Abstract
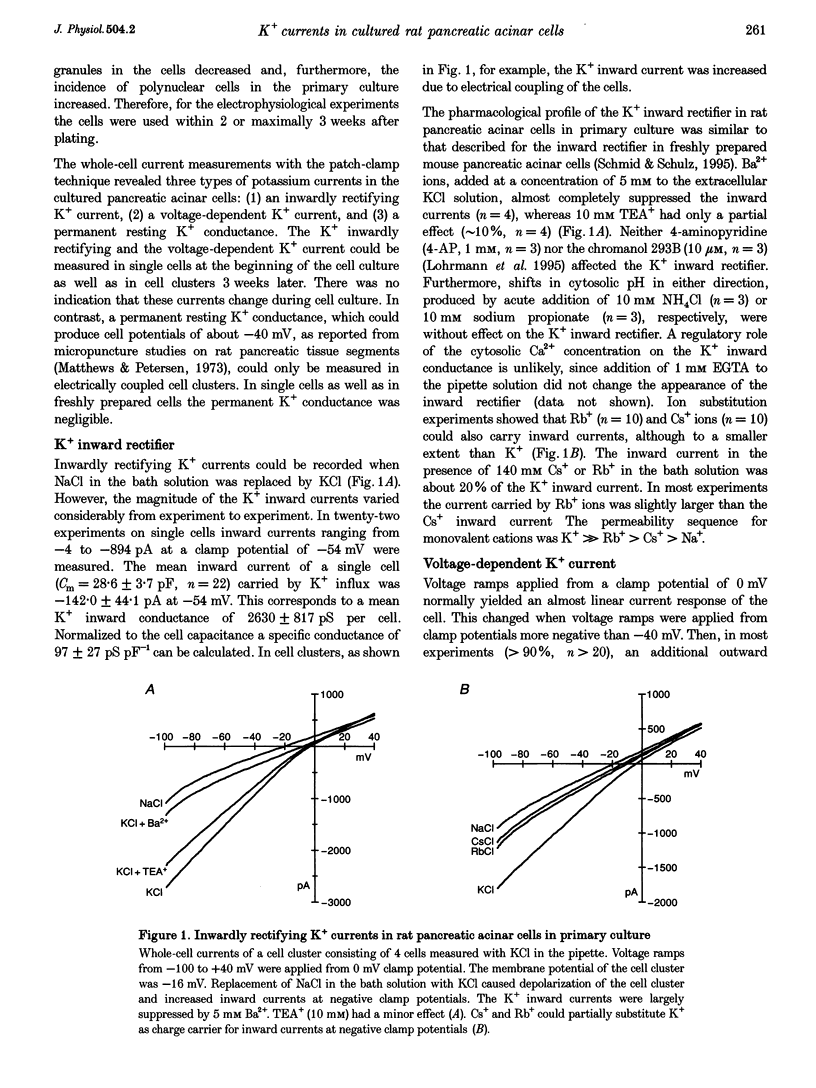
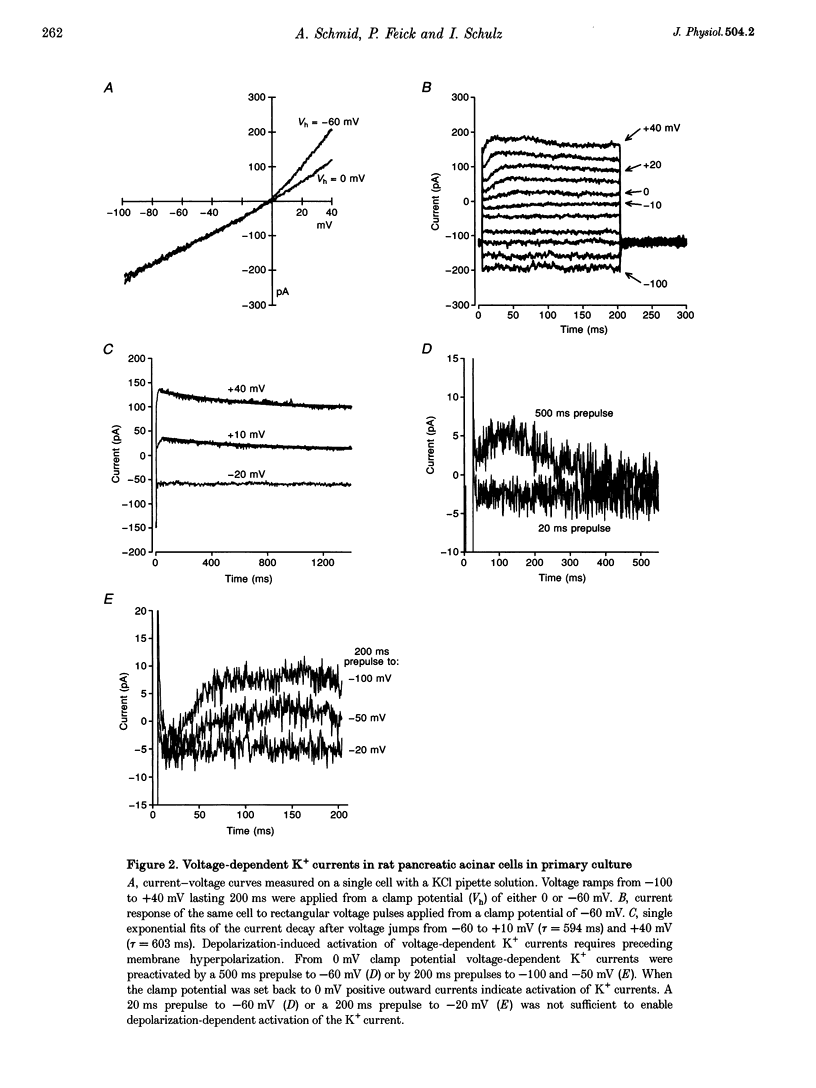
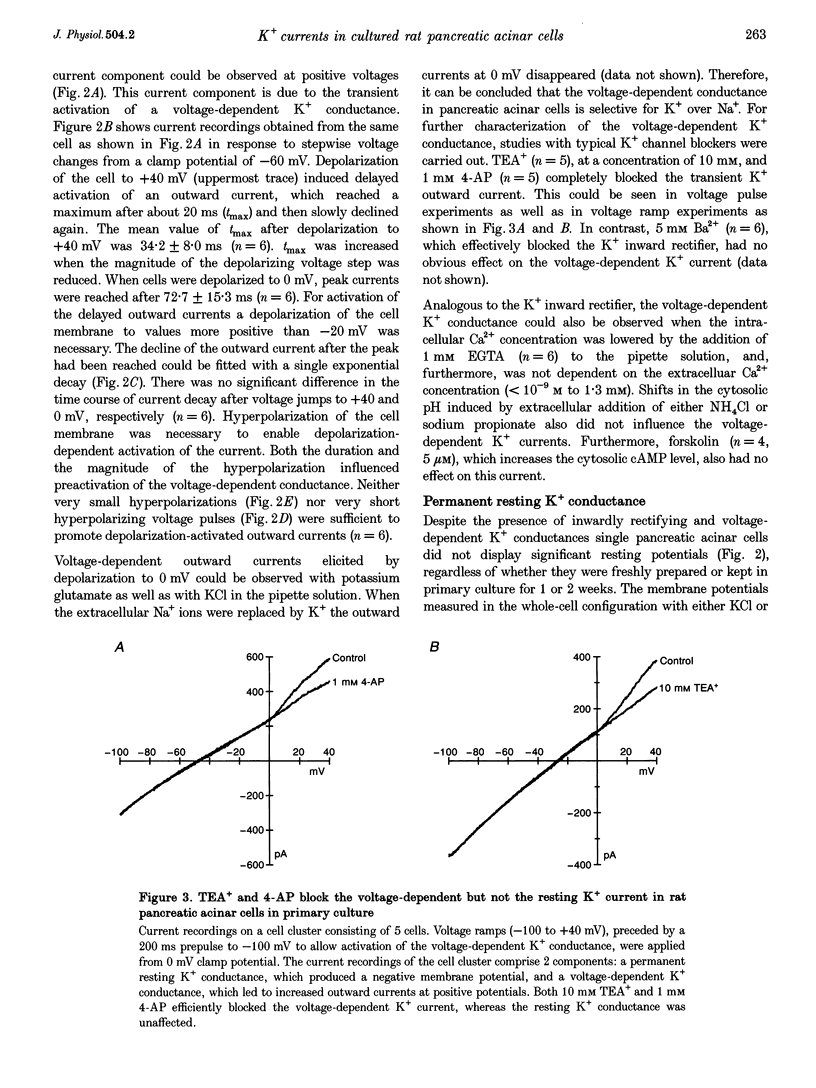
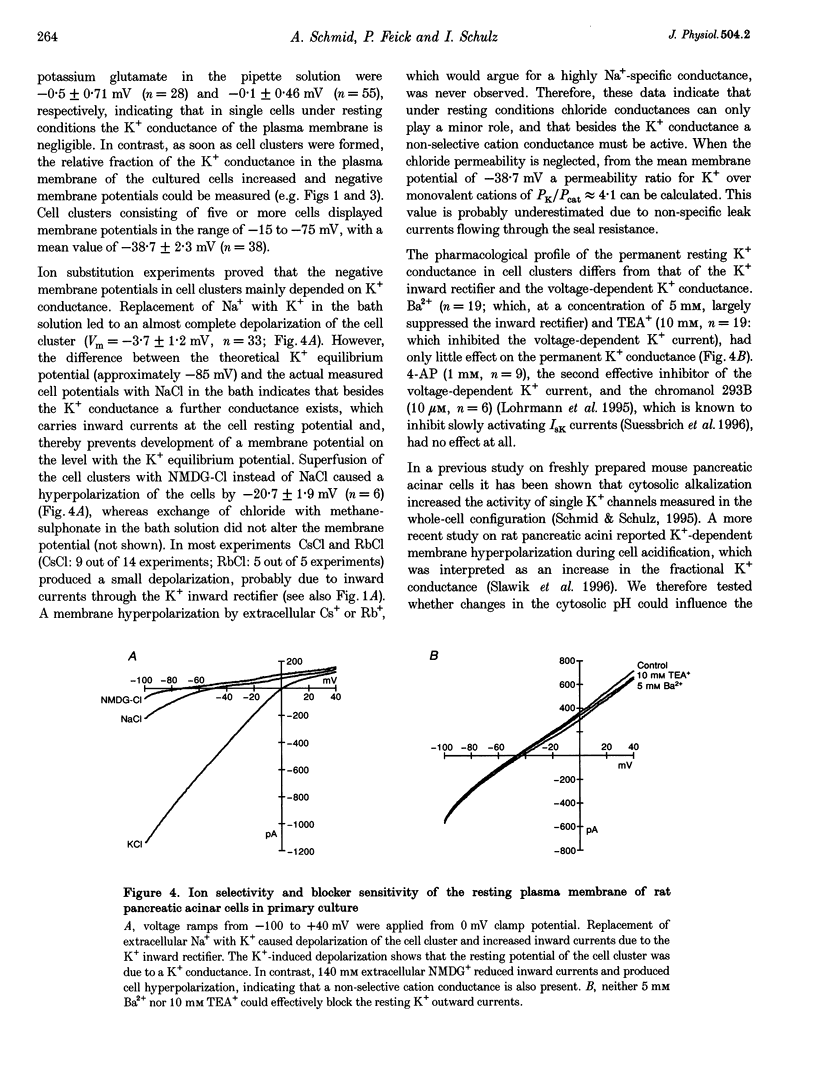
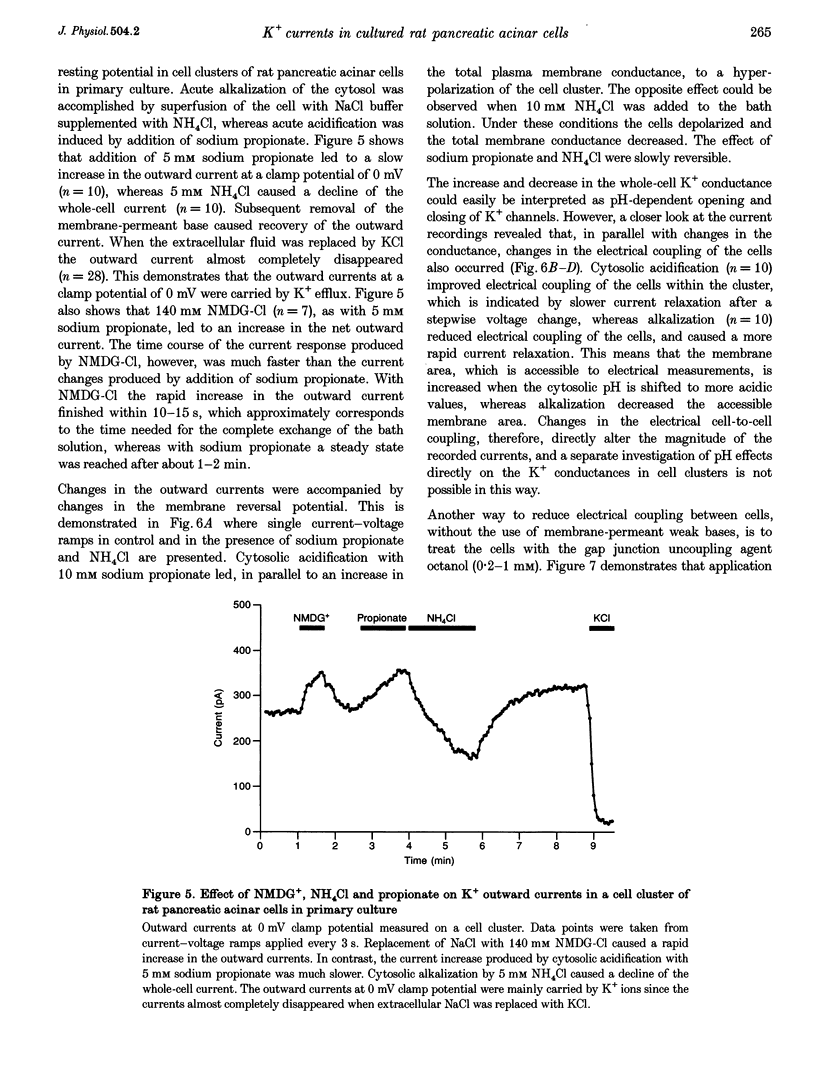
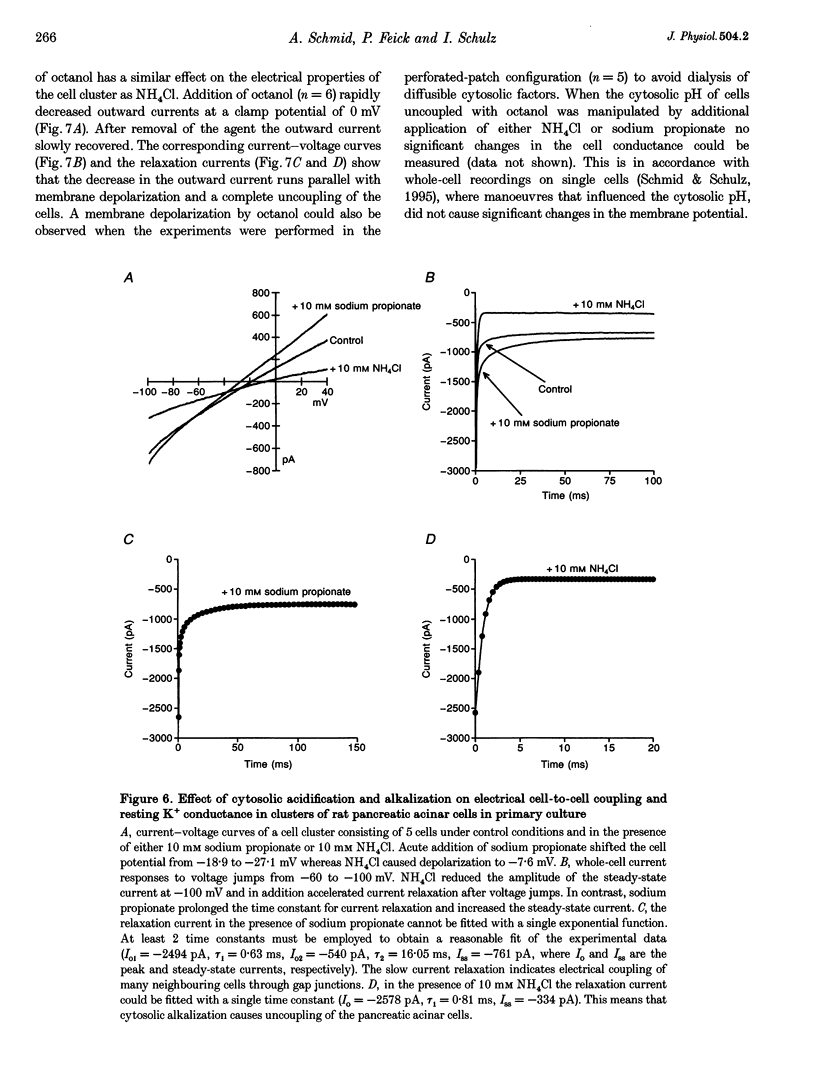
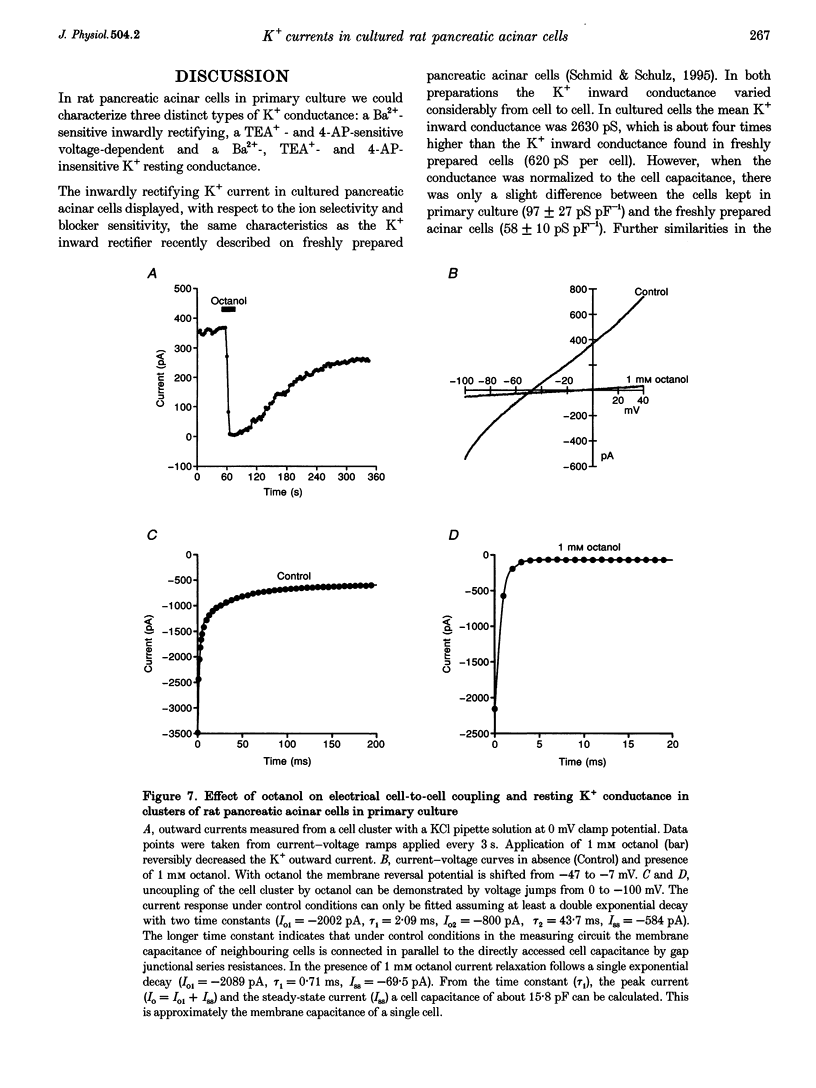
1. In exocrine pancreatic acinar cells in primary culture an inwardly rectifying, a voltage-dependent and a permanent resting K+ current were characterized. 2. Inwardly rectifying K+ currents could be elicited by elevation of the extracellular K+ concentration. The K+ inward currents were almost completely blocked by 5 mM Ba2+, whereas 10 mM TEA+ had only a partial effect. 3. Depolarizing voltage steps from negative clamp potentials evoked transient activation of a voltage-dependent K+ current. This voltage-dependent current could be blocked by 10 mM TEA+ and 1 mM 4-aminopyridine, but not by 5 mM Ba2+. 4. Neither the K+ inward rectifier nor the voltage-dependent K+ conductance produced a significant negative cell potential. Stable membrane potentials (-38.7 +/- 2.3 mV, n = 38) could only be recorded on cell clusters (> or = 5 cells). 