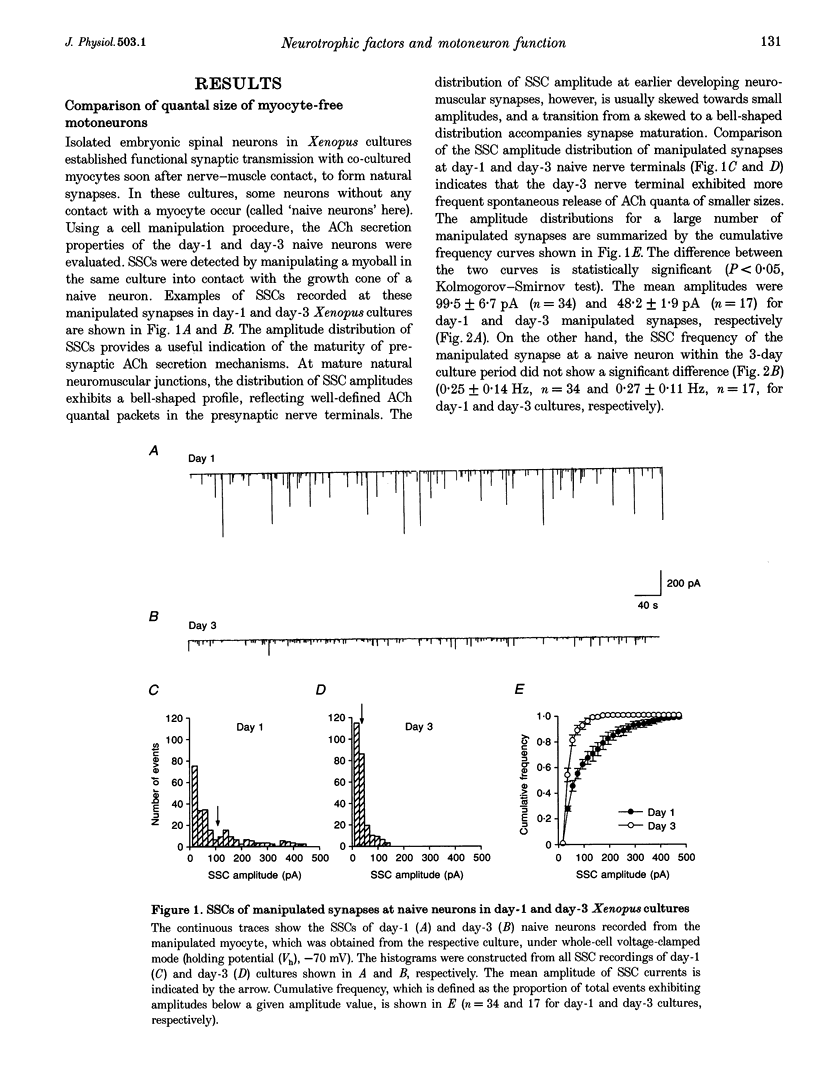
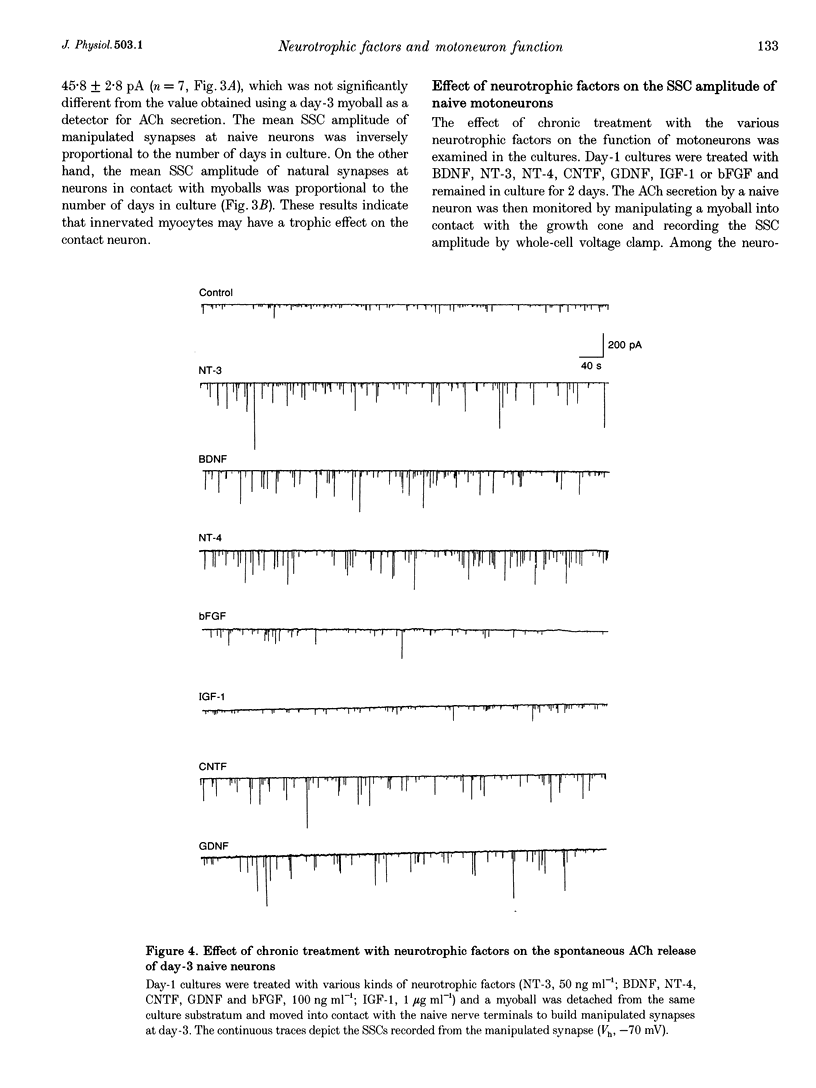
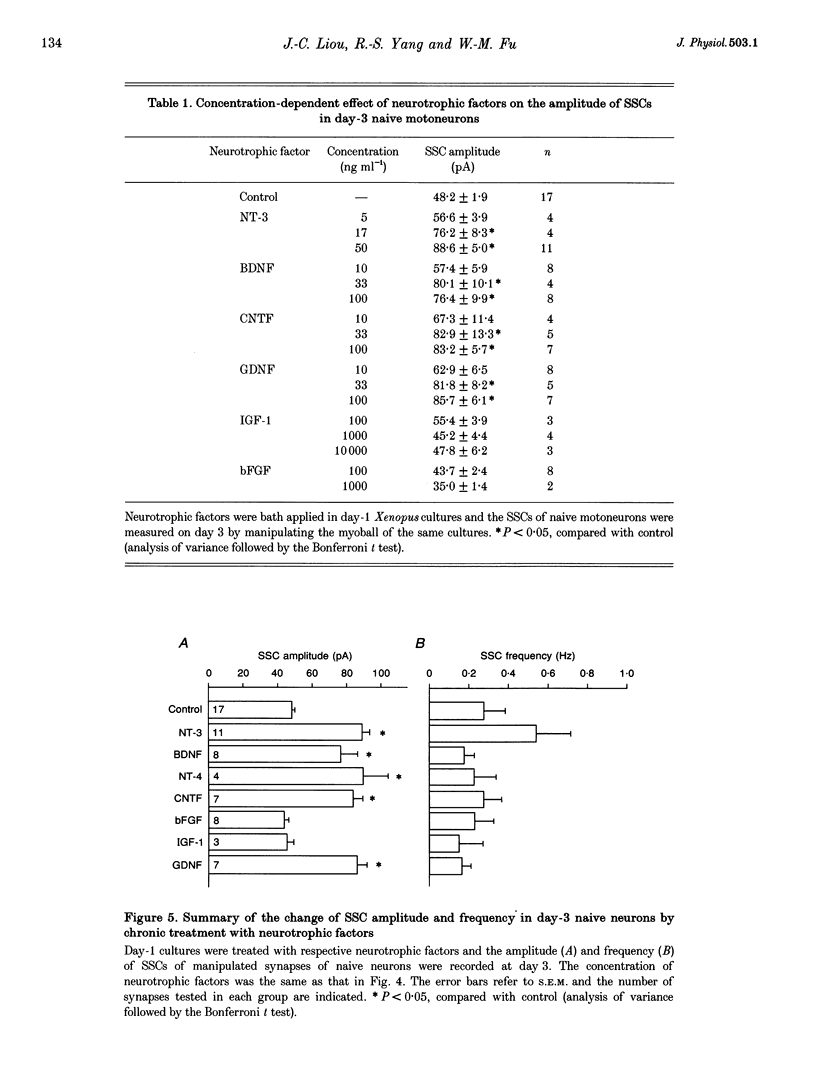
1. The ability of different neurotrophic factors to maintain and regulate synaptic function at the developing motoneuron was studied in Xenopus nerve-muscle co-cultures. Spontaneous synaptic currents (SSCs) were measured by using whole-cell voltage-clamped myocytes. 2. Compared with natural synapses, motoneurons without contact on a myocyte (naive neurons) released ACh in smaller quantal packets, the amplitude being inversely proportional to the days in culture. The mean SSC amplitudes of naive neurons, which were measured by manipulating a myoball into contact with the myocyte-free nerve terminals to form a manipulated synapse, were 99.5 +/- 6.7 and 48.2 +/- 1.9 pA for day-1 and day-3 cultures, respectively. 3. Chronic treatment of day-1 cultures with brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3), neurotrophin-4 (NT-4), ciliary neurotrophic factor (CNTF) or glial cell line-derived neurotrophic factor (GDNF) for 2 days, increased the ACh quantal size of naive motoneurons in a concentration-dependent manner, whereas insulin-like growth factor-1 (IGF-1) and basic fibroblast growth factor (bFGF) had no effect, even at high concentrations. 4. The interaction of various neurotrophic factors was examined, using concentrations that gave maximal effects. Combination of CNTF plus BDNF or CNTF plus NT-3 had synergistic effects in potentiating SSC amplitude of the manipulated synapse of naive neurons, whereas NT-3 plus BDNF, NT-3 plus GDNF, BDNF plus GDNF or CNTF plus GDNF had no synergistic action. 5. Chronic treatment with d-tubocurarine for 2 days resulted in a reduction of the quantal size of natural synapses. Concomitant treatment with BDNF, NT-3, GDNF, CNTF but not bFGF or IGF-1, reconstituted the SSC amplitude. 6. Taken together, these findings suggest that BDNF, NT-3, NT-4, CNTF and GDNF may regulate and maintain the synaptic function of developing motoneurons, and different neurotrophic factors utilizing distinct signalling mechanisms may have synergistic actions.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Sendtner M., Thoenen H. Survival effect of ciliary neurotrophic factor (CNTF) on chick embryonic motoneurons in culture: comparison with other neurotrophic factors and cytokines. J Neurosci. 1990 Nov;10(11):3507–3515. doi: 10.1523/JNEUROSCI.10-11-03507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci. 1995;18:223–253. doi: 10.1146/annurev.ne.18.030195.001255. [DOI] [PubMed] [Google Scholar]
