Abstract
Background: Immune thrombocytopenia (ITP) is an uncommon but serious adverse reaction after vaccination. However, its association with vaccines other than the measles–mumps–rubella vaccine remains debatable. This study aimed to analyze ITP cases following influenza vaccination and assess any potential association. Methods: We performed a systematic search of the Web of Science, Embase, and PubMed databases from their inception to 15 April 2024. Cases were characterized qualitatively, and relative risk was assessed using either fixed or random models. Results: A total of 24 studies were analyzed, including 16 patients from 14 case reports. Patients averaged 56.7 years old, half were female, and ten patients had a history of prior illness. The mean time between vaccination and diagnosis was 13.3 days. Treatment primarily involved corticosteroids or intravenous immunoglobulin, with most recovering within a month. The pooled odds ratio for ITP post-influenza vaccination was 0.94 (95%CI: 0.85–1.03). Subgroup analyses conducted according to the study design and vaccine type did not reveal any significant results. Conclusion: No evidence of an association between influenza vaccination and ITP was found. Further observational studies are required to verify this relationship.
Keywords: Immune thrombocytopenia, purpura, influenza, vaccination, meta-analysis
1. Introduction
Seasonal influenza, typically referred to as the flu, is a highly contagious viral infection that primarily targets the respiratory system. It can result in various mild-to-severe symptoms, particularly in vulnerable populations such as older adults, young children, pregnant individuals, and those with weakened immune systems [1]. Influenza causes approximately 1 billion infections yearly, of which 3–5 million are serious illnesses [2]. Vaccination is crucial for disease prevention and is one of the most cost-effective public health measures available. Annual influenza vaccination significantly reduces influenza-related morbidity and mortality. Influenza vaccines have beneficial effects on patients with chronic diseases, cardiovascular diseases, and immunocompromised patients, such as reducing the risk of acute respiratory infections in patients with chronic obstructive pulmonary disease and hospitalization in patients with heart failure [3,4]. There are three types of influenza vaccines: inactivated, recombinant, and live attenuated. The exact type of vaccination is determined based on the patient’s age and physical condition [5].
Vaccination can occasionally lead to adverse reactions, such as herpes zoster and meningitis from the varicella vaccine and allergic reactions from DTaP or Tdap [6]. These reactions are less frequent and severe than those caused by natural infections; however, they are still of concern. Immunization-related autoimmune diseases are among the most worrisome adverse reactions [7]. Aside from the rare associations of the measles–mumps–rubella (MMR) vaccine with immune thrombocytopenia and that of the influenza vaccine with Guillain–Barre syndrome (GBS), a definitive relationship between vaccines and the development of autoimmune diseases remains unproven [8]. Similar to other vaccines, the influenza vaccine can cause local or systemic adverse effects, commonly manifesting as fever, injection site pain, erythema, swelling, and induration. The attenuated influenza vaccine may also lead to headache, nasal congestion, and sore throat [9]. In specific seasons, the influenza vaccine has been associated with an increased risk of GBS, a rare neurological disorder [10]. Additionally, serious adverse effects such as stroke, encephalitis, peripheral neuropathy, and immune thrombocytopenia have been reported following influenza vaccination [11].
Immune thrombocytopenia (ITP), formerly known as idiopathic thrombocytopenic purpura, is an autoimmune disorder that is primarily defined by the exclusion of other causes of low platelet counts (<100 × 109/L). This condition arises from the immune system mistakenly targeting and destroying platelets, leading to an increased risk of bleeding and related complications. Clinical signs include skin hemorrhages, such as purpura, rash, and bruises, and mucosal hemorrhage, such as epistaxis and gingival bleeding. However, in severe cases, bleeding can extend to the subarachnoid or intracerebral regions, the lower gastrointestinal tract, or other internal areas [12]. The prevalence of ITP is estimated to range from approximately 1.6–3.9 cases per 100,000 person-years. Additionally, epidemiological studies have shown that the overall average incidence of this disorder tends to increase with age and is more prevalent among women [13,14].
