Abstract
An understanding of the nature of immunity to serogroup B meningococci in childhood is necessary in order to establish the reasons for poor responses to candidate vaccines in infancy. We sought to examine the nature of humoral immune responses following infection in relation to age. Serum bactericidal activity was poor in children under 12 months of age despite recent infection with Neisseria meningitidis. The highest levels of bactericidal activity were seen in children over 10 years of age. However, infants produced levels of total immunoglobulin G (IgG) and IgG subclass antibodies similar to those in older children in a meningococcal enzyme-linked immunosorbent assay. Most antibody was of the IgG1 and IgG3 subclasses. This striking age dependency of bactericidal antibody response following infection is not apparently due to failure of class switching in infants but might be due to qualitative differences in antibody specificity or affinity.
In the 111 years since the first isolation of Diplococcus intracellularis meningitidis (Neisseria meningitidis), investigation of the nature of human immunity to the organism has been considerable (17). It now seems clear that for serogroup A and C organisms the most important mechanism which confers protection is complement-mediated lysis by bactericidal antibody, because (i) there is an inverse relationship between serum bactericidal activity and the age-related incidence of disease (13), (ii) military conscripts with low bactericidal activity were more susceptible to disease (14, 16), (iii) individuals with complement deficiency are susceptible to repeated attacks of meningococcal disease (33), and (iv) serogroup A and C polysaccharide vaccine induces bactericidal antibody and confers protection (3, 37, 45, 55).
These bactericidal responses to serogroup A and C meningococci are thought to be directed chiefly at polysaccharide. The antibody response following serogroup C polysaccharide vaccination is predominantly immunoglobulin G2 (IgG2) in children (30), and both IgG1 and IgG2 are generated in response to serogroup A meningococcal polysaccharide vaccination (40). Since there is a relative deficiency of IgG2 in early childhood, poor bactericidal responses to bacteria encapsulated in polysaccharide would not be unexpected (41, 51).
For serogroup B organisms, the nature of acquired immunity is less clear. The same inverse relationship between age and bactericidal activity of serum against serogroup B organisms has been described for both healthy children (13) and vaccinees (31). There are also data showing a relationship between age and absolute antibody levels (7), but there are no data showing that low bactericidal antibody levels precede invasive disease. Indeed, both Rosenqvist et al. (42) and Harthug et al. (21) have found similar enzyme-linked immunosorbent assay (ELISA) antibody levels in controls and in patients with meningococcal disease on admission to a hospital. One study, however, found that the development of bactericidal antibodies after vaccination was age dependent and in good agreement with the results of a case-control study (31).
The polysaccharide capsule of serogroup B organisms is poorly immunogenic (56), and vaccine interest has therefore focused on outer membrane proteins (OMPs) (39). Immunogenicity studies have shown that bactericidal antibody following vaccination with various OMP preparations is directed chiefly at the serosubtype PorA protein, and recent vaccine trials have concentrated on this component in the vaccines used (36, 43). Antibody directed against OMPs has been shown to be bactericidal (24). However, there have been poor responses to OMP vaccines in young children in terms of both efficacy and bactericidal antibody induction, and bactericidal activity has been strain specific (6, 10, 35, 47, 50). The dose schedule may be important in both vaccine response and induction of cross-protective antibodies (38, 44). After both infection and vaccination, the major antibodies produced are of subclasses IgG1 and IgG3, and these antibodies have the highest activity against OMP antigens (19, 48). IgG1 and IgG3 are both complement-fixing antibodies, and generation of adequate levels of these subclasses might be important in bactericidal activity against serogroup B meningococci and could be age dependent. Poor production of antibody subclasses in response to bacteria, particularly that of IgG2 (8, 41) and IgG3 (12), in early childhood has been previously documented.
An outer membrane vesicle (OMV) vaccine, developed in The Netherlands, is hexavalent by virtue of its preparation from two genetically modified strains based on H44/76 (B:15:P1.7,16), each expressing three different PorA proteins (54). One or more of these porins are expressed by a majority of the serogroup B strains currently causing infection in the United Kingdom. If it is satisfactorily immunogenic, the vaccine could give the potential for immunization against most clinically relevant strains of N. meningitidis of all serogroups (9, 34). In order to study the development of bactericidal activity in response to this hexavalent vaccine, a set of six strains based on H44/76, each differing only in their surface expression of PorA, were constructed (52, 53). With these six strains, the present study was undertaken in order to document changes in serum bactericidal antibody activity in relation to (i) age and (ii) PorA expression by N. meningitidis both in children who had recovered from invasive meningococcal infection and in healthy children.
Since vaccine immunogenicity and efficacy are poor in young children, we postulated that there might be age-dependent differences in bactericidal activity or specific immunoglobulin subclass levels after infection. Evidence was sought for age-related changes in (i) functional antibody by the serum bactericidal assay and (ii) immunoglobulin production by whole-cell ELISA both following meningococcal infection and in healthy controls.
MATERIALS AND METHODS
Subjects.
Venous blood was obtained 9 weeks (median) after the onset of meningococcal sepsis from 71 children who presented to the pediatric intensive care unit in the Department of Paediatrics at St. Mary’s Hospital with a clinical diagnosis of meningococcal disease in 1997. All children had typical clinical features of severe meningococcal disease, and meningococcal infection in 47 children was confirmed by isolation of N. meningitidis from blood culture or throat swab, by detection of capsular antigen in blood or cerebrospinal fluid, or by detection of the meningococcal genome by PCR analysis of blood. Typing was available for 36 clinical isolates (Tables 1 and 2). Blood was also obtained from 19 siblings of meningococcal disease patients and from 109 otherwise healthy control children who were either undergoing routine surgery or recruited as controls in another study of genetic factors in meningococcal infection. Sixteen adult volunteers from the Department of Paediatrics also provided a blood sample. Fewer sera were available for bactericidal study (Tables 1 and 2). Serum was separated from whole blood, and aliquots of each sample were stored at −70°C until use. Ethical approval was obtained from the St. Mary’s Hospital local research ethics committee, and informed parental consent for blood sampling was obtained.
TABLE 1.
Bactericidal antibody study subjects
| Study group and strain | No. of subjects
|
Median age (yr) (range) | ||||
|---|---|---|---|---|---|---|
| Total | <1 yr | 1–4 yr | 4–10 yr | >10 yr | ||
| Postinfection | 60 | 13 | 18 | 17 | 12 | 3.83 (0.42–16.83) |
| Group C | 21 | 3 | 7 | 7 | 4 | 4.75 (0.7–13.25) |
| C:unknown | 8 | |||||
| C:2a:NT | 4 | |||||
| C2a:P1.5 | 5 | |||||
| C:2a:P1.5,2 | 1 | |||||
| C:NT:P1.2 | 1 | |||||
| C:NT:P1.5,2 | 1 | |||||
| C:NT:P1.5 | 1 | |||||
| Group B | 26 | 7 | 8 | 4 | 7 | 2.67 (0.42–15) |
| B:unknown | 3 | |||||
| B:15:P1.7,16 | 4 | |||||
| B:NT:P1.4 | 2 | |||||
| B:4:P1.4 | 6 | |||||
| B:4:P1.5 | 1 | |||||
| B:NT:NT | 2 | |||||
| B:NT:P1.9 | 1 | |||||
| B:4:NT | 1 | |||||
| B:4:P1.15 | 1 | |||||
| B:2b:P1.10 | 1 | |||||
| B:NT:P1.3,6 | 1 | |||||
| B:4:P1.3,6 | 2 | |||||
| B:4:P1.14 | 1 | |||||
| Control children | 59 | 5 | 15 | 14 | 25 | 8.1 (0.75–16) |
TABLE 2.