- Caroni P. Activity-sensitive signaling by muscle-derived insulin-like growth factors in the developing and regenerating neuromuscular system. Ann N Y Acad Sci. 1993 Aug 27;692:209–222. doi: 10.1111/j.1749-6632.1993.tb26219.x. [DOI] [PubMed] [Google Scholar]
- Chapman T., Liddle L. F., Kalb J. M., Wolfner M. F., Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995 Jan 19;373(6511):241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Dan Y., Poo M. M. Retrograde interactions during formation and elimination of neuromuscular synapses. Curr Opin Neurobiol. 1994 Feb;4(1):95–100. doi: 10.1016/0959-4388(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Hall Z. W., Sanes J. R. Synaptic structure and development: the neuromuscular junction. Cell. 1993 Jan;72 (Suppl):99–121. doi: 10.1016/s0092-8674(05)80031-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Camu W., Mettling C., Gouin A., Poulsen K., Karihaloo M., Rullamas J., Evans T., McMahon S. B., Armanini M. P. Neurotrophins promote motor neuron survival and are present in embryonic limb bud. Nature. 1993 May 20;363(6426):266–270. doi: 10.1038/363266a0. [DOI] [PubMed] [Google Scholar]
- Henderson C. E., Phillips H. S., Pollock R. A., Davies A. M., Lemeulle C., Armanini M., Simmons L., Moffet B., Vandlen R. A., Simpson LC corrected to Simmons L. GDNF: a potent survival factor for motoneurons present in peripheral nerve and muscle. Science. 1994 Nov 11;266(5187):1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Henderson C. E. Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol. 1996 Feb;6(1):64–70. doi: 10.1016/s0959-4388(96)80010-9. [DOI] [PubMed] [Google Scholar]
- Heumann R. Neurotrophin signalling. Curr Opin Neurobiol. 1994 Oct;4(5):668–679. doi: 10.1016/0959-4388(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Ikeda K., Kinoshita M., Iwasaki Y. Basic fibroblast growth factor has neuroprotective effects on axotomy-induced spinal motoneuron death and wobbler mouse motoneuron disease. Muscle Nerve. 1996 Jun;19(6):794–795. [PubMed] [Google Scholar]
