Abstract
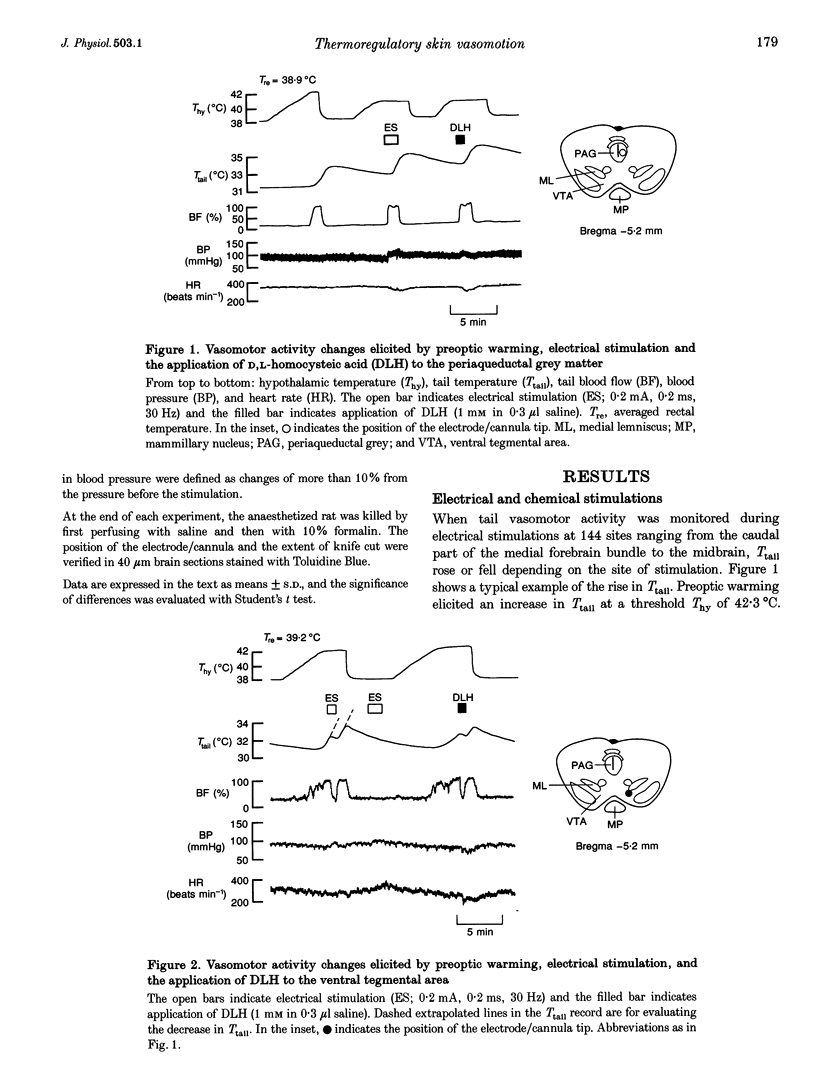
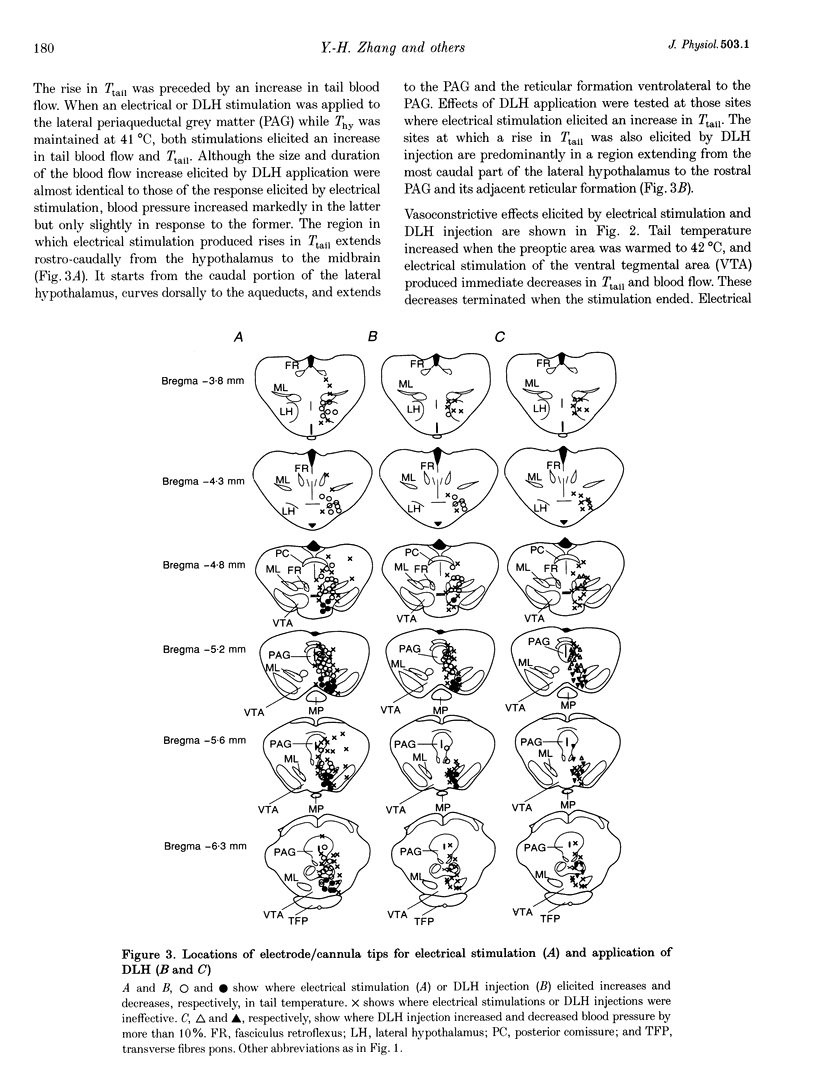
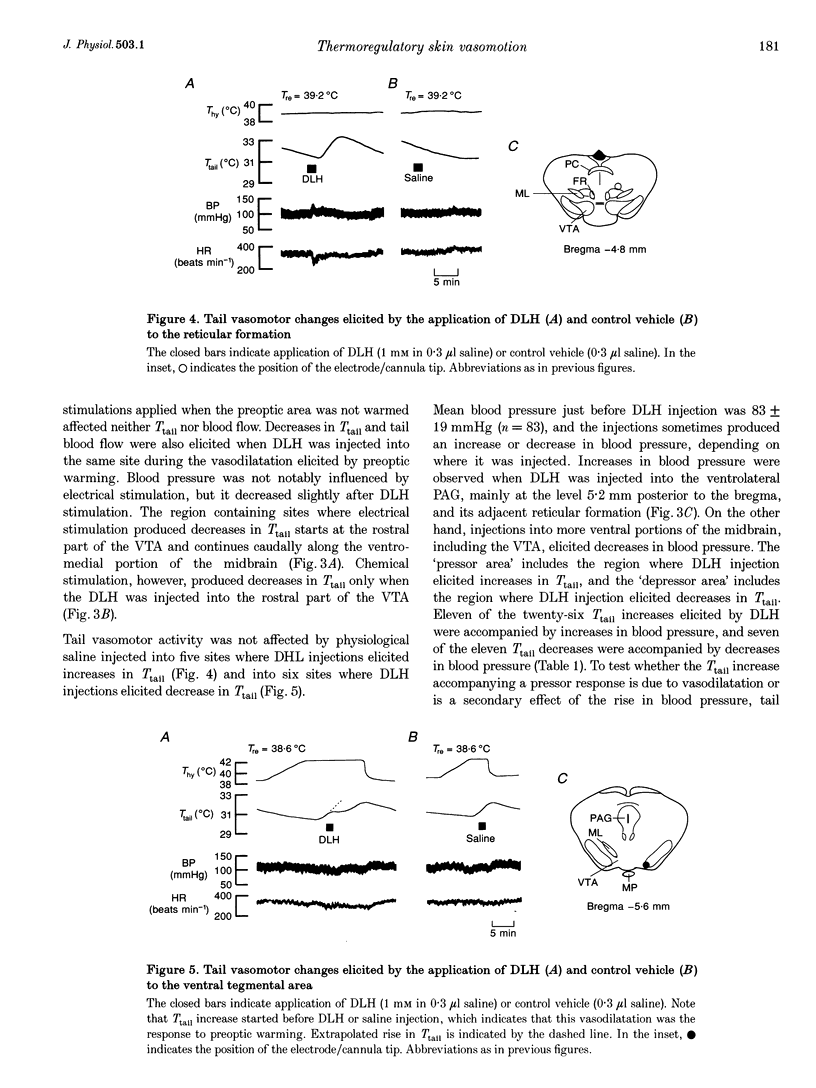
1. Efferent projections eliciting vasodilatation when the preoptic area is warmed were investigated by monitoring tail vasomotor responses of ketamine-anaesthetized rats when brain areas were stimulated electrically (0.2 mA, 200 microseconds, 30 Hz) or with the excitatory amino acid D,L-homocysteic acid (1 mM, 0.3 microliter). 2. Both stimulations elicited vasodilatation when applied within a region extending from the most caudal part of the lateral hypothalamus to the ventrolateral periaqueductal grey matter (PAG) and the reticular formation ventrolateral to the PAG. 3. Vasodilatation elicited by preoptic warming was suppressed when either stimulation was applied within the rostral part of the ventral tegmental area (VTA). 4. Sustained vasodilatation was elicited by knife cuts caudal to the VTA, and vasodilatation elicited by preoptic warming was suppressed by cuts either rostral to the VTA or in the region including the PAG and the reticular formation ventrolateral to it. 5. These results, together with the results of earlier physiological and histological studies, suggest that warm-sensitive neurones in the preoptic area