Antibody study subjects
| Study group and strain | No. of subjects
|
Median age (yr) (range) | ||||
|---|---|---|---|---|---|---|
| Total | <1 yr | 1–4 yr | 4–10 yr | >10 yr | ||
| Postinfection | 71 | 15 | 25 | 22 | 9 | 3.2 (0.42–17.9) |
| Group C | 29 | 4 | 9 | 11 | 5 | 4.9 (0.7–17.6) |
| C:unknown | 7 | |||||
| C:2a:NT | 4 | |||||
| C2a:P1.5 | 8 | |||||
| C:NT:P1.5,2 | 1 | |||||
| C:2a:P1.5,2 | 3 | |||||
| C:NT:P1.2 | 1 | |||||
| C:NT:NT | 1 | |||||
| C:2a:P1.3,6 | 1 | |||||
| C:2b:P1.5,2 | 3 | |||||
| Group B | 27 | 8 | 11 | 5 | 3 | 2 (0.42–17.9) |
| B:unknown | 3 | |||||
| B:15:P1.7,16 | 4 | |||||
| B:NT:P1.4 | 1 | |||||
| B:4:P1.4 | 7 | |||||
| B:4:P1.5 | 1 | |||||
| B:NT:NT | 3 | |||||
| B:4:NT | 1 | |||||
| B:4:P1.15 | 1 | |||||
| B:2b:P1.10 | 1 | |||||
| B:NT:P1.3,6 | 1 | |||||
| B:4:P1.3,6 | 1 | |||||
| B:4:P1.14 | 1 | |||||
| B:NT:P1.9 | 1 | |||||
| B:NT:P1.19 | 1 | |||||
| Control children | 109 | 4 | 15 | 40 | 50 | 9.00 (0.75–16) |
| Siblings | 19 | 0 | 5 | 10 | 4 | 6.5 (1.08–17.5) |
| Adults | 16 | 16 | 31.5 (27–40) | |||
Bacterial strains.
N. meningitidis MC58 and H44/76 and its derivatives are described in Table 3. H44/76 and its derivatives were constructed at the Laboratory of Vaccine Development and Immune Mechanisms, National Institute for Public Health and the Environment, Bilthoven, The Netherlands. The strains were derived by exchange of the parent porA gene with functional porA alleles from five different donor strains (36). Whole-cell ELISAs (1, 2) were performed with a panel of epitope-specific monoclonal antibodies to verify the pattern of OMP expression for each of the bacterial strains. Strains were grown overnight on gonococcal medium base (Difco, Detroit, Mich.) containing 1% Vitox (Oxoid, Basingstoke, United Kingdom) at 37°C in 5% CO2. The six strains were harvested by using a sterile cotton swab and stored in aliquots at −70°C suspended in Mueller-Hinton broth (Oxoid) with 15% glycerol, and the same set of vials was used for all experiments.
TABLE 3.
Strains
| Strain | Derivation | Serosubtype | Donor strain | Reference(s) |
|---|---|---|---|---|
| H44/76 | Parent strain | B:15:P1.7,16 | 22 | |
| TR52 | Exchange with P1.5,2 allele | B:15:P1.5,2 | 2996 (B:2b:P1.5,2) | 36, 52 |
| TR15 | Exchange with P1.19,15 allele | B:15:P1.19,15 | MC51 (C:nt:P1.19,15) | 36 |
| TR10 | Exchange with P1.5c,10 allele | B:15P1.5c,10 | 870227 (B:4:P1.5c,10) | 36 |
| TR4 | Exchange with P1.7h,4 allele | B:15:P1.7h,4 | 892257 (B:4:P1.7h,4) | 36 |
| TR1213 | Exchange with P1.12,13 allele | B:15:P1.12,13 | 870446 (B:14:P1.12,13) | 36 |
| MC58 | B:15:P1.7,16b | 29 |
Antibodies.
The PorA-, PorB-, and class 4-specific mouse monoclonal antibodies for whole-cell typing (from the Laboratory of Vaccine Development and Immune Mechanisms, National Institute for Public Health and the Environment, Bilthoven, The Netherlands) were as follows: MN16C13F4 (anti-P1.2), MN22A9.19 (anti-P1.5), MN14C11.6 (anti-P1.7), MN20A7.10 (anti-P1.12), MN24H10.75 (anti-P1.13), MN3C5C (anti-P1.15), and MN5C11G (anti-P1.16). Class 4 OMP was detected with MN2D6D, and PorB (serotype 15) was detected with MN15A14H6. B306 anti-Opc was kindly provided by M. Achtman, Berlin, Germany. Anti-human IgG and IgM were purchased from Sigma (Poole, United Kingdom). Anti-human IgG1, IgG2, and IgG3 were from ICN (Costa Mesa, Calif.), and IgG4 was from Pharmingen (San Diego, Calif.).
Whole-cell ELISA for immunoglobulin subclasses.
Immunoglobulin class or subclass titers to H44/76 (B:15:P1.7,16) were determined by ELISA, using an adaptation of an ELISA developed for OMP serology (1, 2), as described below. Briefly, H44/76 was grown overnight on gonococcal medium base (Difco) containing 1% Vitox (Oxoid) at 37°C in 5% CO2. Bacteria were harvested by using a sterile cotton swab and suspended in phosphate-buffered saline (PBS) prior to heat killing in a water bath for 1 h at 56°C. Ninety-six-well high-binding ELISA plates (Greiner, Stonehouse, United Kingdom) were coated with 100 μl of these heat-killed whole meningococci (optical density at 600 nm of 0.1 in PBS) and dried in an incubator overnight at 37°C without humidification. When all of the solution had evaporated, the plates were washed with PBS-Tween 20 and blocked for 1 h at 37°C with 1% skim milk powder (Oxoid) in PBS at 200 μl/well. After washing, 100 μl of serum diluted in PBS–Tween–1% skim milk powder was added at the optimal concentration for each ELISA (1/100 for IgG and IgM and 1/25 for subclass ELISAs) and left for 1 h at 37°C. The plates were washed again and incubated with (100 μl/well) mouse anti-human immunoglobulin (class or subclass) horseradish peroxidase-conjugated antibody diluted in PBS–Tween–1% skim milk powder for a further hour. After thorough washing, the plates were developed with the substrate o-phenylenediamine dihydrochloride (Sigma) at 10 mg/50 ml of citrate buffer with 10 μl of hydrogen peroxide (pH 5), and the reaction was stopped with 2 M sulfuric acid. The absorbance was measured in an automated ELISA plate reader (Thermomax microplate reader; Molecular Devices, Menlo Park, Calif.) at 490 nm. Immunoglobulin concentrations were calculated by using ELISA software (Softmax; Molecular Devices) from a standard curve run in duplicate on every plate. The standard curve was produced from doubling dilutions of an equal mixture of serum from an adult volunteer with high antimeningococcal titers and commercially available immunoglobulin (Sandoglobulin; Phoenix Pharmaceuticals, Gloucester, United Kingdom). Antibody concentrations are reported as the concentration calculated from the standard curve with background subtracted and are expressed in arbitrary units. Negative controls run on each plate were wells with no heat-killed meningococci and wells with no serum. Positive controls were the standards and two positive sera and were run on every plate.
Serum bactericidal assay.