- Jones K. R., Fariñas I., Backus C., Reichardt L. F. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994 Mar 25;76(6):989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y., Saito M. Early cross-striation formation in twitching Xenopus myocytes in culture. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1978–1982. doi: 10.1073/pnas.85.6.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R., Silos-Santiago I., Smeyne R. J., Lira S. A., Brambilla R., Bryant S., Zhang L., Snider W. D., Barbacid M. Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature. 1994 Mar 17;368(6468):249–251. doi: 10.1038/368249a0. [DOI] [PubMed] [Google Scholar]
- Koliatsos V. E., Clatterbuck R. E., Winslow J. W., Cayouette M. H., Price D. L. Evidence that brain-derived neurotrophic factor is a trophic factor for motor neurons in vivo. Neuron. 1993 Mar;10(3):359–367. doi: 10.1016/0896-6273(93)90326-m. [DOI] [PubMed] [Google Scholar]
- Korsching S. The neurotrophic factor concept: a reexamination. J Neurosci. 1993 Jul;13(7):2739–2748. doi: 10.1523/JNEUROSCI.13-07-02739.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993 May 21;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liou J. C., Fu W. M. Regulation of quantal secretion from developing motoneurons by postsynaptic activity-dependent release of NT-3. J Neurosci. 1997 Apr 1;17(7):2459–2468. doi: 10.1523/JNEUROSCI.17-07-02459.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManaman J. L., Oppenheim R. W., Prevette D., Marchetti D. Rescue of motoneurons from cell death by a purified skeletal muscle polypeptide: effects of the ChAT development factor, CDF. Neuron. 1990 Jun;4(6):891–898. doi: 10.1016/0896-6273(90)90142-3. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H., Ikeda K., Klinkosz B., Cedarbaum J. M., Wong V., Lindsay R. M. Arrest of motor neuron disease in wobbler mice cotreated with CNTF and BDNF. Science. 1994 Aug 19;265(5175):1107–1110. doi: 10.1126/science.8066451. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Yin Q. W., Collins F., MacDonald J. Control of embryonic motoneuron survival in vivo by ciliary neurotrophic factor. Science. 1991 Mar 29;251(5001):1616–1618. doi: 10.1126/science.2011743. [DOI] [PubMed] [Google Scholar]
- Pennica D., Arce V., Swanson T. A., Vejsada R., Pollock R. A., Armanini M., Dudley K., Phillips H. S., Rosenthal A., Kato A. C. Cardiotrophin-1, a cytokine present in embryonic muscle, supports long-term survival of spinal motoneurons. Neuron. 1996 Jul;17(1):63–74. doi: 10.1016/s0896-6273(00)80281-0. [DOI] [PubMed] [Google Scholar]
