Abstract
Platinum (Pt) oxides are vital catalysts in numerous reactions, but research indicates that they decompose at high temperatures, limiting their use in high-temperature applications. In this study, we identify a two-dimensional (2D) crystalline Pt oxide with remarkable thermal stability (1,200 K under nitrogen dioxide) using a suite of in situ methods. This 2D Pt oxide, characterized by a honeycomb lattice of Pt atoms encased between dual oxygen layers forming a six-pointed star structure, exhibits minimized in-plane stress and enhanced vertical bonding due to its unique structure, as revealed by theoretical simulations. These features contribute to its high thermal stability. Multiscale in situ observations trace the formation of this 2D Pt oxide from α-PtO2, providing insights into its formation mechanism from the atomic to the millimetre scale. This 2D Pt oxide with outstanding thermal stability and distinct surface electronic structure subverts the previously held notion that Pt oxides do not exist at high temperatures and can also present unique catalytic capabilities. This work expands our understanding of Pt oxidation species and sheds light on the oxidative and catalytic behaviours of Pt oxide in high-temperature settings.
Subject terms: Two-dimensional materials, Characterization and analytical techniques, Heterogeneous catalysis
Pt oxides are essential catalysts in many critical reactions, but are typically unstable and prone to evaporation above 700 K. A two-dimensional layered Pt oxide with exceptional thermal stability is introduced, capable of surviving at high temperatures.
Main
In a range of industrial applications, platinum (Pt) is subject to oxidation under demanding conditions, including exposure to oxidizing atmospheres and high temperatures. Therefore, a thorough understanding of the oxidation behaviour of Pt is essential to optimize the efficiency and longevity of catalysts1. This process plays a vital role in various heterogeneous reactions such as CO/NOx oxidation (three-way catalysts)2, ammonia oxidation3, fuel cell operations4 and electrochemical oxygen reduction5–7. Understanding the formation and characteristics of Pt oxides is essential, as they significantly influence the reactivity of Pt-based catalysts in these diverse reactions and are central to the efficiency and functionality of Pt-based catalysts8–11.
A multitude of preceding investigations have examined the oxidation phenomena of Pt, probing diverse conditions encompassing exposure to oxygen, ozone and nitrogen dioxide12–18. Such studies, often conducted via traditional surface science techniques under ultrahigh-vacuum (UHV) conditions, revealed that at 300 K, the Pt(111) surface becomes cloaked with a layer of adsorbed oxygen atoms, attaining coverage levels up to 0.25 monolayer (ML) in a p(2 × 2)-O configuration19. Using atomic oxygen sources, such as ozone or nitrogen dioxide, facilitates achieving higher oxygen coverage on Pt surfaces. In particular, the interaction of NO2 with Pt(111) at 450 K has been observed to induce the formation of 0.75 ML of chemisorbed oxygen15, whereas ozone at 300 K can elevate the oxygen coverage to as much as 2.4 ML (ref. 13). When subjected to high temperatures and high pressures, Pt surfaces exhibit various morphologies, including one-dimensional oxidic rows, triangular oxides and the more unstable α-PtO2 (refs. 14,16,17,20). The detailed Pt–O phase diagram is shown in Supplementary Note 1. Despite extensive research efforts, the dynamic evolution and structural intricacies of various Pt oxide species remain enveloped in a veil of scientific ambiguity21–25.
Understanding the oxidation of Pt requires examining the structural and electronic changes at atomic resolution during oxidation processes in realistic reaction conditions26. By using a comprehensive suite of in situ real-space instruments, we observe the transformation of surface oxides across scales—from atomic to millimetre—bridging the pressure gap between UHV and practical environments27. This approach integrates localized atomistic structures from in situ scanning tunnelling microscopy (STM) and transmission electron microscopy with broader insights from in situ scanning electron microscopy (SEM)27–30. The combined use of in situ imaging and ambient-pressure X-ray photoelectron spectroscopy (AP-XPS) enables us to correlate detailed atomic structures with macroscopic transformation dynamics under dynamic, non-equilibrium conditions31–33. We investigate the oxidation of Pt(111) under a NO2 atmosphere from room temperature to 1,000 K with various levels of increase. Our findings indicate that at temperatures below approximately 700 K, the Pt(111) surface is enveloped by amorphous α-PtO2. However, α-PtO2 demonstrates instability at temperatures exceeding 750 K, undergoing a transition into an oxide phase. Using a combination of complementary in situ methods, we characterize the surface structure of this oxide. Our integrated approach indicates that the observed Pt oxide forms a honeycomb lattice of Pt atoms sandwiched between dual oxygen layers, creating a distinct out-of-plane six-pointed star structure. It exhibits two-dimensional (2D) crystalline properties and exceptional thermal stability above 750 K. Further in situ SEM and AP-XPS observations reveal that this 2D Pt oxide retains its integrity even at 1,200 K. Such observed exceptional thermal stability challenges the prevailing understanding that the structure of Pt oxides becomes unstable above 700 K (refs. 34,35). Comprehensive theoretical analysis, including density functional theory (DFT) and molecular dynamics (MD) simulations with a machine learning force field, accurately describes the surface structure and confirms the stability of this oxide at the atomic level under various conditions.
Besides remarkable thermal stability, this 2D crystalline Pt oxide also presents a unique surface electronic structure, facilitating the selective oxidation of CO in environments containing a mixture of methane, oxygen and CO. Furthermore, the Pt oxide formed in the presence of NO2 can also be produced under other oxidizing conditions. This research enhances our knowledge of Pt oxidation species and provides an insight into the behaviour of Pt oxide in terms of oxidation and catalysis, particularly in high-temperature and oxidizing settings. These findings have important implications for the design and development of advanced catalysts. In particular, the outstanding thermal stability under oxidative duress indicates that this 2D Pt oxide may represent the primary active phase of Pt in such environments, a revelation that redefines the understanding of the role of Pt oxide in catalysis.
Results and discussion
Observing the oxidation of Pt
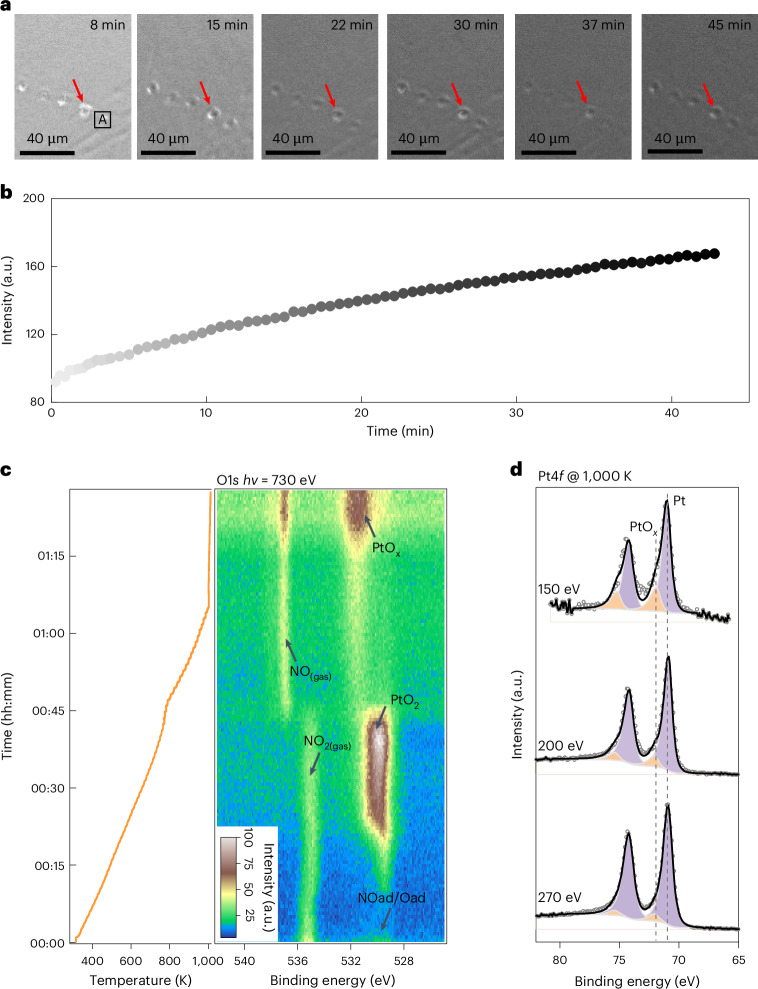
The surface oxide was grown by annealing the Pt(111) substrate in the presence of 1 mbar NO2 at 1,000 K. The Pt(111) surface was cleaned by cycles of sputtering and annealing in advance (Supplementary Note 2). In situ SEM images (Fig. 1a) captured the oxidation process of Pt. The homogeneously grey level increasing in the secondary-electron image reflects the changes in the surface work function during NO2 oxidation (Fig. 1b and Supplementary Video 1). Therefore, the in situ SEM observations imply the formation of uniform layered species on the Pt(111) surface under a NO2 atmosphere at 1,000 K.