send excitatory signals to vasodilatative neurones in the caudal part of the lateral hypothalamus, ventrolateral PAG and reticular formation, and send inhibitory signals to vasoconstrictive neurones in the rostral part of the VTA.
Full text
PDF

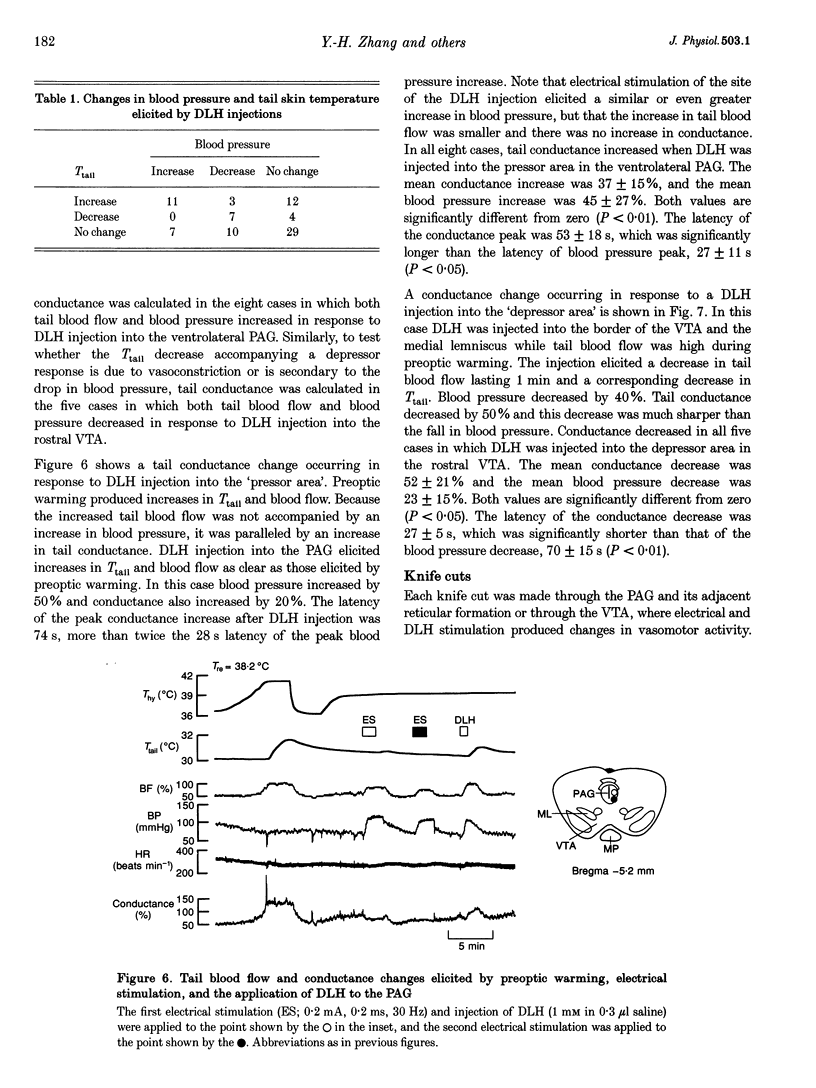
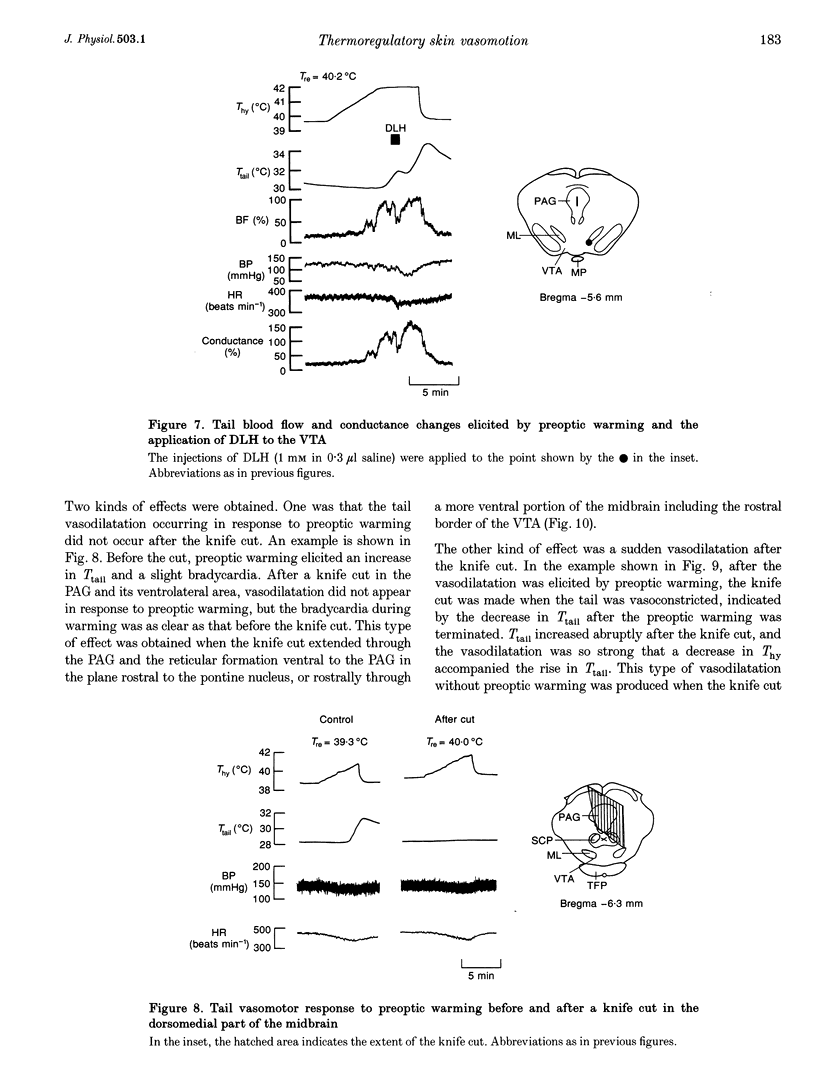
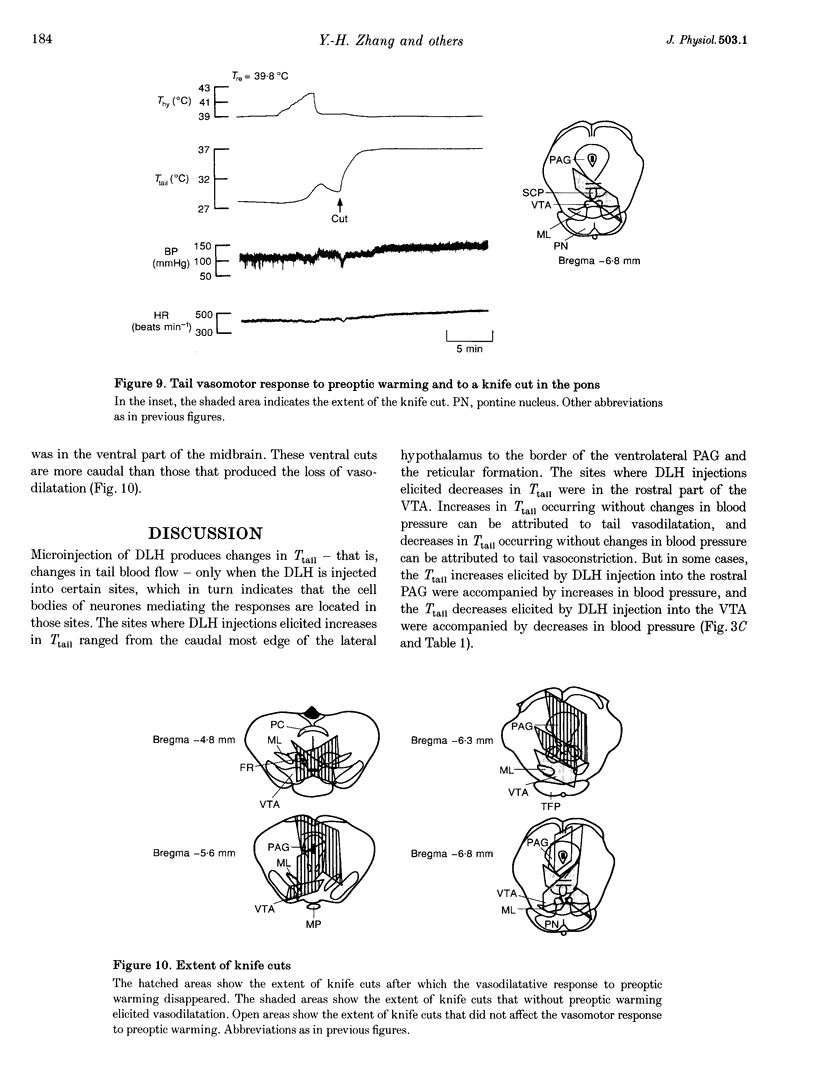


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band D. M., McClelland M., Phillips D. L., Saunders K. B., Wolff C. B. Sensitivity of the carotid body to within-breath changes in arterial PCO2. J Appl Physiol Respir Environ Exerc Physiol. 1978 Nov;45(5):768–777. doi: 10.1152/jappl.1978.45.5.768. [DOI] [PubMed] [Google Scholar]
- Black A. M., McCloskey D. I., Torrance R. W. The responses of carotid body chemoreceptors in the cat to sudden changes of hypercapnic and hypoxic stimuli. Respir Physiol. 1971 Oct;13(1):36–49. doi: 10.1016/0034-5687(71)90063-6. [DOI] [PubMed] [Google Scholar]
- Blanco C. E., Dawes G. S., Hanson M. A., McCooke H. B. The response to hypoxia of arterial chemoreceptors in fetal sheep and new-born lambs. J Physiol. 1984 Jun;351:25–37. doi: 10.1113/jphysiol.1984.sp015229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatteis C. M., Haar H., Banet M., Hensel H. A simple microknife manipulator. Electroencephalogr Clin Neurophysiol. 1982 Aug;54(2):237–238. doi: 10.1016/0013-4694(82)90167-5. [DOI] [PubMed] [Google Scholar]