ITP is typically idiopathic; however, it can also be secondary to autoimmune diseases, infections, cancers, and drugs [15]. Regarding drug-related ITP, approximately half of the cases can be attributed to vaccines, and the mechanism for the increased risk of ITP after vaccination appears to be the same as that for the induction of antiplatelet autoantibodies by microbial infections [16]. The MMR vaccine is currently the only vaccine proven to be associated with thrombocytopenia, with an estimated 1 case per 20,000–40,000 doses [17]. Numerous surveillance data and cases have also reported the occurrence of ITP following other vaccinations, including influenza, Bacillus Calmette–Guerin, COVID-19, encephalitis B, hepatitis B virus, polio, diphtheria–tetanus–acellular pertussis, varicella, and enterovirus [18,19,20,21]. Therefore, ITP is receiving more and more attention. However, as the most used vaccine globally, no conclusive causal link exists between the influenza vaccine and ITP. Grimaldi-Bensouda et al. and Garbe et al. conducted separate case–control studies to evaluate the potential association between the influenza vaccine and ITP; however, they had contradictory results [22,23]. Therefore, whether influenza vaccination truly increases the risk of ITP or whether the reported cases are coincidental remains unclear. In addition, studies have reported vaccine hesitancy in the general population towards influenza vaccination due to concerns and misconceptions about the risk of adverse reactions [24]. Collecting, analyzing, and evaluating data on suspected abnormal vaccination reactions after influenza vaccination can effectively improve public confidence in the safety of influenza vaccines and provide a reference basis for developing and implementing immunization strategies.
Thus, to clarify the relationship between ITP and influenza vaccination, a systematic review and meta-analysis was performed after summarizing relevant epidemiological studies.
2. Materials and Methods
2.1. Data Sources
This study was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines to ensure transparency and methodological rigor in the research process. Our study was registered in INPLASY (ID: INPLASY2024100107).
We performed a comprehensive search of all relevant articles on the connection between ITP and influenza vaccination, published up to 15 April 2024, in the Embase, Web of Science, and PubMed databases. The main search terms were “Purpura, Thrombocytopenic, Idiopathic”, “Immune Thrombocytopenia”, “Immune Thrombocytopenic Purpura”, “Thrombocytopenic Purpura”, “Idiopathic Thrombocytopenic Purpura”, “influenza”, “flu”, “vaccin*”, and immuni*”. Furthermore, references to the retrieved articles were checked for additional relevant articles.
2.2. Eligibility Criteria
The inclusion criteria for the studies were defined as follows: (1) case reports of ITP following influenza vaccination and (2) observational studies that reported an odds ratio (OR), incidence rate ratio (IRR), or risk ratio (RR) with a 95% confidence interval (CI) of ITP after influenza vaccination. We excluded (1) duplicate articles; (2) reviews, conference abstracts, or comments; and (3) papers without data or full text.
2.3. Study Selection
All search results were imported into the EndNote X9 software for deduplication and reference management. The remaining articles underwent an initial screening based on their titles and abstracts, and full-text searches were performed on those deemed eligible. Studies were ultimately included after screening by two independent researchers who strictly followed the predefined inclusion and exclusion criteria. Inconsistent results were addressed by mutual discussion or consultation with a third researcher.
2.4. Data Extraction and Quality Assessment
Two researchers independently completed data collection using a standardized data extraction form. The following critical information was extracted from the case reports: first author, year of publication, patient age and sex, medical history, number of doses, platelet count before and after vaccination, time from vaccination to diagnosis, time to recovery from ITP, presentation, treatment strategy, and outcomes. For observational studies, we extracted the first author, study design, study area, age, vaccine type, and OR, RR, or IRR with 95% CI. The quality assessment of the observational studies included in this analysis was conducted using the Newcastle–Ottawa Scale (NOS). Scores on the NOS range from 0 to 9, with ≥7 being considered high quality and included in this study. Inconsistent results were addressed by mutual discussion or consultation with a third researcher.