5. Cell clusters, in contrast to single cells, had a permanent resting K+ conductance in addition to the inward rectifier and the voltage-dependent current. This resting K+ conductance was not blocked by TEA+, Ba2+, 4-aminopyridine or by the chromanol 293B. 6. Cytosolic alkalization by addition of NH4Cl to the bath solution decreased the resting K+ current. In parallel, electrical uncoupling of the cells and breakdown of the resting potential could be observed. The same effects could be produced when the cells were uncoupled by 0.2-1.0 mM n-octanol. It can be concluded that cell coupling is essential for maintenance of stable resting membrane potentials in pancreatic acinar cells.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., McNiven M. A. Vesicle dynamics during regulated secretion in a novel pancreatic acinar cell in vitro model. Eur J Cell Biol. 1995 Jan;66(1):25–38. [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Kasai H., Li Y. X., Miyashita Y. Subcellular distribution of Ca2+ release channels underlying Ca2+ waves and oscillations in exocrine pancreas. Cell. 1993 Aug 27;74(4):669–677. doi: 10.1016/0092-8674(93)90514-q. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H. Cholecystokinin activation of single-channel currents is mediated by internal messenger in pancreatic acinar cells. Nature. 1982 Nov 4;300(5887):61–63. doi: 10.1038/300061a0. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Petersen O. H., Flanagan P., Pearson G. T. Quantification of Ca2+-activated K+ channels under hormonal control in pig pancreas acinar cells. Nature. 1983 Sep 15;305(5931):228–232. doi: 10.1038/305228a0. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H. Pancreatic acinar cells: ionic dependence of the membrane potential and acetycholine-induced depolarization. J Physiol. 1973 Jun;231(2):283–295. doi: 10.1113/jphysiol.1973.sp010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meda P., Bruzzone R., Knodel S., Orci L. Blockage of cell-to-cell communication within pancreatic acini is associated with increased basal release of amylase. J Cell Biol. 1986 Aug;103(2):475–483. doi: 10.1083/jcb.103.