- Carroll J. L., Bamford O. S., Fitzgerald R. S. Postnatal maturation of carotid chemoreceptor responses to O2 and CO2 in the cat. J Appl Physiol (1985) 1993 Dec;75(6):2383–2391. doi: 10.1152/jappl.1993.75.6.2383. [DOI] [PubMed] [Google Scholar]
- Conrad L. C., Pfaff D. W. Efferents from medial basal forebrain and hypothalamus in the rat. I. An autoradiographic study of the medial preoptic area. J Comp Neurol. 1976 Sep 15;169(2):185–219. doi: 10.1002/cne.901690205. [DOI] [PubMed] [Google Scholar]
- Cross B. A., Davey A., Guz A., Katona P. G., MacLean M., Murphy K., Semple S. J., Stidwill R. The ph oscillations in arterial blood during exercise; a potential signal for the ventilatory response in the dog. J Physiol. 1982 Aug;329:57–73. doi: 10.1113/jphysiol.1982.sp014290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies R. O., Nishino T., Lahiri S. Sympathectomy does not alter the response of carotid chemoreceptors to hypoxemia during carboxyhemoglobinemia or anemia. Neurosci Lett. 1981 Jan 20;21(2):159–164. doi: 10.1016/0304-3940(81)90375-x. [DOI] [PubMed] [Google Scholar]