Fig. 1. Formation of PtOx layer on Pt(111) under NO2.
a, In situ SEM images obtained during the oxidation of Pt(111) by 1 mbar NO2 under 1,000 K. b, Change in intensity in region A (shown in a). c, Evolution of the O1s spectra during the oxidation process on a Pt(111) surface under 1 mbar NO2 from 300 to 1,000 K. d, Pt4f spectra of the PtOx collected with various photon energies, demonstrating that PtOx was located at the topmost surface. The red arrows indicate identical positions on the Pt surface. A complete oxidation process is shown in Supplementary Videos 1 and 2.
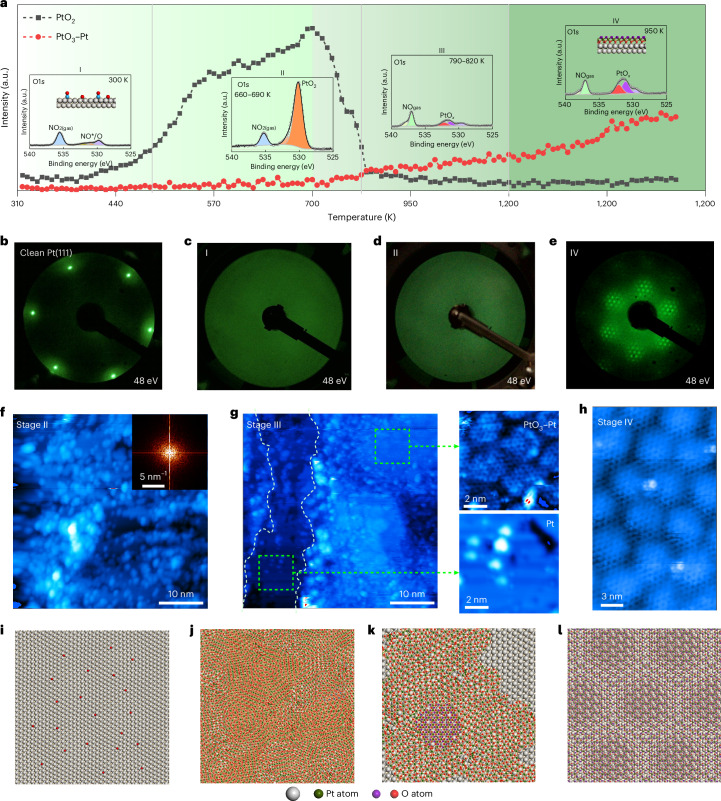
To identify these surface processes, the chemical state of the surface species was monitored by AP-XPS to fingerprint the composition and evolution behaviour of surface species. Figure 1c and Supplementary Video 2 show the evolution of O1s spectra gathered under NO2 conditions from 300 to 1,000 K, and the fitted spectra are shown in Supplementary Fig. 3. Initially, the surface was covered with adsorbed NO* (531.6 eV) and chemisorbed oxygen atoms (529.8 eV) from room temperature to 450 K (refs. 36,37). As the temperature increased, PtO2 gradually formed, as evidenced by the O1s peak (530.4 eV) and Pt4f5/2 peak (72.0 eV) (Supplementary Fig. 3 and Fig. 1c)16,36,38. Similar PtO2 formation processes have been observed under oxygen or ozone conditions12,13.
By further increasing to 1,000 K, an oxygen-containing species emerged, as depicted by the O1s peak at approximately 531.5 eV (Fig. 1c and Supplementary Fig. 3). The change in peak intensity (Fig. 1c) at higher temperatures could be attributed to the thermal expansion of the sample holder (Supplementary Note 3 and Supplementary Video 3). The depth profile shown in Fig. 1d indicates that the oxide species formed on this surface (Supplementary Note 4). In particular, the Pt(111) surface displayed neither N nor other contaminations (Supplementary Fig. 7). Hence, the surface species consists of Pt and O with a ratio of approximately 1 to 2.7 (Pt/O) (Supplementary Note 5). Thus, we temporarily assigned the surface thin film as PtOx. Considering that the experimental conditions between in situ SEM and AP-XPS were identical, we can correlate the evolution of the electronic structure of PtOx with the uniform secondary-electron contrast change of the Pt surface recognized by SEM observations.
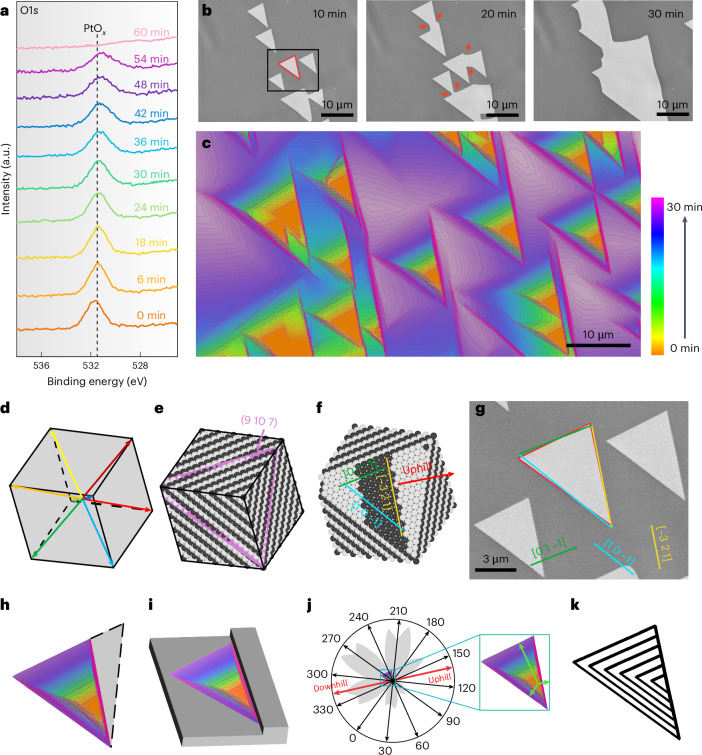
To further elucidate the chemical properties of PtOx, the evolution of the O1s spectra was monitored during reduction by simply switching from NO2 to hydrogen. The O1s spectra under 0.1 mbar H2 at 1,000 K show the reduction process of the PtOx film (Fig. 2a). The intensity of the O1s peak decreased slowly and required approximately 60 min to disappear entirely. The in situ SEM provides real-space details of the surface reduction processes. As shown in Fig. 2b, the area with a high grey level (high intensity) in the SEM image corresponds to the regions covered by PtOx, whereas the low-grey-level (low-intensity) areas represent the bare Pt surface (vacancy island). The formation of aligned vacancy islands with similar triangular shapes emerged and expanded over time during exposure to the H2 atmosphere. When neighbouring aligned vacancy islands met, the contact region exhibited a sharp concave corner and gradually became roundish with time (Fig. 2b, red arrows). The propagation of the roundish concave corners expanded rapidly and replaced the original smooth edges (Fig. 2c and Supplementary Video 4). Such an evolution behaviour is a signature of the inherent seamless coalescence behaviour of a 2D crystalline film27. This observation implies that the chemical configuration of PtOx reveals a highly 2D crystalline film, and provides an indirect indication that the PtOx overlayer could even be a single-crystalline film.