The serum bactericidal assay was performed as described by Peeters et al. (36) with modifications. For each experiment the strains of N. meningitidis were streaked from frozen stock onto separate GC agar plates (gonococcal medium base; Difco) containing 1% Vitox (Oxoid). After overnight incubation at 37°C in 5% CO2, the colonies were harvested onto fresh GC agar plates and incubated for a further 4 h to the exponential phase of growth (11, 28). After 4 h, the colonies were harvested into 10 ml of PBS and adjusted to an optical density at 600 nm of between 0.22 and 0.24. The suspension was then diluted to a working concentration of 105 CFU/ml in Gey’s balanced salt solution (Gibco, Paisley, United Kingdom) supplemented with 1% (wt/vol) bovine serum albumin (Sigma) (GBSS-BSA). Test sera were heat inactivated for 30 min at 56°C in a water bath. Doubling dilutions of sera were made in GBSS-BSA in polystyrene V-bottom microtiter plates (Greiner, Stonehouse, United Kingdom), and bacteria were added to the wells and incubated at room temperature for 10 min. Finally, 6 μl of either 25% baby rabbit complement in GBSS (Serotec, Oxford, United Kingdom) or undiluted human serum was added to the wells as the complement source. The final volume in each well was 24 μl, consisting of 12 μl of serum diluted in GBSS-BSA, 6 μl of bacteria suspended in GBSS-BSA, and 6 μl of complement. Controls included wells with no complement, wells with no serum, and a positive serum control with serum from an adult with serum bactericidal activity against all test strains. A time zero plate (100% survival plate) was then prepared by spotting 7 μl from the control samples with buffer, bacteria, and complement onto a GC agar plate containing 1% Vitox. The microtiter plate was then incubated for 60 min at 37°C in 5% CO2 before 7 μl was harvested from each well onto a square-formed GC agar plate containing 1% Vitox by using a multichannel pipette.
After overnight incubation at 37°C in 5% CO2, the colonies from the time zero and 1-h plates were counted, and the reciprocal of the titer yielding ≥50% killing was reported. The lowest titer used was 1:4, and for analysis sera showing titers of <1:4 were assigned the titer 1:2.
Statistical methods.
Data were analyzed by using the statistical software package Epi Info (Centers for Disease Control and Prevention, Atlanta, Ga.), and the Kruskal-Wallis test was employed throughout for testing the differences between groups. For the serum bactericidal assays, the geometric mean titers were calculated by using Excel (Microsoft) and statistical analysis was performed by using the natural logarithms of the reciprocals of the absolute bactericidal titers.
RESULTS
Serum bactericidal assay.
Preliminary experiments established that organisms from stationary phase (added to the assay mixture directly from overnight growth on GC plates) were highly sensitive to both human and baby rabbit complement in this system. Therefore, plate-grown organisms in log phase (4 h after subculture from an overnight culture on GC agar) were used for the assay as recommended by Maslanka et al. (28).
A donor with no serum bactericidal activity against MC58 was identified as a human complement source for the study. However, the strains derived from H44/76 proved to be highly sensitive to the serum from this individual as well as to several others tested. Baby rabbit complement was therefore used as the complement source for these strains, and human complement was used for assays with MC58.
Bactericidal activity against strains derived from H44/76 with rabbit complement. (i) Bactericidal activity after infection.
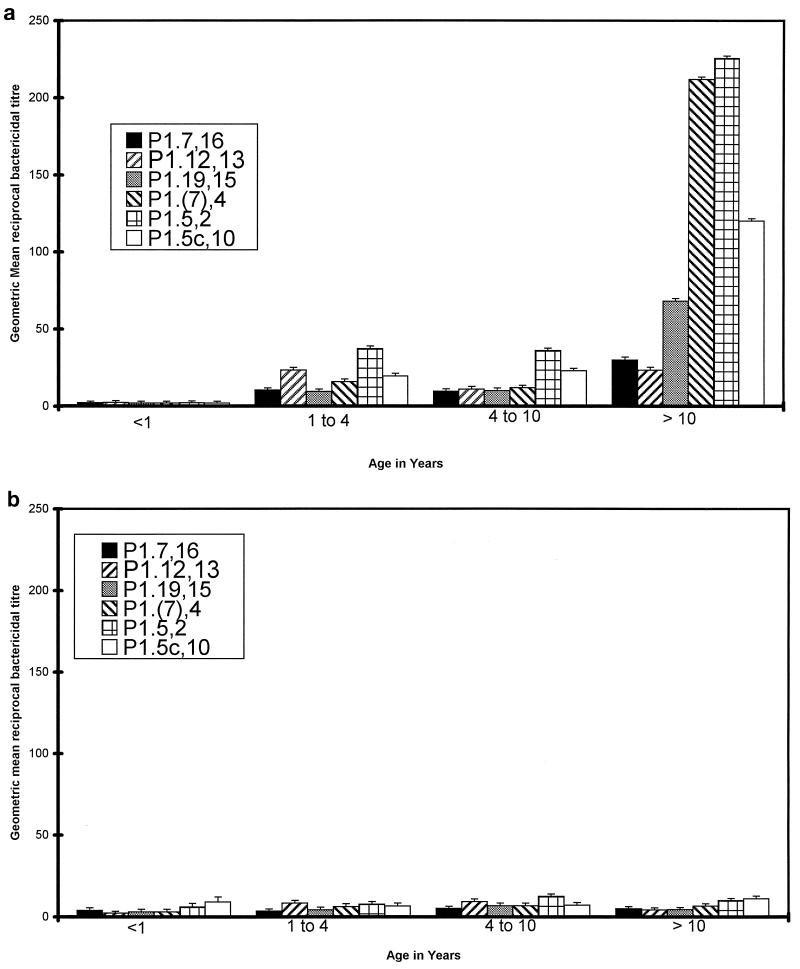
Following meningococcal infection, infants under 1 year of age had no detectable bactericidal activity against the six strains tested (geometric mean titer of <1:4 for all strains) (Fig. 1a). Children from 1 to 10 years of age had significantly greater titers than the infants (P < 0.005 for all six strains), with geometric mean titers in this age group of between 1:9 and 1:36 for the different strains. The spread of titers showed no systematic difference when results from 1- to 4-year-olds were compared with those from children aged 4 to 10 years (P > 0.1). After infection, children over 10 years of age had the highest titers (1:23 to 1:225). In all age groups there were individual variations in bactericidal activity and intraindividual variations in bactericidal activity against strains expressing different serosubtype antigens.
FIG. 1.
Serum bactericidal activity against six different PorA variants of N. meningitidis in sera. Geometric mean bactericidal titers and standard errors of the means either after meningococcal infection (a) or in healthy children (b) are reported in relation to age.
(ii) Bactericidal activity in control children.
Bactericidal antibody levels in control children under 10 years of age were not significantly different from the levels seen in the children after infection (Fig. 1b). Between 1 and 10 years of age there was a trend to higher bactericidal antibody levels in children after infection compared with controls, but this only reached significance for two strains in the 1- to 4-year age group (P1.7,16, P = 0.03; P1.5,2, P = 0.01). Control children over 10 years of age had significantly lower bactericidal titers than children after infection (P < 0.01 for all six strains). There was no significant increase in geometric mean titer with age in control children (P > 0.05).
(iii) Bactericidal activity in relation to strain of N. meningitidis.
There was no significant difference in bactericidal activity between the children who had infections with serogroup B N. meningitidis infection and those who had serogroup C infections (P > 0.05), regardless of age. Indeed, there was no significant relationship between the serosubtype of the infecting strain and the bactericidal response. The highest bactericidal titers were directed against strains expressing P1.5,2 and P1.5c,10 in children >10 years for both control children and convalescent children and against P1.7h,4 in the older children after infection.
Bactericidal activity against strain MC58 with human complement.
Only children under 1 year of age and children ≥10 years of age were studied. With human serum as the complement source and MC58 as the target strain, the sera from children under 1 year of age did not kill meningococci in the bactericidal assay (mean titer, <1:4). Children over 10 years of age had mean bactericidal titers of 1:16, but the titers ranged from 1:2 to 1:256 (Table 4).
TABLE 4.