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Pancreatic acinar cells: membrane potential and resistance change evoked by acetylcholine. J Physiol. 1974 Apr;238(1):145–158. doi: 10.1113/jphysiol.1974.sp010515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappas C. A., Rioult M. G., Ransom B. R. Octanol, a gap junction uncoupling agent, changes intracellular [H+] in rat astrocytes. Glia. 1996 Jan;16(1):7–15. doi: 10.1002/(SICI)1098-1136(199601)16:1<7::AID-GLIA2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Petersen O. H., Findlay I., Iwatsuki N., Singh J., Gallacher D. V., Fuller C. M., Pearson G. T., Dunne M. J., Morris A. P. Human pancreatic acinar cells: studies of stimulus-secretion coupling. Gastroenterology. 1985 Jul;89(1):109–117. doi: 10.1016/0016-5085(85)90751-6. [DOI] [PubMed] [Google Scholar]
- Petersen O. H. Stimulus-secretion coupling: cytoplasmic calcium signals and the control of ion channels in exocrine acinar cells. J Physiol. 1992 Mar;448:1–51. doi: 10.1113/jphysiol.1992.sp019028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A., Schulz I. Characterization of single potassium channels in mouse pancreatic acinar cells. J Physiol. 1995 May 1;484(Pt 3):661–676. doi: 10.1113/jphysiol.1995.sp020694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spray D. C., White R. L., de Carvalho A. C., Harris A. L., Bennett M. V. Gating of gap junction channels. Biophys J. 1984 Jan;45(1):219–230. doi: 10.1016/S0006-3495(84)84150-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer P. L., Zhao H., Luby-Phelps K., Moss R. L., Star R. A., Muallem S. Gap junction communication modulates [Ca2+]i oscillations and enzyme secretion in pancreatic acini. J Biol Chem. 1993 Sep 15;268(26):19769–19775. [PubMed] [Google Scholar]
- Suessbrich H., Bleich M., Ecke D., Rizzo M., Waldegger S., Lang F., Szabo I., Lang H. J., Kunzelmann K., Greger R. Specific blockade of slowly activating I(sK) channels by chromanols -- impact on the role of I(sK) channels in epithelia. FEBS Lett. 1996 Nov 4;396(2-3):271–275. doi: 10.1016/0014-5793(96)01113-1. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Petersen O. H. Patch-clamp study of single-channel and whole-cell K+ currents in guinea pig pancreatic acinar cells. Am J Physiol. 1988 Sep;255(3 Pt 1):G275–G285. doi: 10.1152/ajpgi.1988.255.3.G275. [DOI] [PubMed] [Google Scholar]
- Thorn P., Lawrie A. M., Smith P. M., Gallacher D. V., Petersen O. H. Local and global cytosolic Ca2+ oscillations in exocrine cells evoked by agonists and inositol trisphosphate. Cell. 1993 Aug 27;74(4):661–668. doi: 10.1016/0092-8674(93)90513-p. [DOI] [PubMed] [Google Scholar]
- Thorn P., Petersen O. H. A voltage-sensitive transient potassium current in mouse pancreatic acinar cells. Pflugers Arch. 1994 Oct;428(3-4):288–295. doi: 10.1007/BF00724509. [DOI] [PubMed] [Google Scholar]
- Zhao H., Muallem S. Agonist-specific regulation of [Na+]i in pancreatic acinar cells. J Gen Physiol. 1995 Dec;106(6):1243–1263. doi: 10.1085/jgp.106.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]