Fig. 2. H2 etching process of a PtOx layer.
a, Evolution of the O1s spectra of PtOx under 1 mbar H2 at 1,000 K; the spectra were collected under a photon energy of 1,486.6 eV. b, In situ SEM images recorded the etching behaviour of PtOx on a Pt(9 10 7) surface showing anisotropic evolution under 1 mbar H2 at 1,000 K (Supplementary Video 3). Note: the sequence of images shows the appearance of new edges at the concave corner during the coalescence of etching pits (highlighted by red arrows). c, Shape evolution of the etching pits during H2 etching, reproduced as the colour-coded superposition of outlines abstracted from images recorded at 53 s intervals. d, Orientation of the Pt substrate is determined by EBSD and presented in the Pt unit cell. The coloured arrows indicate the <111> directions for the unit cell. e, The corresponding ball model. f, Ball model of the unreconstructed (9 10 7) surface orientation of the underlying Pt grain. The yellow line highlights the surface step, with the uphill direction indicated by the red labelled arrow. g, Representative details of the vacancy islands (etching pits) in b. The shape of the vacancy islands almost perfectly overlaps the triangle surrounded by the stripes along [1 0 −1], [0 1 −1] and the line along the (9 10 7) surface step. h, Colour-coded shape evolution of an etching pit according to the growth time provided in the colour legend. The black dotted line indicates the missing half of the regular triangle shape. i, Schematic of the attachment of one of the vacancy island edges to the Pt step site. j, Shape evolution of the etching pits represented in polar coordinates shows anisotropic growth behaviour. Note: the initiation site of the etching pits is from the pole of the polar coordinates. k, Simulated kinetic Wulff construction of growth.
Additionally, we analysed the shape of the vacancy islands to validate this crystalline nature. Under the assumption that PtOx is a 2D crystalline film, the triangular shape of the vacancy island should be related to the crystallographic orientation of the Pt grain. The spatial and angular distributions of crystallographic orientations (9 10 7) are clearly visualized by electron backscatter diffraction (EBSD) observation in the Pt cubic cell (Fig. 2d,e). The corresponding surface atomic structure can be deduced39. The surface of Pt(9 10 7) exhibits a distinct terrace–step–kink structure in the ideal state (Fig. 2f). Because the surface step and kink play a key role in the nucleation of a 2D crystal and define the in-plane orientation of the overlayer40,41, the observed orientations of the vacancy islands should correlate with the atomic structure of the (9 10 7) surface. The shape of the vacancy island almost perfectly overlaps with the triangle surrounded by stripes along [1 0 –1], [0 1 –1] and the line along the (9 10 7) surface step (Fig. 2g), signifying that the PtOx film underwent epitaxial growth on the (9 10 7) surface42.
Moreover, the individual vacancy islands grew self-similarly in the shape of a truncated equilateral triangle (Fig. 2h,i), indicating that each vacancy island was co-oriented and similarly attached to a Pt surface step43. Thus, the substrate structure and associated barriers along and across the steps led to an in-plane anisotropic evolution (Fig. 2j). To rationalize the experimental observations, we performed kinetic Wulff construction simulations of the vacancy island shapes during etching based on the experimentally obtained anisotropic evolution rates. The relative expansion rates along different directions were obtained by measuring the distances from the position of the initial etching to the respective edge (Fig. 2j, green arrows). Only the expansion speed of the edges was utilized as the parameter for simulating anisotropic evolution. The resulting polar plots of the orientation-dependent expansion rates are exhibited in Fig. 2k. The striking consistency between the experimental observation and simulated evolution further validates our proposed model in which the PtOx overlayer is 2D single crystalline.
Characterization of the platinum oxide lattice constant
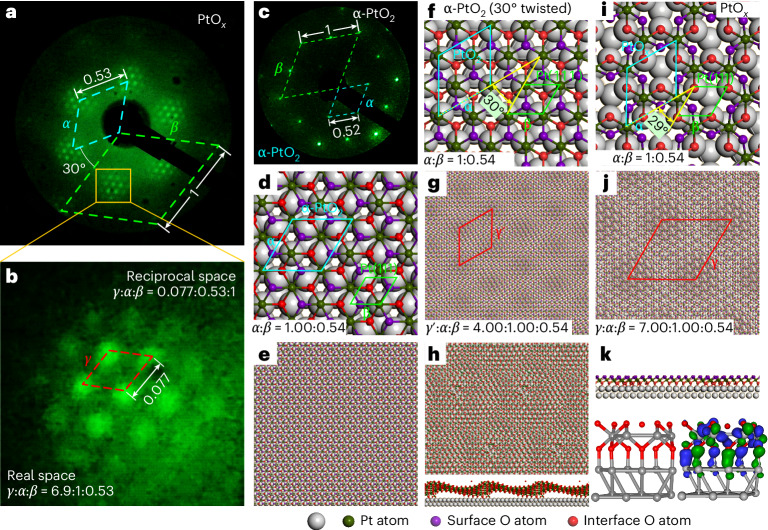
Thus far, by using spatially resolved and time-resolved observations, we have confirmed that the etching behaviours of PtOx share the characteristic features of a 2D crystalline material. To pinpoint the atomic structure and chemical configuration of the PtOx film, we now turn to reciprocal space by using low-energy electron diffraction (LEED) to predict the possible atomic structure of PtOx (refs. 44,45).
LEED measurements reveal a set of six outermost spots originating from the Pt(111) substrate (Fig. 3a, green dashed rhombus labelled β), and another set of six spots arising from the PtOx film (Fig. 3a, bright blue dashed rhombus labelled α). Additionally, the LEED observations across diverse energy ranges and spatial extents confirm that the as-grown PtOx exhibits high crystallinity, manifesting as a large-area, 2D single-crystalline film (Supplementary Figs. 8 and 9 and Supplementary Video 5). There is a discernible relative rotation angle of 30° between the PtOx film and the underlying Pt(111) substrate (Supplementary Fig. 10). The moiré superlattice formed between PtOx and Pt(111) appears as a series of satellite points surrounding six PtOx spots (Fig. 3a), which are rotated by 30° with respect to the Pt(111) lattice (Fig. 3b (red dashed rhombus) and Supplementary Fig. 10). The single set of LEED points for both PtOx and the corresponding moiré pattern indicate strong interactions between the PtOx film and the substrate27,46. The LEED patterns reveal that the crystal lattice ratio of the PtOx–Pt(111) system in reciprocal space is α:β:γ = 0.53:1:0.077, corresponding to a proportional lattice ratio of α:β:γ = 1:0.53:6.9 in real space. By utilizing the known crystal lattice constant of Pt(111), we can determine that the surface lattice for PtOx is α = 0.53 nm, and the moiré period for PtOx on Pt(111) in real space is γ = 3.65 nm. Surprisingly, the lattice constant of PtOx closely resembles that of α-PtO2 (Fig. 3c–e), which aligns with the Pt(111) surface without rotation47. We, thus, attempt to construct an atomic model for PtOx by directly rotating α-PtO2 by 30° on Pt(111) (Fig. 3f). This rotation operation results in a moiré superstructure rotated by 30° with respect to Pt(111). In particular, this moiré superstructure measures 2.07 nm (Fig. 3g), which does not match our LEED observations. Furthermore, the MD simulations demonstrate that the surface atomic arrangement of a 30°-rotated α-PtO2 is thermodynamically unstable and prone to corrugation due to in-plane stress; such a surface arrangement cannot exist specially at relevant high temperatures (Fig. 3h, Supplementary Fig. 11 and Supplementary Videos 6 and 7).
Fig. 3. Characterization of the PtOx layer.
a, LEED pattern of the surface covered with a PtOx layer. b, Enlarged image of the region in the yellow box in a. c, LEED pattern of the α-PtO2 overlayer on the Pt(111) surface. d,e, Schematic of the models for the α-PtO2 overlayer on Pt(111). f,g, Atomic model of the predicted structure of the PtOx film (30°-rotated α-PtO2). h, MD simulation of the stability of the rotated α-PtO2. i, Atomic structure after removing a Pt atom from the 30°-rotated α-PtO2. j, Formation of moiré pattern after structural relaxation. k, Cross-sectional view of the relaxed structure in j, depicting the interfacial bonding interactions between Pt and O.