Individual bactericidal titers against strain MC58 with human complement and against strain H44/76 and serosubtype B:15P1.5,2 with baby rabbit complement after meningococcal infection in children under 1 year of age or over 10 years of age
| Group and serum no. | Age (yr) of child | Reciprocal titer with the following complement source and target strain:
|
|||
|---|---|---|---|---|---|
| Human 1, MC58 (B:15:P1.7,16b) | Human 2, MC58 (B:15:P1.7,16b) | Rabbit, H44/76 (B:15:P1.7,16) | Rabbit, B:15:P1.5,2 | ||
| Children <1 yr | |||||
| M2615 | 0.42 | 2 | 2 | 2 | 4 |
| M2383 | 0.58 | 2 | 2 | 2 | 2 |
| G3084 | 0.67 | 2 | 2 | 2 | 4 |
| M2605 | 0.72 | 2 | 2 | 2 | 2 |
| M2598 | 0.75 | 2 | 2 | 2 | 2 |
| M2486 | 0.92 | 2 | 2 | 2 | 2 |
| Geometric mean | 2 | 2 | 2 | 2.5 | |
| Children >10 yr | |||||
| M2699 | 10.9 | 2 | 2 | 1,024 | 1,024 |
| G3458 | 11.45 | 4 | 4 | 32 | 512 |
| G0969 | 12.1 | 256 | 256 | 32 | 1,024 |
| N0171 | 12.6 | 2 | 16 | 2 | 1,024 |
| G3905 | 12.7 | 4 | 4 | 16 | 512 |
| M2514 | 13.1 | 32 | 32 | 256 | 128 |
| M2515 | 13.25 | 32 | 16 | 128 | 256 |
| T0183 | 15 | 64 | 64 | 128 | 256 |
| M2595 | 16.8 | 64 | 64 | 2 | 16 |
| Geometric mean | 16.0 | 18.6 | 40.3 | 322.5 | |
Percentage of children with bactericidal titers of ≥1:4.
The results of this study were compared with the bactericidal titers found in children in the United States by Goldschneider et al. (13). They considered a bactericidal titer of ≥1:4 to reflect immunity. Applying this definition to our patients, immunity rose from 8 to 23% (depending on the target strain) in children under 1 year to 75 to 100% in those over 10 years of age after infection. Among control children, 20 to 40% of infants and 28 to 68% of those over 10 years of age were immune.
Immunoglobulin levels.
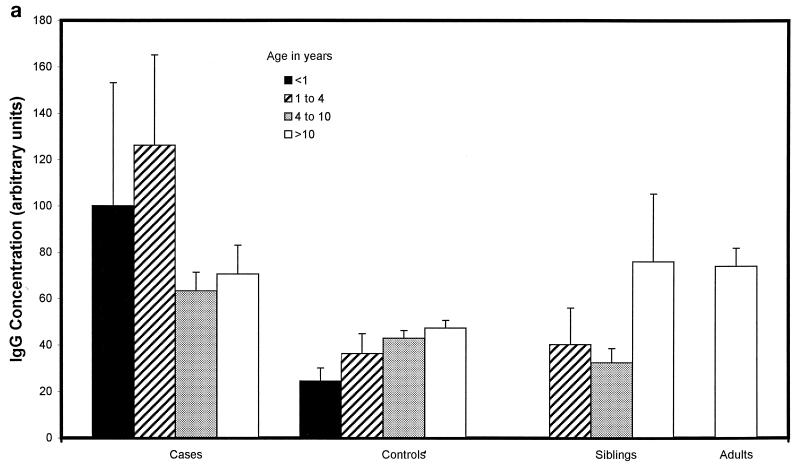
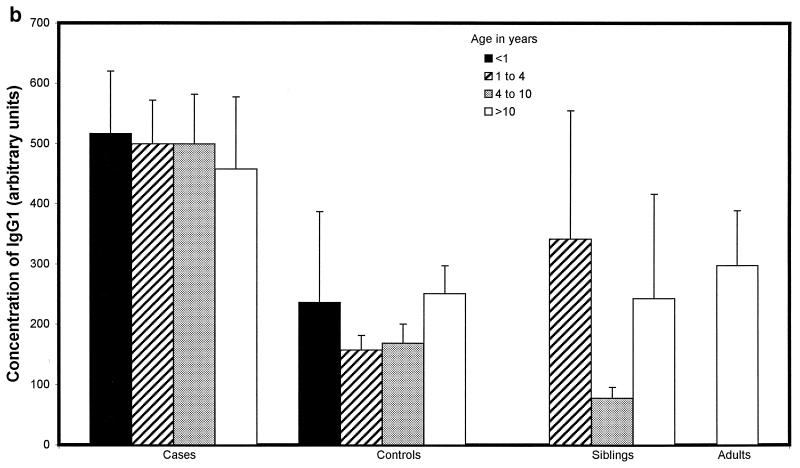
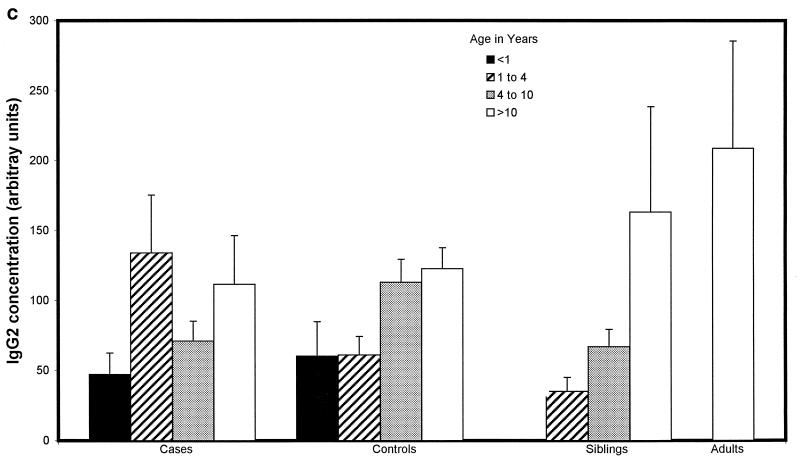
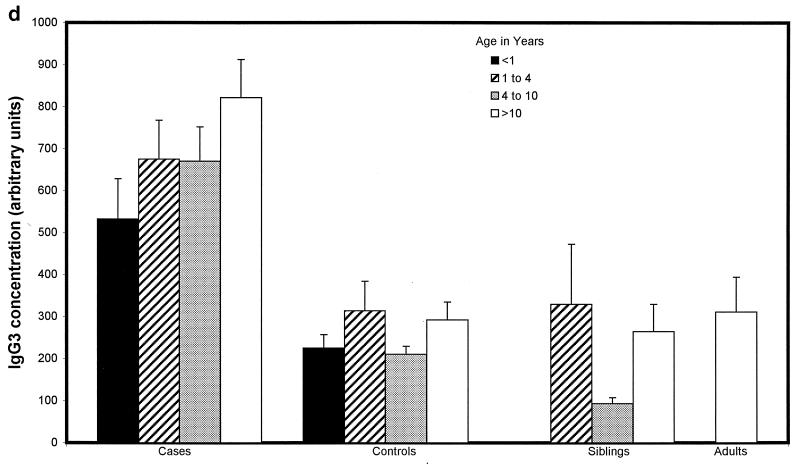
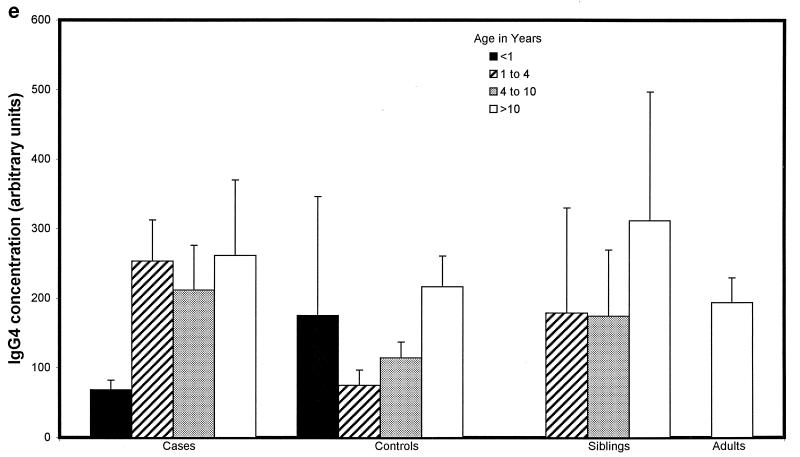
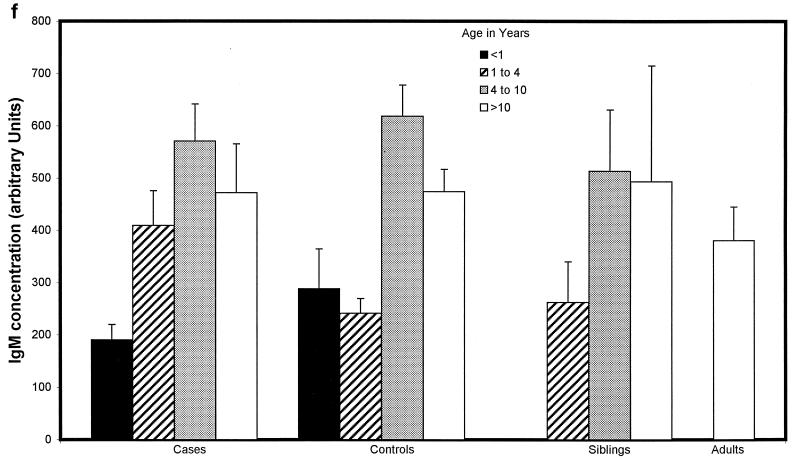
Children with proven meningococcal infection had significantly greater levels of antimeningococcal total IgG (94 ± 34 U) (Fig. 2a), IgG1 (498 ± 83 U) (Fig. 2b), and IgG3 (662 ± 91 U) (Fig. 2d) than controls (45 ± 4 [P = 0.01], 204 ± 44 [P < 0.000001], and 273 ± 52 [P < 0.000001], respectively). Conversely, IgG2 (93 ± 31 U) (Fig. 2c), IgG4 (202 ± 62 U) (Fig. 2e), and IgM (421 ± 72 U) (Fig. 2f) were not significantly raised at 9 weeks (median) following natural infection compared with controls (112 ± 20, 155 ± 41, and 476 ± 58 U, respectively). There was no difference in the level of total IgG or IgG subclass antibody to strain H44/76 between sera from children following serogroup B or C meningococcal infection. There was also no significant difference in ELISA antibody level among children who had recovered from infection with different serogroup B strains, although there was a trend to higher antibody levels in children after B:15:P1.7,16 infection.
FIG. 2.
Whole-cell ELISA antibody levels against strain H44/76 in children with meningococcal infection, controls, siblings of children with meningococcal infection, and adults in relation to age. Means of the antibody concentration and standard errors of the means are reported for total IgG (a), IgG1 (b), IgG2 (c), IgG3 (d), IgG4 (e), and IgM (f).
There was no correlation between age and IgG1 or IgG3 antibody level in children who had meningococcal disease (age range, 0.42 to 17.9 years) or in control children (0.75 to 16 years) (Fig. 2b and d). Children under 1 year of age had lower levels of IgG2 (47 ± 29 U) (Fig. 2c) and IgG4 (69 ± 26 U) (Fig. 2e) after infection than children over 1 (106 ± 32 U [P = 0.02] and 238 ± 62 [P = 0.02], respectively), although these were not different from the levels seen in controls. Adults had higher levels of IgG2 (209 ± 150 U) than convalescent children or pediatric controls (123 ± 29 U [P < 0.001]) (Fig. 2c). Control children >4 years of age had higher levels of IgG2 than those under 4 (118 ± 21 and 61 ± 21 U, respectively [P = 0.01]) and also higher levels of IgG4 (171 ± 52 and 96 ± 66 U [P = 0.013]) and IgM (538 ± 70 and 251 ± 49 U [P = 0.0001]).
Siblings of children who had recovered from meningococcal disease had antibody levels similar to those of unrelated control children.
IgM levels were lower in children under 1 year of age after serogroup B infection (135 ± 54 U) than after serogroup C infection (313 ± 114 U) (P = 0.02). This difference was not apparent in older children and was unrelated to the time of sampling after infection.