- Fahrbach S. E., Morrell J. I., Pfaff D. W. Identification of medial preoptic neurons that concentrate estradiol and project to the midbrain in the rat. J Comp Neurol. 1986 May 15;247(3):364–382. doi: 10.1002/cne.902470307. [DOI] [PubMed] [Google Scholar]
- Fleming P. J., Azaz Y., Wigfield R. Development of thermoregulation in infancy: possible implications for SIDS. J Clin Pathol. 1992 Nov;45(11 Suppl):17–19. [PubMed] [Google Scholar]
- HORNBEIN T. F., GRIFFO Z. J., ROOS A. Quantitation of chemoreceptor activity: interrelation of hypoxia and hypercapnia. J Neurophysiol. 1961 Nov;24:561–568. doi: 10.1152/jn.1961.24.6.561. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y., Nakayama T., Kanosue K., Matsumura K. Activation of central warm-sensitive neurons and the tail vasomotor response in rats during brain and scrotal thermal stimulation. Pflugers Arch. 1984 Mar;400(3):222–227. doi: 10.1007/BF00581551. [DOI] [PubMed] [Google Scholar]
- Kanosue K., Yanase-Fujiwara M., Hosono T. Hypothalamic network for thermoregulatory vasomotor control. Am J Physiol. 1994 Jul;267(1 Pt 2):R283–R288. doi: 10.1152/ajpregu.1994.267.1.R283. [DOI] [PubMed] [Google Scholar]
- Kanosue K., Zhang Y. H., Yanase-Fujiwara M., Hosono T. Hypothalamic network for thermoregulatory shivering. Am J Physiol. 1994 Jul;267(1 Pt 2):R275–R282. doi: 10.1152/ajpregu.1994.267.1.R275. [DOI] [PubMed] [Google Scholar]