To satisfy the diffraction pattern in reciprocal space and thermodynamic stability, reconstructing the surface atomic arrangement by periodically removing a Pt atom from the 30°-rotated α-PtO2 is necessary (Fig. 3i). The resulting reconstructed structure exhibits similarities to layered CrI3 (ref. 48). This approach allows for the preservation of the lattice constant of α-PtO2 and achieve perfect matching with LEED observations in terms of in-plane rotation and moiré superstructure on Pt(111) (Fig. 3b,j). MD simulations indicate that this structural configuration helps release the in-plane stress and flatten the oxide overlayer (Fig. 3k, Supplementary Figs. 12 and 13 and Supplementary Videos 8 and 9)49. In particular, within each vacancy created by removing a Pt atom, lower-layer O atoms form strong bonds with metallic Pt atoms located above them (Supplementary Note 6). We, thus, revise the chemical formula of PtOx to be PtO3–Pt. This Pt oxide exhibits a honeycomb lattice configuration, where Pt atoms are sandwiched between dual layers of O atoms, resulting in a distinctive six-pointed star structure when viewed from out of plane.
Imaging of the Pt oxide structure in real space
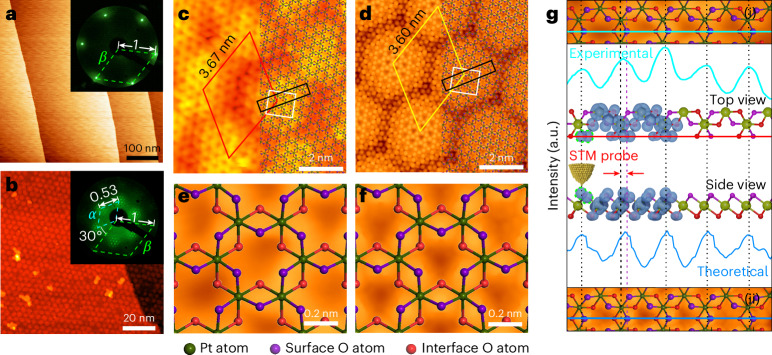
To corroborate the geometric inferences drawn from the LEED observations in reciprocal space, we undertook STM measurements to directly visualize the structure of PtO3–Pt in the real-space atomic level.
Figure 4a displays the STM topography of the pristine Pt(111) surface with large and flat terraces, showing the excellent quality of the substrate. On oxidation under 1 mbar NO2 at 1,000 K, our STM topography reveals that the PtO3–Pt film is fully covered on the Pt(111) surface (Fig. 4b), where remarkable hexagonal moiré patterns are observed. Both the surface structure of PtO3–Pt with a lattice constant of 0.54 nm and the periodicity of the moiré patterns of 3.67 nm relative to Pt(111) are measured from the STM atomic-resolution images (Fig. 4c (rhombus) and Supplementary Fig. 14). Our STM results are close to our LEED observations (Fig. 4a,b (insets) and Fig. 3a,b), which reveal a lattice constant of 0.53 nm for PtO3–Pt and a period of 3.54 nm for the moiré superstructure (Supplementary Fig. 14).
Fig. 4. Real-space image of the PtO3–Pt structure.
a,b, STM images of the clean Pt(111) surface (a) and the fully covered PtO3–Pt layer (b). The insets in a and b are the corresponding LEED patterns. c, Atomic-resolution STM images of the PtO3–Pt layer. d, Simulated STM image of the PtO3–Pt layer based on the structure shown in Fig. 3j. e,f, Enlarged image of the white box in c (e) and d (f). g, The top and bottom panels show the enlarged image of the black box in c and d, respectively; the line profile is a comparison between the experimental (along the green line in g(i)) and theoretical (along the blue line in g(ii)) results; the middle panel is the electron density isosurface map from the top and side views. The LEED patterns were collected under the same energy (48 eV) and followed the same direction. Size and tunnelling parameters: (a) 500 nm × 500 nm, Vs = 50 mV, I = 1 nA; (b) 100 nm × 100 nm, Vs = 1.74 V, I = 0.07 nA; (c) 9.5 nm × 9.5 nm, Vs = 1.06 V, I = 0.07 nA.
Furthermore, our STM topography is fully consistent with the purposed six-pointed star structure of PtO3–Pt mentioned above. The dark spots in the topographic image (Fig. 4c,e) result from the absence of Pt atoms on the surface, and then we can directly superimpose the purposed structure of PtO3–Pt onto our STM image (Fig. 4c, right). Figure 4e shows the cropped image of the area highlighted by the white square in Fig. 4c. Both unit cells of the dark spots corresponding to the absence of a Pt atom and the period of the moiré patterns fit well with our purposed structure of PtO3–Pt and the corresponding theoretical simulation results (Fig. 4d,f).
Besides, the brightest spots are not directly above the surface O in both experimental and simulated STM images (Fig. 3g,j), suggesting a position offset of surface O, which can be understood by analysing the projected density of states from the side view (Fig. 4e). Figure 4e illustrates the schematic of the models and the corresponding electron density isosurfaces of the p orbitals of surface O, which exhibit a fusiform shape when viewed from the side (Fig. 4e, green line). The relative position of the scanning tip and its interaction with the isosurface along the scanning line demonstrates that the region with the highest electron density is not directly above the surface O atom, consistent with our experimental observations (Supplementary Videos 10 and 11). Consequently, by the combination of experiments and theoretical simulations, we can clearly demonstrate that the spatial distribution of the p orbitals of surface O results from the observed positional offset of surface O atoms in the STM image (Fig. 4e, red arrows).
The core-level shift of the O1s spectra for the PtO3–Pt structure was also calculated (Supplementary Fig. 15). The result shows that the core-level shift of upper-layer O is lowered by about 1.01 eV compared with the lower-layer O, which is close to the experimental value of 1.29 eV. Furthermore, the number of the two forms of O atoms is the same with those in the atomic model, suggesting that the peak area of these two O1s peaks would be nearly equal. This is also consistent with the experimental results, showing that the peak area ratio of Oint./Osurf. is close to 1. For the valence state of Pt oxide, we calculated the Bader charges of PtO3–Pt and bulk α-PtO2 systems. The results show that the valence state of Pt in PtO3(–Pt) should be slightly higher than +4 (Supplementary Note 7).
Until now, we have elucidated the structure of 2D layered PtO3–Pt and gained insights into the influence of the interaction between the six-pointed star overlayer and the substrate on this structure. To further investigate the spatial structure of PtO3–Pt, we used in situ scanning transmission electron microscopy (STEM) for operando cross-sectional imaging, capturing the growth process of PtO3–Pt in a NO2 atmosphere.
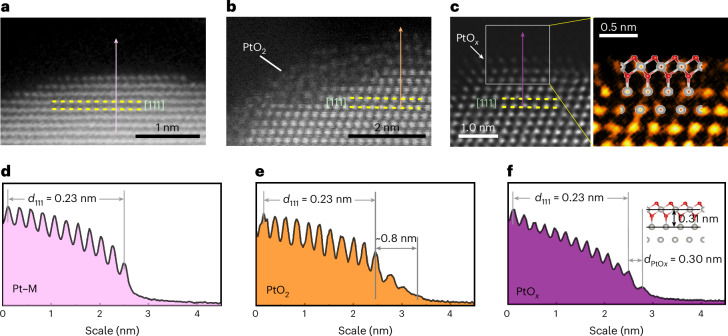
Figure 5a–c illustrates the evolution behaviour of surface Pt oxides on the (111) plane from an out-of-plane perspective under 300, 620 and 1,000 K, respectively. As a reference, a high-angle annular dark-field STEM image of a Pt nanoparticle obtained at 300 K is demonstrated in the cleaned Pt(111) plane (Fig. 5a). The interspacing of the (111) plane is 0.23 nm, which remains uniform across the gas–solid interface (Fig. 5d). Increasing the temperature led to the distinct oxidation states of the Pt particle. Within the temperature range of 500–700 K, a disordered oxide with a thickness of several atomic layers is developed on Pt (Fig. 5b), and this structure is assigned to α-PtO2 (refs. 50–52). The profile of the contrast plot along the cross section shows that the thickness of α-PtO2 is ~0.8 nm (Fig. 5e). With additional annealing up to 1,000 K, the gas–solid interface underwent a transformation, resulting in a monolayer oxide (Fig. 5c and Supplementary Fig. 17). This oxide layer is positioned 0.30 nm away from the (111) plane (Fig. 5f). The electron energy loss spectroscopy (EELS) analysis (Supplementary Fig. 18) confirms the formation of an oxide layer on the Pt surface. An atomically resolved monolayer oxide together with the atomic model structure of PtO3–Pt is proposed from previous STM observations (Fig. 5c). The in situ STEM imaging of Pt(111) oxidation processes under NO2 reveals the disordered α-PtO2 layer in the transition state during PtO3–Pt formation.