- Schecterson L. C., Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992 Sep;9(3):449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Segal R. A., Greenberg M. E. Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci. 1996;19:463–489. doi: 10.1146/annurev.ne.19.030196.002335. [DOI] [PubMed] [Google Scholar]
- Sendtner M., Holtmann B., Kolbeck R., Thoenen H., Barde Y. A. Brain-derived neurotrophic factor prevents the death of motoneurons in newborn rats after nerve section. Nature. 1992 Dec 24;360(6406):757–759. doi: 10.1038/360757a0. [DOI] [PubMed] [Google Scholar]
- Stoop R., Poo M. M. Synaptic modulation by neurotrophic factors: differential and synergistic effects of brain-derived neurotrophic factor and ciliary neurotrophic factor. J Neurosci. 1996 May 15;16(10):3256–3264. doi: 10.1523/JNEUROSCI.16-10-03256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M., Rydén M., Jörnvall H., Funakoshi H., Timmusk T., Arenas E., Ibáez C. F. Peripheral expression and biological activities of GDNF, a new neurotrophic factor for avian and mammalian peripheral neurons. J Cell Biol. 1995 Jul;130(1):137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong V., Arriaga R., Ip N. Y., Lindsay R. M. The neurotrophins BDNF, NT-3 and NT-4/5, but not NGF, up-regulate the cholinergic phenotype of developing motor neurons. Eur J Neurosci. 1993 May 1;5(5):466–474. doi: 10.1111/j.1460-9568.1993.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Xie Z. P., Poo M. M. Initial events in the formation of neuromuscular synapse: rapid induction of acetylcholine release from embryonic neuron. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7069–7073. doi: 10.1073/pnas.83.18.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Elliott J. L., Matheson C., Sun J., Zhang L., Mu X., Rex K. L., Snider W. D. Influences of neurotrophins on mammalian motoneurons in vivo. J Neurobiol. 1993 Dec;24(12):1555–1577. doi: 10.1002/neu.480241202. [DOI] [PubMed] [Google Scholar]
- Zafra F., Lindholm D., Castrén E., Hartikka J., Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci. 1992 Dec;12(12):4793–4799. doi: 10.1523/JNEUROSCI.12-12-04793.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]