DISCUSSION
This study demonstrated a striking relationship between age and immune responses to N. meningitidis following invasive infection. Serum bactericidal responses in children following infection increased with age despite the finding that children of all ages, including infants, produced similar levels of specific total IgG and IgG1 and IgG3 subclasses in response to meningococcal infection.
The 13 infants under age 1 year studied had very low bactericidal titers, there were significantly higher titers in 35 1- to 10-year-olds, and the highest titers were in the 12 children over 10 years of age. This finding of a lack of bactericidal response to infection in infants is supported by another study in which three infants had no bactericidal activity following mild meningococcal infection (4). Griffiss et al. (18) also found that following serogroup C infection, a 4-month-old infant had no detectable bactericidal activity against the infecting strain in convalescence. However, in contrast to the findings presented here, they also noted that all of 25 children infected with serogroup B meningococci developed bactericidal antibody following infection, but the ages of the children were not specified (18). In another study, Idanpaan-Heikkila et al. (23) found only 21% of teenagers who recovered from meningococcal infection developed serum bactericidal activity to a test strain (B:15:P1.7,16) in an assay based on human complement, but nearly 80% of teenagers in our study produced bactericidal activity. However, in the study of Idanpaan-Heikkila et al. (23), all teenagers infected with an organism homologous to the test strain produced bactericidal antibody. Similarly, in our study, there was one child over 10 years of age infected with a B:15:P1.7,16 strain, and he had high bactericidal titers against the homologous test strain. However, infants seem to be different. The two infants in our study who were previously infected with B:15:P1.7,16 strains did not produce bactericidal activity against the homologous strains (H44/76 or MC58). Furthermore, the porA genes of most infecting strains were expressed in at least one of our target strains, but there was no activity in any infant against strains bearing the homologous PorA. There was insufficient serum to test activity against the patient’s own strains, but these may express relevant bactericidal epitopes other than those used in serological typing.
The lack of bactericidal antibody found in these infants after infection is in accord with the poor responses noted in young children after vaccination with OMP or OMV vaccines (6, 10, 35). However, one study which examined serum bactericidal activity following vaccination with the Norwegian OMV vaccine in South America found a significant rise in bactericidal activity, although responses seemed to be limited to the vaccine strain (50). There may be important differences between our population and the children included in these vaccine trials. Such differences include the route of immunization (nasopharyngeal versus intramuscular), the nature of the immunogen (live organisms versus OMVs), and possibly the individual’s immunological background, since our children were susceptible to infection with N. meningitidis (but none were complement deficient). The lack of bactericidal activity against the strains in infants in the present study does not rule out the possibility that these infants might have such activity against their own infecting strain. However, it is clear that there is no cross-reactive bactericidal activity in this age group.
The observed age-dependent changes in bactericidal activity in our study parallel the well-recognized age-related responses to T-independent polysaccharide antigens. It has been suggested that the predominant anti-B meningococcal bactericidal antibody when the complement source is rabbit complement is directed against B polysaccharide and is of the IgM class (26, 27, 49, 59) and that this is not a target for bactericidal activity when human complement is used (57, 58). This antipolysaccharide bactericidal activity can be blocked with serogroup B polysaccharide that may nonspecifically interfere with binding of complement to opsonizing antibody (18). In contrast, others have found that most bactericidal activity, even with rabbit complement, is directed against noncapsular antigens (18, 24, 26), and even human complement binds anti-B polysaccharide IgM under the right assay conditions (26). The situation in vivo remains unclear.