Fig. 5. Direct observation of Pt oxidation by in situ STEM.
a–c, STEM images of Pt nanoparticles under NO2 at 300 K (a), 620 K (b) and 1,000 K (c). The right panel in c is a magnified image of the grey-box area (c, left) together with a superimposed PtO3–Pt model. d–f, Intensity profiles of the arrow-marked regions in a (d), b (e) and c (f).
Mechanism of PtO3–Pt formation
To further understand the layered PtO3–Pt formation, we compare the in situ LEED, STEM and AP-XPS observations across the entire oxidation process. The in situ LEED observations of the Pt(111) oxidation process under conditions identical to the above STEM experiments are shown in Fig. 6c–e, starting from the clean Pt(111) surface (Fig. 6b). The LEED patterns of the Pt(111) substrate gradually become blurred and diffused with increasing temperature (Fig. 6c,d). This observation implies that the surface structure became disordered, consistent with the previous in situ STEM measurement (Fig. 5b). With further annealing to 1,000 K, the disordered surface converted to a well-ordered structure (Fig. 6e), agreeing with the in situ STEM visualizations (Fig. 5c). The corresponding evolution of the O1s spectra at different oxidation stages is presented in Fig. 6a (insets). Therefore, the in situ AP-XPS experiments prove that the comparison between in situ STEM/LEED and in situ environmental scanning electron microscopy (ESEM) is legitimate. In particular, the evolution of the O1s spectra indicates a rapid disappearance of α-PtO2 at the inception of PtO3–Pt growth. Nonetheless, at this juncture, we remain limited in our ability to elucidate the detailed transition from α-PtO2 to PtO3–Pt on an atomic scale, which constrains our comprehensive understanding of the formation processes. Therefore, we used quasi-in situ STM to capture the atomic structure of the surface at various oxidation stages, guided by the AP-XPS findings (see the ‘Quasi-in situ STM’ section). The quasi-in situ STM observations reveal that at the stage of α-PtO2 formation, the surface displays a disordered structure (Fig. 6f). This observation aligns well with the in situ STEM data (Fig. 5b).
Fig. 6. Evolution process from Pt to PtO3–Pt.
a, O1s peak area evolution of PtO2 and PtO3–Pt in Fig. 1c as a function of temperature. The insets show the O1s spectra under different stages. b, LEED pattern of the clean Pt(111) surface. c–e, Corresponding LEED patterns at stage I (c), stage II (d) and stage IV (e). f,g, STM images collected at stage II (f) and stage III (g) in a. i–l, Atomic models during the oxidation process. Tunnelling parameters: (f) Vs = 500 mV, I = 0.14 nA; (g) Vs = 1.5 V, I = 0.21 nA; g(i) Vs = 1.5 V, I = 0.21 nA; g(ii) Vs = 1.5 V, I = 0.21 nA; (h) Vs = 2.6 V, I = 0.18 nA.
As the temperature went to 700 K, α-PtO2 began to desorb from the Pt(111) surface, leading to the appearance of the O1s spectra of PtO3–Pt (Fig. 6a, stage III). Subsequent quasi-in situ STM observations revealed a surface with intricate topographic features (Fig. 6g), characterized by the coexistence of flat areas and undulating regions attributed to α-PtO2. On detailed examination, these flat areas were categorized into two distinct types: plain and plateau. High-resolution STM measurements confirmed that the plain areas represent the unaltered Pt(111) surface (Fig. 6g), whereas the plateau regions correspond to areas covered by PtO3–Pt, displaying the characteristic moiré corrugation due to the overlayer–Pt(111) interaction. The simultaneous presence of α-PtO2 and PtO3–Pt suggests that PtO3–Pt evolves from α-PtO2 (the atomic model of the PtO3–Pt evolution process is shown in Fig. 6i–l), rather than forming directly on the pristine Pt(111) surface.
Thermodynamic stability of PtO3–Pt
To understand the thermodynamic stability of PtO3–Pt on the Pt(111) substrate, the free energy changes (ΔG) associated with oxidizing the Pt(111) surface by O2 or NO2 to form either a single-layer PtO3/Pt or α-PtO2/Pt system were further investigated by DFT calculations. The results shown in Supplementary Fig. 19 suggest that under the investigated conditions of temperature from 300 to 1,300 K and 1 mbar O2 or NO2, the ΔG value of all the oxidation processes is always negative. This indicates that all the oxidation processes are thermodynamically favourable.
The formation of 2D PtO3–Pt (PtOx) structures under O2 conditions requires relatively elevated temperatures, as demonstrated in Supplementary Note 8. To assess the stability of PtO3–Pt formed in O2 and NO2 environments, we pumped out the oxygen as the sample was held at 1,300 K. After the sample was cooled under UHV, the X-ray photoelectron spectroscopy (XPS) spectra and LEED patterns were obtained to identify the PtO3–Pt species (Supplementary Fig. 21). Comparing them with those formed under NO2 conditions, we conclude that the 2D structure of PtO3–Pt is stable under both O2 and NO2 conditions, even in UHV, at elevated temperatures.
The transition from α-PtO2 to PtO3–Pt on heating can be ascribed to the weaker affinity of α-PtO2 for the Pt substrate than PtO3–Pt (Supplementary Note 9). Due to the instability of α-PtO2 oxides, which readily sublimate from the Pt surface35,53, metallic Pt typically exhibits linear rates of weight loss in oxidizing atmospheres, forming volatile oxides at high temperatures (Supplementary Fig. 22). However, our findings reveal that the six-pointed star structure of Pt oxide exhibits exceptional thermodynamic stability, enduring even at temperatures as high as 1,200 K. Consequently, this 2D crystalline PtO3–Pt serves as a protective layer, which was very similar to the 2D rafts of PdOx (refs. 54,55), effectively shielding the substrate from continuous oxidation and sublimation (Supplementary Fig. 22).
Conclusion
In summary, our comprehensive study of Pt(111) oxidation under NO2 conditions, covering temperatures from room temperature to 1,000 K, reveals that the Pt(111) surface was initially covered with adsorbed oxygen and NO* species. As the temperature increased, an amorphous α-PtO2 layer formed on the surface. However, this layer became unstable above 750 K and changed into an oxide phase—PtO3–Pt. PtO3–Pt demonstrates exceptional thermal stability and endurance under high-temperature conditions.
Structural analyses and theoretical studies revealed the unique six-pointed star structure of PtO3–Pt, critical in minimizing in-plane stress and promoting unidirectional domain nucleation. This leads to the epitaxial growth of large-area, single-crystal PtO3–Pt. Theoretical models were crucial in understanding the key spectroscopic aspects of 2D PtO3–Pt formation and confirming the structure and stability of this oxide. Additionally, this Pt oxide displays a distinctive surface electronic structure, which can selectively oxidize CO in mixed hydrocarbon, oxygen and CO environments (Supplementary Note 10). Moreover, the remarkable stability of PtO3–Pt suggests that under high-temperature oxidizing conditions, the active phase of Pt is a 2D layered Pt oxide.
Our findings reveal that 2D PtO3–Pt plays a crucial role not just as a transient phase but as a stable, active entity even above 700 K, which could revolutionize the design and application of Pt-based catalysts. The identification of a stable Pt oxide phase at these temperatures provides a perspective on catalyst behaviour under operating conditions typically considered detrimental to oxide stability. This discovery has profound implications for catalytic science, potentially leading to the development of more effective catalysts that leverage the unique properties of Pt oxides.