- Kholwadwala D., Donnelly D. F. Maturation of carotid chemoreceptor sensitivity to hypoxia: in vitro studies in the newborn rat. J Physiol. 1992;453:461–473. doi: 10.1113/jphysiol.1992.sp019239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuipers I. M., Maertzdorf W. J., De Jong D. S., Hanson M. A., Blanco C. E. Effect of mild hypocapnia on fetal breathing and behavior in unanesthetized normoxic fetal lambs. J Appl Physiol (1985) 1994 Apr;76(4):1476–1480. doi: 10.1152/jappl.1994.76.4.1476. [DOI] [PubMed] [Google Scholar]
- Kumar P., Hanson M. A. Re-setting of the hypoxic sensitivity of aortic chemoreceptors in the new-born lamb. J Dev Physiol. 1989 Apr;11(4):199–206. [PubMed] [Google Scholar]
- Kumar P., Nye P. C., Torrance R. W. Do oxygen tension variations contribute to the respiratory oscillations of chemoreceptor discharge in the cat? J Physiol. 1988 Jan;395:531–552. doi: 10.1113/jphysiol.1988.sp016933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFramboise W. A., Guthrie R. D., Standaert T. A., Woodrum D. E. Pulmonary mechanics during the ventilatory response to hypoxemia in the newborn monkey. J Appl Physiol Respir Environ Exerc Physiol. 1983 Sep;55(3):1008–1014. doi: 10.1152/jappl.1983.55.3.1008. [DOI] [PubMed] [Google Scholar]