In our study bactericidal antibody does not seem to be related to the presence of serogroup B polysaccharide, since the levels produced by patients infected with documented serogroup C organisms were not significantly different from the levels in those who had had serogroup B infections. The bactericidal activity after infection was related to the serosubtype antigen, with the highest titers against P1.(7),4, P1.5c,10, and P1.5,2. All six strains had the same genetic background (H44/76) apart from their porA gene, and they all possessed serogroup B capsule and lipopolysaccharide (L3,7,9), suggesting that the differences in bactericidal response were directed more against the differences in their class 1 OMPs than against those in other structures. This has also been suggested by Kasper et al. (24), who found that bactericidal activity in convalescent-phase sera correlated best with titers of antibodies to serotype antigens. Moreover, the age dependency persisted in our study even with human complement, such that infants under 1 year of age had no bactericidal response to infection with MC58, while 80% of those over 10 years of age had bactericidal activity against this strain.
There may be both bacterial strain and human population differences in acquisition of bactericidal activity as measured in healthy children. Goldschneider et al. (14) showed an age-related acquisition of bactericidal activity against a single test strain, such that 80% of children had bactericidal activity of ≥1:4 by 12 years of age. In contrast, in the United Kingdom, Zorgani et al. (60) found that only 25% of 106 healthy 12- to 18-year-olds had bactericidal titers of ≥1:4 to three different serogroup B strains, using human complement. This is similar to data presented here that show bactericidal titers of ≥1:4 against strain H44/76 in 28% of children over 10 years of age. However, 68% in our study had bactericidal titers of ≥1:4 against strain B:15:P1.5,2. In the control children as well as in children after infection, the highest titers were directed against strains with P1.5,2, P1.5c,10, and P1.(7),4 as the PorA protein, which could reflect immunity arising from carriage as a result of the increased circulation of strains expressing these proteins in recent years in the United Kingdom (34).
Even the youngest infants produced high levels of IgG1 and IgG3 subclass antibody against whole meningococci despite documented deficiencies in antibody production in response to other pathogens (12). Others have also found increased levels of IgG1 and IgG3 after infection (20, 48) and vaccination (32). In our study levels of IgM, IgG2, and IgG4 were not elevated in response to infection compared with levels in controls, and the youngest children had the lowest levels of these antibodies. Therefore, it seems unlikely that these antibodies are important in the acquisition of immunity to meningococci after infection, although they may play a part in the development of natural immunity. Granoff et al. (15) also found lower IgM levels in convalescent sera from children aged 3 to 9 years than in older children and adults. Since a deficiency of IgG2 causes enhanced susceptibility to encapsulated bacteria, including meningococci (5, 41, 46, 51), low levels of IgG2 in the youngest children may be an important factor reflected in the age-dependent incidence of disease. IgG4 does not activate the classical complement pathway but could be important in vivo in phagocytosis or alternative pathway activation. Kristiansen et al. (25), measuring total IgG, found that levels in Norwegian children were low until 12 to 15 years of age and then steadily increased. They did not undertake subclass analysis, but based on the data here, we speculate that they would have found that the increase correlated with a rise in IgG2 and IgG4 with age.
Our data showed no differences in the anti-B:15:P1.7,16 IgG antibody level in children infected with serogroup B or serogroup C meningococci, although the level of IgM was slightly lower in infants after infection with serogroup B organisms. This suggests that most antibody is directed at noncapsular conserved epitopes on the organism. It has been shown before that anti-serogroup B meningococcal antibody is directed against surface-exposed and not strain-specific epitopes (20, 42). There was also little difference in the level of antibody among children who had recovered from infection with different B strains, although there was a nonsignificant trend to higher antibody levels in children after B:15:P1.7,16 infection.
This study has shown striking age-dependent differences in the bactericidal response to infection with N. meningitidis, which parallel the poor protection afforded to young children in several serogroup B meningococcal vaccine studies (6, 10, 35, 47). Epidemiological data also show that susceptibility to infection is greatest in young children (13, 34). These differences were not related to levels of IgG subclasses or IgM. The failure of young children to develop bactericidal antibodies after infection, despite similar levels of immunoglobulin subclasses, might be explained by differences in antibody specificity, affinity, or avidity. Understanding whether the differences lie in the T-cell help for antibody production or in the cytokine milieu which directs the elaboration of specificity, affinity, or avidity of antibody may be the key to developing immunogenic vaccines for infants.
ACKNOWLEDGMENTS
We thank the medical and nursing staff of the pediatric intensive care and infectious disease units and the pediatric outpatient clinic at St. Mary’s Hospital; Jayne Farrant and Kate Dunn for microbiological assistance; and Barbara Chan and Graham Shennan for advice on ELISA development. We also thank Betsy Kuipers and Harry van Dijken (Laboratory of Vaccine Development and Immune Mechanisms, Bilthoven, The Netherlands) for technical advice.
A.J.P. is funded by an Action Research Fellowship, R.G. is supported by a grant from the Meningitis Research Foundation, and R.B. is supported by a Wellcome Trust Clinical Epidemiology Fellowship.
REFERENCES
- 1.Abdillahi H, Poolman J T. Neisseria meningitidis group B serosubtyping using monoclonal antibodies in whole-cell ELISA. Microb Pathog. 1988;4:27–32. doi: 10.1016/0882-4010(88)90045-9. [DOI] [PubMed] [Google Scholar]
- 2.Abdillahi H, Poolman J T. Typing of group-B Neisseria meningitidis with monoclonal antibodies in the whole-cell ELISA. J Med Microbiol. 1988;26:177–180. [PubMed] [Google Scholar]
- 3.Amato Neto V, Finger H, Gotschlich E C, Feldman R A, de Avila C A, Konichi S R, Laus W C. Serologic response to serogroup C meningococcal vaccine in Brazilian preschool children. Rev Inst Med Trop Sao Paulo. 1974;16:149–153. [PubMed] [Google Scholar]
- 4.Baltimore R S, Hammerschlag M. Meningococcal bacteremia: clinical and serologic studies of infants with mild illness. Am J Dis Child. 1977;131:1001–1004. doi: 10.1001/archpedi.1977.02120220067011. [DOI] [PubMed] [Google Scholar]
- 5.Bass J L, Nuss R, Mehta K A, Morganelli P, Bennett L. Recurrent meningococcemia associated with IgG2-subclass deficiency N. Engl J Med. 1983;309:430. doi: 10.1056/NEJM198308183090711. [DOI] [PubMed] [Google Scholar]