This research highlights the importance of using multiple in situ methods to get a comprehensive view of surface reactions from the atomic level to the millimetre scale, and bridges the gap between UHV techniques and real-world industrial conditions. Our methods and results not only deepen our understanding of surface oxidation but also open up new possibilities for designing high-temperature industrial catalysts.
Methods
The Pt(111) single crystal was purchased from MaTecK. The gas lines for NO2 were baked at 120 °C for 24 h and flushed several times using high-purity NO2 (99.995%) before introducing NO2 into the chamber to minimize contamination.
In situ SEM
The in situ observational experiments were conducted within the confines of a chamber in a specially modified commercial ESEM instrument (Thermo Fisher Quattro S). The vacuum system of the ESEM instrument utilized oil-free pre-vacuum pumps. A custom-made laser-heating stage was incorporated into the instrument, in addition to a gas supply module facilitated by mass flow controllers from Bronkhorst, and a mass spectrometer (Pfeiffer HiQuad) was used for atmospheric analysis within the chamber. The ESEM chamber was subjected to a thorough cleaning process using plasma before the experiments. Temperature was determined using a K-type thermocouple, which was meticulously spot-welded onto the substrate. The microscope operation was maintained at an acceleration voltage ranging from 2 kV. A large field detector was utilized for image capture. Throughout the experiments, no influence of the electron beam on the oxidation process was detected.
In situ XPS
In situ XPS measurements were performed utilizing the advanced synchrotron-based AP-XPS system located at beamline 02B01 of the Shanghai Synchrotron Radiation Facility. Additionally, a complementary laboratory-based SPECS AP-XPS system was used. Both systems were outfitted with high-transmission analysers, integrated with a preparation chamber and a µ-metal main chamber maintained at a base pressure of 1 × 10−9 mbar. The synchrotron-based AP-XPS system is capable of achieving an energy resolution of up to 13,000, with a focused beam spot size of approximately 460 µm (horizontal) × 15 µm (vertical). The laboratory-based AP-XPS system features a spot size of roughly 300 µm in diameter. Sample temperatures were precisely controlled within the range of 300–1,000 K using an infrared laser-heating unit. A mass spectrometer, connected to the first differential pumping stage, was used for the real-time monitoring of gas-phase components within the analysis chamber.
To mitigate issues related to carbon segregation from the bulk, the Pt(111) single crystal was subjected to an iterative cleaning procedure involving Ar+ sputtering (15 min, 1 keV) followed by annealing at 700 °C for 10 min. This process effectively removed the dissolved carbon and other surface contaminants, resulting in a pristine Pt(111) surface.
Unless specified otherwise, spectral analysis was conducted using CasaXPS software (Version 2.3.25PR1.0) with a Shirley-type background correction (10.1016/j.apsadv.2021.100112). The O1s spectra were fitted with a GL(30) line shape, representing a mixture of 70% Gaussian and 30% Lorentzian components, and the asymmetric Pt4f spectra were fitted using a DS(0.02,100)GL(30) line shape, which combines Doniach–Sunjic asymmetry with a Gaussian−Lorentzian (product) function.
EBSD
EBSD patterns were obtained using an EDAX Velocity Super EBSD detector on a Thermo Fisher ApreoSEM instrument. The analysis, encompassing phase identification and orientation-map generation, was performed using EDAX’s OIM Analysis 8 software (Version: 8.6 108 x64).
Quasi-in situ STM
Quasi-in situ STM experiments were conducted using SPECS JT-STM, a commercial STM system. For these experiments, the sample was transported to the STM setup via a UHV transfer suitcase. After loading into the STM chamber, the sample was transferred to a cryogenic stage and maintained at 5 K to ensure optimal imaging conditions. The commercial Pt–Ir tip, utilized for both imaging and tunnelling, had been previously calibrated on silver islands formed on p-type Si(111) surfaces with a 7 × 7 reconstruction.
In situ TEM
Pt particles (ADAMAS-BETA) were dispersed in pure ethanol. The mixture was then drop-cast onto a commercial microelectromechanical system chip (Protochips) followed by drying in air. A closed cell with electron-transparent SiNx window and heating device were assembled on an in situ transmission electron microscopy (TEM) holder. An electrical current was applied through the gold contacts to the SiC heating membrane. Using an external gas-feeding system, the in situ observations were conducted under the flowing-gas mode. An integrated residual gas analyser was used to verify the gas mixture applied to the cell. The observation was conducted in an aberration-corrected JEM Grand ARM 300F TEM instrument (JEOL) operated in the scanning mode (STEM) at 300 kV. The microscope is equipped with high-angle annular dark-field STEM detector. In situ observations of Pt oxidation were made at various temperatures from 300 to 1,000 K in a flowing NO2 gas environment (0.1 bar, 0.12 s.c.c.m.). Due to the large temperature span for investigation, a single-particle observation was impossible as a result of sample rotation and the tilt-off of the appropriate zone axis. Therefore, multilocation assessments were applied to obtain the surface-layer structure with Pt-[111] atomic arrangement.
In high-angle annular dark-field STEM imaging mode, the electron signal is mainly derived from the Rutherford scattering of incoherent electrons. Due to this, it is not expected that any phase contrast from the edge of the particle, as well as the atomic arrangements of Pt, can be well interpreted by the high-angle annular dark-field intensity distribution. Additionally, the mass-thickness contrast (particularly Z contrast) is promoted for Pt through the avoidance of the O-lattice Bragg scattering at low angles. In particular, the O intensity is suppressed (therefore, no O atom can be ‘seen’) in this mode to facilitate a clear interpretation of atomic locations for Pt. On the basis of this, we carefully examine the atomic location for the Pt overlayer with respect to the bulk Pt atoms (Fig. 5c). The imaged atomic position demonstrates reasonable agreement with the simulated result (Fig. 5c, inset), despite a slightly compromised resolution at elevated temperature (1,000 K). A spatial filter of 5 × 5 convolution is applied to the original image to enhance the contrast of Pt atoms (both images are shown in Supplementary Fig. 17).
EELS
Environmental STEM analysis of the Pt(111) surface was conducted using Hitachi HF5000, an aberration-corrected microscope, operated at 200 kV. To prepare a clean Pt(111) surface, Pt particles were dispersed on a microelectromechanical system chip and subsequently treated in 1 Pa H2 at temperatures above 600 °C for 3 h. The temperature was then increased to 700 °C, and NO2 was introduced at a flow rate of 1.5 ml min–1 for a total of 75 ml for subsequent analyses. Annular dark-field imaging was carried out under standard dose rates, and no beam-induced damage was detected. Elemental analysis of the Pt(111) surface was performed using EELS line scan in HF5000, equipped with a Gatan 965 EELS spectrometer. The EELS spectra were collected in the range of 300–812 eV, with a dispersion setting of 0.25 eV per channel.
Calculation details
DFT calculation
DFT calculations were performed using the Vienna ab initio simulation package code56,57. The Perdew–Burke–Ernzerhof exchange–correlation functional was used to set the plane-wave basis, and the cutoff energy was set to 380 eV (ref. 58). The Brillouin zone was sampled using a Monkhorst−Pack 1 × 1 × 1 k-point grid, and the wavefunction was converged to 1 × 10–4 eV. The DFT-D3 method of Grimme was applied to account for the van der Waals interactions59. Structure optimizations were performed with on-the-fly machine learning force field implemented using the Vienna ab initio simulation package software, which has been shown to accurately predict the DFT data60.
MD simulation
MD simulations were carried out in LAMMPS package with LASP machine learning force field in a constant volume and constant temperature (NVT) system61,62. The MD simulations were performed for 10 ps with a time step of 1 fs. The Pt substrate was fixed, and PtOx was allowed to move during structure optimizations and MD simulations.
XPS/STM simulation and molecular orbital analysis
The STM image of the surfaces was calculated and analysed in the Vienna ab initio simulation package (https://www.vasp.at/wiki/index.php/STM_of_graphene).
The XPS simulations were performed by calculating the core-level binding energy changes with the final-state approximation. In this approximation, the core-level binding energies were calculated following the procedure described elsewhere63,64.