- LaFramboise W. A., Tuck R. E., Woodrum D. E., Guthrie R. D. Maturation of eupneic respiration in the neonatal monkey. Pediatr Res. 1984 Oct;18(10):943–948. doi: 10.1203/00006450-198410000-00007. [DOI] [PubMed] [Google Scholar]
- Marchal F., Bairam A., Haouzi P., Crance J. P., Di Giulio C., Vert P., Lahiri S. Carotid chemoreceptor response to natural stimuli in the newborn kitten. Respir Physiol. 1992 Feb;87(2):183–193. doi: 10.1016/0034-5687(92)90058-5. [DOI] [PubMed] [Google Scholar]
- McQueen D. S., Evrard Y., Gordon B. H., Campbell D. B. Ganglioglomerular nerves influence responsiveness of cat carotid body chemoreceptors to almitrine. J Auton Nerv Syst. 1989 Jun;27(1):57–66. doi: 10.1016/0165-1838(89)90129-x. [DOI] [PubMed] [Google Scholar]
- North R. G., Petersen S. A., Wailoo M. P. Lower body temperature in sleeping supine infants. Arch Dis Child. 1995 Apr;72(4):340–342. doi: 10.1136/adc.72.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper D. R., Landauer R. C., Kumar P. Postnatal development of CO2-O2 interaction in the rat carotid body in vitro. J Physiol. 1995 Jun 1;485(Pt 2):531–541. doi: 10.1113/jphysiol.1995.sp020749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simerly R. B., Swanson L. W. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988 Apr 8;270(2):209–242. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Torrance R. W., Bartels E. M., McLaren A. J. Update on the bicarbonate hypothesis. Adv Exp Med Biol. 1993;337:241–250. doi: 10.1007/978-1-4615-2966-8_34. [DOI] [PubMed] [Google Scholar]
- Upton C. J., Milner A. D., Stokes G. M., Wilson A. J. Dynamic responses to tube breathing during the first 10 days of life. Pediatr Pulmonol. 1990;9(2):72–79. doi: 10.1002/ppul.1950090203. [DOI] [PubMed] [Google Scholar]
- Wolsink J. G., Berkenbosch A., DeGoede J., Olievier C. N. Maturation of the ventilatory response to CO2 in the newborn piglet. Pediatr Res. 1993 Oct;34(4):485–489. doi: 10.1203/00006450-199310000-00020. [DOI] [PubMed] [Google Scholar]
- Wolsink J. G., Berkenbosch A., DeGoede J., Olievier C. N. Ventilatory sensitivities of peripheral and central chemoreceptors of young piglets to inhalation of CO2 in air. Pediatr Res. 1991 Nov;30(5):491–495. doi: 10.1203/00006450-199111000-00018. [DOI] [PubMed] [Google Scholar]
- Young A. A., Dawson N. J. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol. 1982 Mar;60(3):392–398. doi: 10.1139/y82-057. [DOI] [PubMed] [Google Scholar]