- 6.Boslego J, Garcia J, Cruz C, Zollinger W, Brandt B, Ruiz S, Martinez M, Arthur J, Underwood P, Silva W, et al. Efficacy, safety, and immunogenicity of a meningococcal group B (15:P1.3) outer membrane protein vaccine in Iquique, Chile. Vaccine. 1995;13:821–829. doi: 10.1016/0264-410x(94)00037-n. [DOI] [PubMed] [Google Scholar]
- 7.Burian V, Gotschlich E, Kuzemenska P, Svandova E. Naturally occurring antibodies to Neisseria meningitidis. Bull W H O. 1977;55:653–657. [PMC free article] [PubMed] [Google Scholar]
- 8.Castagliuolo P P, Nisini R, Quinti I, Fattorossi A, D’Amelio R. Immunoglobulin deficiencies and meningococcal disease. Ann Allergy. 1986;57:68–70. [PubMed] [Google Scholar]
- 9.Claassen I, Meylis J, van der Ley P, Peeters C, Brons H, Robert J, Borsboom D, van der Ark A, van Straaten I, Roholl P, Kuipers B, Poolman J. Production, characterization and control of a Neisseria meningitidis hexavalent class 1 outer membrane protein containing vesicle vaccine. Vaccine. 1996;14:1001–1008. doi: 10.1016/0264-410x(96)00020-5. [DOI] [PubMed] [Google Scholar]
- 10.de Moraes J C, Perkins B A, Camargo M C, Hidalgo N T, Barbosa H A, Sacchi C T, Landgraf I M, Gattas V L, Vasconcelos H D G, Gral I M, et al. Protective efficacy of a serogroup B meningococcal vaccine in Sao Paulo, Brazil. Lancet. 1992;340:1074–1078. doi: 10.1016/0140-6736(92)93086-3. [DOI] [PubMed] [Google Scholar]
- 11.Frasch C E, Robbins J D. Protection against group B meningococcal disease. III. Immunogenicity of serotype 2 vaccines and specificity of protection in a guinea pig model. J Exp Med. 1978;147:629–644. doi: 10.1084/jem.147.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldblatt D, Turner M W, Levinsky R J. Branhamella cattarhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 13.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldschneider I, Gotschlich E C, Artenstein M S. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granoff D M, Kelsey S K, Bijlmer H A, Van Alphen L, Dankert J, Mandrell R E, Azmi F H, Scholten R J. Antibody responses to the capsular polysaccharide of Neisseria meningitidis serogroup B in patients with meningococcal disease. Clin Diagn Lab Immunol. 1995;2:574–582. doi: 10.1128/cdli.2.5.574-582.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiss J M. Epidemic meningococcal disease: synthesis of a hypothetical immunoepidemiologic model. Rev Infect Dis. 1982;4:159–172. doi: 10.1093/clinids/4.1.159. [DOI] [PubMed] [Google Scholar]
- 17.Griffiss J M. Mechanisms of host immunity. In: Cartwright K, editor. Meningococcal disease. Chichester, United Kingdom: John Wiley & Sons; 1995. pp. 35–70. [Google Scholar]
- 18.Griffiss J M, Brandt B L, Broud D D, Goroff D K, Baker C J. Immune response of infants and children to disseminated infections with Neisseria meningitidis. J Infect Dis. 1984;150:71–79. doi: 10.1093/infdis/150.1.71. [DOI] [PubMed] [Google Scholar]
- 19.Guttormsen H K, Wetzler L M, Naess A. Humoral immune response to the class 3 outer membrane protein during the course of meningococcal disease. Infect Immun. 1993;61:4734–4742. doi: 10.1128/iai.61.11.4734-4742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttormsen H K, Wetzler L M, Solberg C O. Humoral immune response to class 1 outer membrane protein during the course of meningococcal disease. Infect Immun. 1994;62:1437–1443. doi: 10.1128/iai.62.4.1437-1443.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harthug S, Rosenqvist E, Hoiby E A, Gedde-Dahl T W, Froholm L O. Antibody response in group B meningococcal disease determined by enzyme-linked immunosorbent assay with serotype 15 outer membrane antigen. J Clin Microbiol. 1986;24:947–953. doi: 10.1128/jcm.24.6.947-953.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holten E. Serotypes of Neisseria meningitidis isolated from patients in Norway during the first six months of 1978. J Clin Microbiol. 1979;9:186–188. doi: 10.1128/jcm.9.2.186-188.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Idanpaan-Heikkila I, Hoiby E A, Chattopadhyay P, Airaksinen U, Michaelsen T M, Wedege E. Antibodies to meningococcal class 1 outer-membrane protein and its variable regions in patients with systemic meningococcal disease. J Med Microbiol. 1995;43:335–343. doi: 10.1099/00222615-43-5-335. [DOI] [PubMed] [Google Scholar]
- 24.Kasper D L, Winkelhake J L, Brandt B L, Artenstein M S. Antigenic specificity of bactericidal antibodies in antisera to Neisseria meningitidis. J Infect Dis. 1973;127:378–387. doi: 10.1093/infdis/127.4.378. [DOI] [PubMed] [Google Scholar]
- 25.Kristiansen B E, Lind K W, Mevold K, Sorensen B, Froholm L O, Bryn K, Tjade T, Bovre K. Meningococcal phenotypic and genotypic characteristics and human antibody levels. J Clin Microbiol. 1988;26:1988–1992. doi: 10.1128/jcm.26.10.1988-1992.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandrell R E, Azmi F H, Granoff D M. Complement-mediated bactericidal activity of human antibodies to poly alpha 2→8 N-acetylneuraminic acid, the capsular polysaccharide of Neisseria meningitidis serogroup B. J Infect Dis. 1995;172:1279–1289. doi: 10.1093/infdis/172.5.1279. [DOI] [PubMed] [Google Scholar]
- 27.Mandrell R E, Zollinger W D. Measurement of antibodies to meningococcal group B polysaccharide: low avidity binding and equilibrium binding constants. J Immunol. 1982;129:2172–2178. [PubMed] [Google Scholar]
- 28.Maslanka S E, Gheesling L L, Libutti D E, Donaldson K B, Harakeh H S, Dykes J K, Arhin F F, Devi S J, Frasch C E, Huang J C, Kriz-Kuzemenska P, Lemmon R D, Lorange M, Peeters C C, Quataert S, Tai J Y, Carlone G M. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. Clin Diagn Lab Immunol. 1997;4:156–167. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuinness B T, Clarke I N, Lambden P R, Barlow A K, Poolman J T, Jones D M, Heckels J E. Point mutation in meningococcal por A gene associated with increased endemic disease. Lancet. 1991;337:514–517. doi: 10.1016/0140-6736(91)91297-8. [DOI] [PubMed] [Google Scholar]
- 30.Milagres L G, Lemos A P, Meles C E, Silva E L, Ferreira L H, Souza J A, Carlone G M. Antibody response after immunization of Brazilian children with serogroup C meningococcal polysaccharide noncovalently complexed with outer membrane proteins. Braz J Med Biol Res. 1995;28:981–989. [PubMed] [Google Scholar]
- 31.Milagres L G, Ramos S R, Sacchi C T, Melles C E, Vieira V S, Sato H, Brito G S, Moraes J C, Frasch C E. Immune response of Brazilian children to a Neisseria meningitidis serogroup B outer membrane protein vaccine: comparison with efficacy. Infect Immun. 1994;62:4419–4424. doi: 10.1128/iai.62.10.4419-4424.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naess L M, Rosenqvist E, Hoiby E A, Michaelsen T E. Quantitation of IgG subclass antibody responses after immunization with a group B meningococcal outer membrane vesicle vaccine, using monoclonal mouse-human chimeric antibodies as standards. J Immunol Methods. 1996;196:41–49. doi: 10.1016/0022-1759(96)00108-1. [DOI] [PubMed] [Google Scholar]
- 33.Nicholson A, Lepow I H. Host defense against Neisseria meningitidis requires a complement-dependent bactericidal activity. Science. 1979;205:298–299. doi: 10.1126/science.451601. [DOI] [PubMed] [Google Scholar]
- 34.Noah N, Connolly M. Surveillance of bacterial meningitis in Europe 1995. London, United Kingdom: King’s European Meningitis Surveillance Unit; 1996. [Google Scholar]
- 35.Noronha C P, Struchiner C J, Halloran M E. Assessment of the direct effectiveness of BC meningococcal vaccine in Rio de Janeiro, Brazil: a case-control study. Int J Epidemiol. 1995;24:1050–1057. doi: 10.1093/ije/24.5.1050. [DOI] [PubMed] [Google Scholar]