The wavefunction plots were drawn with the help of the VASPMO (https://sourceforge.net/projects/vaspmo/) and Molekel (http://ugovaretto.github.io/molekel/) programs.
A simplified system was modelled in the XPS/STM simulation and molecular orbital analysis. The system was modelled using a Pt substrate on a periodic 2 × 3 Pt(111) slab with two atomic layers. Then, a PtO3–Pt layer with 4 Pt and 12 O atoms were placed in the vacuum layer above the Pt substrate. The lattice of the system was 8.316 × 4.8 × 30 Å3, and the Brillouin zone was sampled using a Monkhorst−Pack 2 × 4 × 1 k-point grid. Structure optimization was performed before the XPS/STM simulation and molecular orbital analysis.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41563-024-02002-y.
Supplementary information
Supplementary Figs. 1–25 and Notes 1–10.
In situ SEM observation of the oxidation process.
In situ XPS study of the oxidation process.
Thermal expansion of the sample holder in the AP-XPS experiment.
In situ SEM observation of the H2 etching process.
LEED with increasing electron energy.
Simulation of α-PtO2 decomposition.
Simulation of the 30°-rotated α-PtO2 decomposition.
PtOx stability simulation.
Structure evolution from PtO2 to PtOx.
STM simulation.
Simulation of position gap between STM and atom model.
Source data
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Acknowledgements
This work was mainly supported by the National Natural Science Foundation of China under grant no. 12027804. Zhi Liu acknowledges the Science and Technology Commission of Shanghai Municipality (no. 22560780300). This work was supported by the National Key R&D Program of China (no. 2022YFA1503802) and the National Natural Science Foundation of China (nos. 21991152, 22002090, 21902179 and 52025023). J.C. and Z.-J.W. thank BL02B01 of the Shanghai Synchrotron Radiation Facility and SPECS AP-XPS instrument supported by the National Natural Science Foundation of China (no. 11227902). J.C. and Z.-J.W. acknowledge X. Kong from the Shanghai Institute of Microsystem and Information Technology for the theoretical simulation. B.Y. acknowledges funding support from the National Natural Science Foundation of China (22322302). We also thank the HPC Platform of ShanghaiTech University for computing time. We acknowledge the Center for High-Resolution Electron Microscopy of ShanghaiTech University for use of the electron microscope. K.L. acknowledges Guangdong Major Project of Basic and Applied Basic Research (2021B0301030002).
Author contributions
Z.-J.W. conceived this project and supervised the research. In situ SEM and EBSD experiments and analysis were conducted by Z.-J.W., J.C., C.X. and M.-G.W. AP-XPS and LEED experiments and analysis were performed by J.C., Y.Z., H.Z., Z.-J.W. and Zhi Liu. Quasi-in situ STM measurements and analysis were performed by L.W., J.C., M.W., Z.-J.W. and Zhongkai Liu. The in situ TEM, high-angle annular dark-field STEM and EELS measurements were performed by Z.C., Y.H., J.C., T.Q., L.C. and X.L. The theoretical simulations and implementation of the obtained results were carried out by J.L., W.S., B.Y., K.L. and P.H. Important contributions to the interpretation of the results, conception and writing of the paper were made by J.C. and Z.-J.W. All authors participated in the scientific discussion.
Peer review
Peer review information
Nature Materials thanks Matteo Cargnello, Abhaya Datye and Jakob Wagner for their contribution to the peer review of this work.
Data availability
The data that support the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Jun Cai, Liyang Wei, Jian Liu, Chaowu Xue.
Contributor Information
Bo Yang, Email: yangbo1@shanghaitech.edu.cn.
Zhongkai Liu, Email: liuzhk@shanghaitech.edu.cn.
Zhi Liu, Email: liuzhi@shanghaitech.edu.cn.
Zhu-Jun Wang, Email: wangzhj3@shanghaitech.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41563-024-02002-y.
References
- 1.Tian, N., Zhou, Z.-Y., Sun, S.-G., Ding, Y. & Wang, Z. L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science316, 732–735 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Kašpar, J., Fornasiero, P. & Hickey, N. Automotive catalytic converters: current status and some perspectives. Catal. Today77, 419–449 (2003). [Google Scholar]
- 3.Chmielarz, L. & Jabłońska, M. Advances in selective catalytic oxidation of ammonia to dinitrogen: a review. RSC Adv.5, 43408–43431 (2015). [Google Scholar]
- 4.Park, S., Vohs, J. M. & Gorte, R. J. Direct oxidation of hydrocarbons in a solid-oxide fuel cell. Nature404, 265–267 (2000). [DOI] [PubMed] [Google Scholar]
- 5.Jones, J. et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping. Science353, 150–154 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Liu, J. et al. High performance platinum single atom electrocatalyst for oxygen reduction reaction. Nat. Commun.8, 15938 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mom, R. et al. The oxidation of platinum under wet conditions observed by electrochemical X-ray photoelectron spectroscopy. J. Am. Chem. Soc.141, 6537–6544 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann, M. D. et al. Structure and reactivity of surface oxides on Pt(110) during catalytic CO oxidation. Phys. Rev. Lett.95, 255505 (2005). [DOI] [PubMed] [Google Scholar]
- 9.Beniya, A. et al. CO oxidation activity of non-reducible oxide-supported mass-selected few-atom Pt single-clusters. Nat. Commun.11, 1888 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, J. et al. Diversity of platinum-sites at platinum/fullerene interface accelerates alkaline hydrogen evolution. Nat. Commun.14, 1711 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu, F.-Y. et al. Pt-O bond as an active site superior to Pt0 in hydrogen evolution reaction. Nat. Commun.11, 490 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmerón, M., Brewer, L. & Somorjai, G. A. The structure and stability of surface platinum oxide and of oxides of other noble metals. Surf. Sci.112, 207–228 (1981). [Google Scholar]
- 13.Saliba, N. A., Tsai, Y. L., Panja, C. & Koel, B. E. Oxidation of Pt(111) by ozone (O3) under UHV conditions. Surf. Sci.419, 79–88 (1999). [Google Scholar]
- 14.Helveg, S. et al. Role of surface elastic relaxations in an O-induced nanopattern on Pt(110)–(1 × 2). Phys. Rev. Lett.98, 115501 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Devarajan, S. P., Hinojosa, J. A. & Weaver, J. F. STM study of high-coverage structures of atomic oxygen on Pt(111): p(2 × 1) and Pt oxide chain structures. Surf. Sci.602, 3116–3124 (2008). [Google Scholar]
- 16.Fantauzzi, D. et al. Growth of stable surface oxides on Pt(111) at near‐ambient pressures. Angew. Chem. Int. Ed.56, 2594–2598 (2017). [DOI] [PubMed] [Google Scholar]
- 17.Van Spronsen, M. A., Frenken, J. W. M. & Groot, I. M. N. Observing the oxidation of platinum. Nat. Commun.8, 429 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salanov, A. N. et al. Oxidation and recrystallization of platinum group metals (Pt, Pd, Rh) in oxygen. Surface and subsurface reconstruction of polycrystalline platinum during annealing in the O2 atmosphere over the temperature range of 600–1400 K. Appl. Surf. Sci.490, 188–203 (2019). [Google Scholar]
- 19.Steininger, H., Lehwald, S. & Ibach, H. Adsorption of oxygen on Pt(111). Surf. Sci.123, 1–17 (1982). [Google Scholar]
- 20.Weaver, J. F., Chen, J.-J. & Gerrard, A. L. Oxidation of Pt(111) by gas-phase oxygen atoms. Surf. Sci.592, 83–103 (2005). [Google Scholar]
- 21.Fryburg, G. C. Enhanced oxidation of platinum in activated oxygen. J. Chem. Phys.24, 175–180 (1956). [Google Scholar]
- 22.Krier, C. A. & Jaffee, R. I. Oxidation of the platinum-group metals. J. Less-Common Met.5, 411–431 (1963). [Google Scholar]
- 23.Ertl, G. Oscillatory kinetics and spatio-temporal self-organization in reactions at solid surfaces. Science254, 1750–1755 (1991). [DOI] [PubMed] [Google Scholar]
- 24.Cirak, F. et al. Oscillatory thermomechanical instability of an ultrathin catalyst. Science300, 1932–1936 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Aitbekova, A. et al. Templated encapsulation of platinum-based catalysts promotes high-temperature stability to 1,100 °C. Nat. Mater.21, 1290–1297 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Tao, F. et al. Break-up of stepped platinum catalyst surfaces by high CO coverage. Science327, 850–853 (2010). [DOI] [PubMed] [Google Scholar]
- 27.Wang, Z.-J. et al. The coalescence behavior of two-dimensional materials revealed by multiscale in situ imaging during chemical vapor deposition growth. ACS Nano14, 1902–1918 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Hansen, P. L. et al. Atom-resolved imaging of dynamic shape changes in supported copper nanocrystals. Science295, 2053–2055 (2002). [DOI] [PubMed] [Google Scholar]
- 29.Cai, J. et al. Formation of different Rh–O species on Rh(110) and their reaction with CO. ACS Catal. 13, 11–18 (2023).