- 36.Peeters C C, Rumke H C, Sundermann L C, Rouppe van der Voort E M, Meulenbelt J, Schuller M, Kuipers A J, van der Ley P, Poolman J T. Phase I clinical trial with a hexavalent PorA containing meningococcal outer membrane vesicle vaccine. Vaccine. 1996;14:1009–1015. doi: 10.1016/0264-410x(96)00001-1. [DOI] [PubMed] [Google Scholar]
- 37.Peltola H, Makela H, Kayhty H, Jousimies H, Herva E, Hallstrom K, Sivonen A, Renkonen O V, Pettay O, Karanko V, Ahvonen P, Sarna S. Clinical efficacy of meningococcus group A capsular polysaccharide vaccine in children three months to five years of age. N Engl J Med. 1977;297:686–691. doi: 10.1056/NEJM197709292971302. [DOI] [PubMed] [Google Scholar]
- 38.Perkins B A, Jonsdottir K, Briem H, Griffiths E, Plikaytis B D, Hoiby E A, Rosenqvist E, Holst J, Nokleby H, Sotolongo F, Sierra G, Campa H C, Carlone G M, Williams D, Dykes J, Kapczynski D, Tikhomirov E, Wenger J D, Broome C V. Immunogenicity of two efficacious outer membrane protein-based serogroup B meningococcal vaccines among young adults in Iceland. J Infect Dis. 1998;177:683–691. doi: 10.1086/514232. [DOI] [PubMed] [Google Scholar]
- 39.Poolman J T. Development of a meningococcal vaccine. Infect Agents Dis. 1995;4:13–28. [PubMed] [Google Scholar]
- 40.Rautonen N, Pelkonen J, Sipinen S, Kayhty H, Makela O. Isotype concentrations of human antibodies to group A meningococcal polysaccharide. J Immunol. 1986;137:2670–2675. [PubMed] [Google Scholar]
- 41.Rijkers G T, Saunders E A M, Zegers B J M. Anti-capsular polysaccharide antibody deficiency states. Immunodeficiency. 1994;5:1–21. [PubMed] [Google Scholar]
- 42.Rosenqvist E, Harthug S, Froholm L O, Hoiby E A, Bovre K, Zollinger W D. Antibody responses to serogroup B meningococcal outer membrane antigens after vaccination and infection. J Clin Microbiol. 1988;26:1543–1548. doi: 10.1128/jcm.26.8.1543-1548.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosenqvist E, Hoiby E A, Bjune G, Bryn K, Closs O, Feiring B, Klem A, Nokleby H, Frolm L O. Human antibody responses after vaccination with the Norwegian group B meningococcal outer membrane vesicle vaccine: results from ELISA studies. NIPH Ann. 1991;14:169–179. , 180–181. [PubMed] [Google Scholar]
- 44.Rosenqvist E, Hoiby E A, Wedege E, Bryn K, Kolberg J, Klem A, Ronnild E, Bjune G, Nokleby H. Human antibody responses to meningococcal outer membrane antigens after three doses of the Norwegian group B meningococcal vaccine. Infect Immun. 1995;63:4642–4652. doi: 10.1128/iai.63.12.4642-4652.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstein N, Levine O, Taylor J P, Evans D, Plikaytis B D, Wenger J D, Perkins B A. Efficacy of meningococcal vaccine and barriers to vaccination. JAMA. 1998;279:435–439. doi: 10.1001/jama.279.6.435. [DOI] [PubMed] [Google Scholar]
- 46.Shackleford P G, Granoff D M, Polmar S H, Scott M G, Goskowicz M C, Madaserry J V, Nahm M H. Subnormal serum concentration of IgG2 in children with frequent infections associated with varied patterns of immunologic dysfunction. J Pediatr. 1990;116:529–538. doi: 10.1016/s0022-3476(05)81598-7. [DOI] [PubMed] [Google Scholar]
- 47.Sierra G V, Campa H C, Varcacel N M, Garcia I L, Izquierdo P L, Sotolongo P F, Casanueva G V, Rico C O, Rodriguez C R, Terry M H. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195–207. , 208–210. [PubMed] [Google Scholar]
- 48.Sjursen H, Wedege E, Rosenqvist E, Naess A, Halstensen A, Matre R, Solberg C O. IgG subclass antibodies to serogroup B meningococcal outer membrane antigens following infection and vaccination. APMIS. 1990;98:1061–1069. doi: 10.1111/j.1699-0463.1990.tb05035.x. [DOI] [PubMed] [Google Scholar]
- 49.Skevakis L, Frasch C E, Zahradnik J M, Dolin R. Class-specific human bactericidal antibodies to capsular and noncapsular surface antigens of Neisseria meningitidis. J Infect Dis. 1984;149:387–396. doi: 10.1093/infdis/149.3.387. [DOI] [PubMed] [Google Scholar]
- 50.Tappero J, Lagos R, Maldonado A, Herrera P, Gheesling L, Williams D, Carlone G, Plikaytis B, Nokleby H, Holst J, Sierra G, Perkins B. Serum bactericidal activity elicited by two outer membrane protein serogroup B meningococcal vaccines among infants, pre-school children, and adults in Santiago, Chile. Baltimore, Md: Neisseria ’96, 110th International Pathogenic Neisseria Conference; 1996. [Google Scholar]
- 51.Trollfors B, Lagergard T, Claesson B A, Thornberg E, Martinell J, Schneerson R. Characterisation of the serum antibody response to the capsular polysaccharide of Haemophilus influenzae type b in children with invasive infections. J Infect Dis. 1992;166:1335–1339. doi: 10.1093/infdis/166.6.1335. [DOI] [PubMed] [Google Scholar]
- 52.Van Der Ley P, Poolman J T. Construction of a multivalent meningococcal vaccine strain based on the class 1 outer membrane protein. Infect Immun. 1992;60:3156–3161. doi: 10.1128/iai.60.8.3156-3161.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Ley P, van der Biezen J, Hohenstein P, Peeters C, Poolman J T. Use of transformation to construct antigenic hybrids of the class 1 outer membrane protein in Neisseria meningitidis. Infect Immun. 1993;61:4217–4224. doi: 10.1128/iai.61.10.4217-4224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van der Ley P, van der Biezen J, Poolman J T. Construction of Neisseria meningitidis strains carrying multiple chromosomal copies of the porA gene for use in the production of a multivalent outer membrane vesicle vaccine. Vaccine. 1995;13:401–407. doi: 10.1016/0264-410x(95)98264-b. [DOI] [PubMed] [Google Scholar]
- 55.Wahdan M H, Sallam S A, Hassan M N, Abdel Gawad A, Rakha A S, Sippel J E, Hablas R, Sanborn W R, Kassem N M, Riad S M, Cvjetanovic B. A second controlled field trial of a serogroup A meningococcal polysaccharide vaccine in Alexandria. Bull W H O. 1977;55:645–651. [PMC free article] [PubMed] [Google Scholar]
- 56.Wyle F A, Artenstein M S, Brandt B L, Tramont E C, Kasper D L, Altieri P L, Berman S L, Lowenthal J P. Immunologic response of man to group B meningococcal polysaccharide vaccines. J Infect Dis. 1972;126:514–521. doi: 10.1093/infdis/126.5.514. [DOI] [PubMed] [Google Scholar]
- 57.Zollinger W D, Mandrell R E. Importance of complement source in bactericidal activity of human antibody and murine monoclonal antibody to meningococcal group B polysaccharide. Infect Immun. 1983;40:257–264. doi: 10.1128/iai.40.1.257-264.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zollinger W D, Mandrell R E, Altieri P, Berman S, Lowenthal J, Artenstein M S. Safety and immunogenicity of a Neisseria meningitidis type 2 protein vaccine in animals and humans. J Infect Dis. 1978;137:728–739. doi: 10.1093/infdis/137.6.728. [DOI] [PubMed] [Google Scholar]
- 59.Zollinger W D, Mandrell R E, Griffiss J M, Altieri P, Berman S. Complex of meningococcal group B polysaccharide and type 2 outer membrane protein immunogenic in man. J Clin Investig. 1979;63:836–848. doi: 10.1172/JCI109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zorgani A A, James V S, Stewart J, Blackwell C C, Elton R A, Weir D M. Serum bactericidal activity in a secondary school population following an outbreak of meningococcal disease: effects of carriage and secretor status. FEMS Immunol Med Microbiol. 1996;14:73–81. doi: 10.1111/j.1574-695X.1996.tb00273.x. [DOI] [PubMed] [Google Scholar]