- 30.Wang, Z.-J. et al. Conversion of chirality to twisting via sequential one-dimensional and two-dimensional growth of graphene spirals. Nat. Mater.23, 331–338 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Salmeron, M. & Schlögl, R. Ambient pressure photoelectron spectroscopy: a new tool for surface science and nanotechnology. Surf. Sci. Rep.63, 169–199 (2008). [Google Scholar]
- 32.Starr, D., Liu, Z., Hävecker, M., Knop-Gericke, A. & Bluhm, H. Investigation of solid/vapor interfaces using ambient pressure X-ray photoelectron spectroscopy. Chem. Soc. Rev.42, 5833–5857 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Cai, J. et al. An APXPS endstation for gas–solid and liquid–solid interface studies at SSRF. Nucl. Sci. Tech.30, 81 (2019). [Google Scholar]
- 34.Chaston, J. C. Reactions of oxygen with the platinum metals. Platin. Met. Rev.9, 51–56 (1965). [Google Scholar]
- 35.Plessow, P. N. & Abild-Pedersen, F. Sintering of Pt nanoparticles via volatile PtO2: simulation and comparison with experiments. ACS Catal.6, 7098–7108 (2016). [Google Scholar]
- 36.Parkinson, C. R., Walker, M. & Mcconville, C. F. Reaction of atomic oxygen with a Pt(111) surface: chemical and structural determination using XPS, CAICISS and LEED. Surf. Sci.545, 19–33 (2003). [Google Scholar]
- 37.Zhu, J. F., Kinne, M., Fuhrmann, T., Denecke, R. & Steinrück, H. P. In situ high-resolution XPS studies on adsorption of NO on Pt(111). Surf. Sci.529, 384–396 (2003). [Google Scholar]
- 38.Miller, D. J. et al. Oxidation of Pt(111) under near-ambient conditions. Phys. Rev. Lett.107, 195502 (2011). [DOI] [PubMed] [Google Scholar]
- 39.Li, X. et al. Single-crystal two-dimensional material epitaxy on tailored non-single-crystal substrates. Nat. Commun.13, 1773 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sutter, P. How silicon leaves the scene. Nat. Mater.8, 171–172 (2009). [DOI] [PubMed] [Google Scholar]
- 41.Wang, L. et al. Epitaxial growth of a 100-square-centimetre single-crystal hexagonal boron nitride monolayer on copper. Nature570, 91–95 (2019). [DOI] [PubMed] [Google Scholar]
- 42.Sutter, P. W., Flege, J.-I. & Sutter, E. A. Epitaxial graphene on ruthenium. Nat. Mater.7, 406–411 (2008). [DOI] [PubMed] [Google Scholar]
- 43.Wang, Z.-J. et al. Formation mechanism, growth kinetics and stability limits of graphene adlayers in metal-catalyzed CVD growth. Adv. Mater. Interfaces5, 1800255 (2018). [Google Scholar]
- 44.Hu, P. & King, D. A. A direct inversion method for surface structure determination from LEED intensities. Nature360, 655–658 (1992). [Google Scholar]
- 45.Gustafson, J. et al. Self-limited growth of a thin oxide layer on Rh(111). Phys. Rev. Lett.92, 126102 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Lundgren, E. et al. Two-dimensional oxide on Pd(111). Phys. Rev. Lett.88, 246103 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Krasnikov, S. A. et al. Self-limited growth of triangular PtO2 nanoclusters on the Pt(111) surface. Nanotechnology21, 335301 (2010). [DOI] [PubMed] [Google Scholar]
- 48.Huang, B. et al. Layer-dependent ferromagnetism in a van der Waals crystal down to the monolayer limit. Nature546, 270–273 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Nair, S. et al. Engineering metal oxidation using epitaxial strain. Nat. Nanotechnol.18, 1005–1011 (2023). [DOI] [PubMed] [Google Scholar]
- 50.Gao, M.-R. et al. Completely green synthesis of colloid Adams’ catalyst α-PtO2 nanocrystals and derivative Pt nanocrystals with high activity and stability for oxygen reduction. Chem. Eur. J.18, 8423–8429 (2012). [DOI] [PubMed] [Google Scholar]
- 51.Tang, M. et al. Recent progresses on structural reconstruction of nanosized metal catalysts via controlled-atmosphere transmission electron microscopy: a review. ACS Catal.10, 14419–14450 (2020). [Google Scholar]
- 52.Salmeron, M. & Eren, B. High-pressure scanning tunneling microscopy. Chem. Rev.121, 962–1006 (2021). [DOI] [PubMed] [Google Scholar]
- 53.Alcock, C. B., Hooper, G. W. & Nyholm, R. S. Thermodynamics of the gaseous oxides of the platinum-group metals. Proc. R. Soc. Lond. A254, 551–561 (1960). [Google Scholar]
- 54.Xiong, H. et al. Metastable Pd↔PdO structures during high temperature methane oxidation. Catal. Lett.147, 1095–1103 (2017). [Google Scholar]
- 55.Xiong, H. et al. Engineering catalyst supports to stabilize PdOx two-dimensional rafts for water-tolerant methane oxidation. Nat. Catal.4, 830–839 (2021). [Google Scholar]
- 56.Hafner, J. Ab-initio simulations of materials using VASP: density-functional theory and beyond. J. Comput. Chem.29, 2044–2078 (2008). [DOI] [PubMed] [Google Scholar]
- 57.Kresse, G. & Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B48, 13115–13118 (1993). [DOI] [PubMed] [Google Scholar]
- 58.Perdew, J. P. et al. Atoms, molecules, solids and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B46, 6671–6687 (1992). [DOI] [PubMed] [Google Scholar]
- 59.Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys.132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Jinnouchi, R., Karsai, F. & Kresse, G. On-the-fly machine learning force field generation: application to melting points. Phys. Rev. B100, 014105 (2019). [Google Scholar]
- 61.Huang, S.-D., Shang, C., Kang, P.-L., Zhang, X.-J. & Liu, Z.-P. LASP: fast global potential energy surface exploration. WIREs Comput. Mol. Sci.9, e1415 (2019). [Google Scholar]
- 62.Thompson, A. P. et al. LAMMPS—a flexible simulation tool for particle-based materials modeling at the atomic, meso and continuum scales. Comput. Phys. Commun.271, 108171 (2023). [Google Scholar]
- 63.Köhler, L. & Kresse, G. Density functional study of CO on Rh(111). Phys. Rev. B70, 165405 (2004). [Google Scholar]
- 64.Zeng, Z., Ma, X., Ding, W. & Li, W.‐X. First-principles calculation of core-level binding energy shift in surface chemical processes. Sci. China Chem.53, 402–410 (2010). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figs. 1–25 and Notes 1–10.
In situ SEM observation of the oxidation process.
In situ XPS study of the oxidation process.
Thermal expansion of the sample holder in the AP-XPS experiment.
In situ SEM observation of the H2 etching process.
LEED with increasing electron energy.
Simulation of α-PtO2 decomposition.
Simulation of the 30°-rotated α-PtO2 decomposition.
PtOx stability simulation.
Structure evolution from PtO2 to PtOx.
STM simulation.
Simulation of position gap between STM and atom model.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Statistical source data.
Data Availability Statement
The data that support the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.