2.5. Data Synthesis
We qualitatively analyzed the case reports. A meta-analysis was performed using Review Manager 5.3 and Stata 15.0 (Stata Corp, College Station, TX, USA). The pooled effect size is expressed as the OR of ITP with influenza vaccination compared with no vaccination, and the corresponding forest plot was obtained. As this outcome is rare in the population, ORs can be considered approximations of RRs or IRRs. Cochran’s Q and I2 statistics were employed to assess the heterogeneity among the included studies, and statistical heterogeneity was defined as I2 > 50%. When I2 was > 50%, we used a random-effects model to amalgamate the effect sizes; conversely, a fixed-effects model was employed. Subgroup analyses were performed according to study design and vaccine type. Sensitivity analyses were performed to test the robustness of the findings using a study-by-study approach to exclude individual studies. Funnel plots, in conjunction with Begg’s test and Egger’s test, were implemented to assess potential publication bias.
3. Results
3.1. Search Results
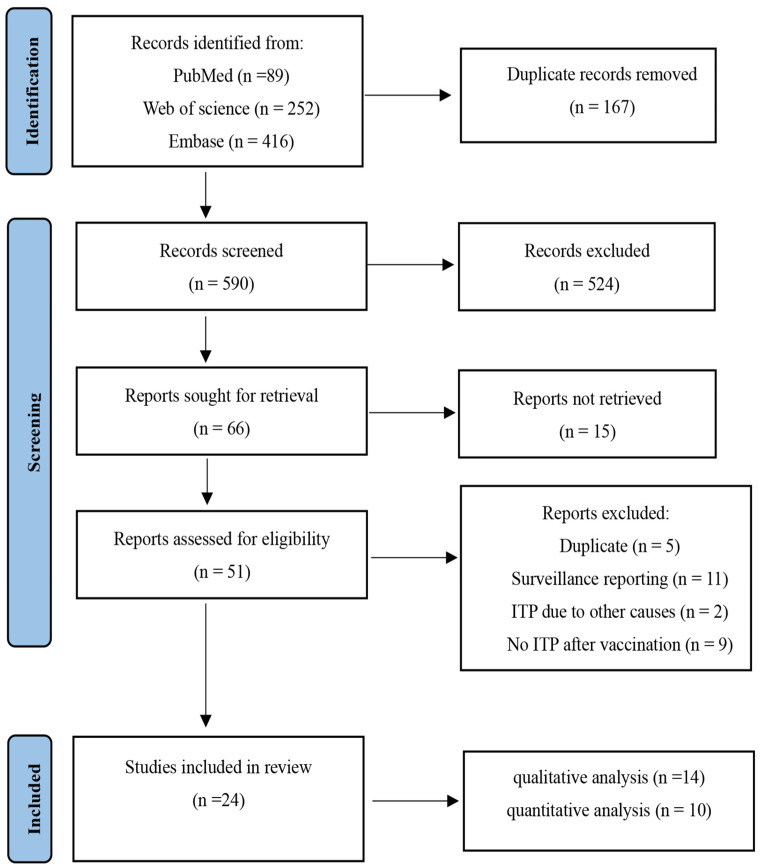
The literature search and selection process is illustrated in Figure 1. Overall, 757 studies were retrieved from three databases, 167 of which were identified as duplicates. Following the screening of the titles and abstracts, 524 articles were disqualified. Finally, 24 studies, all in English, were deemed eligible for inclusion through a full-text screening.
Figure 1.
Flow chart of the included studies.
3.2. Baseline Characteristics of the Included Studies
Among the included studies, fourteen, three, four, and three were case reports, case–control studies, cohort studies, and self-controlled case series, respectively. The 14 case reports collectively involved 16 patients. The mean age at initial presentation was 56.7 (a range of 3–88) years, with an equal sex distribution among the patients. Ten patients had a history of prior illness. Three cases occurred after the first and third doses of the influenza vaccination, respectively, and one occurred after the second dose. Platelet counts were markedly reduced after influenza vaccination in all patients. The mean interval from the vaccination to ITP onset was 13.3 (a range of 2–35) days. Most patients recovered within a month of diagnosis, with two taking approximately 5 months and a year to recover, respectively. Fifteen patients presented with cutaneous mucosal bleeding, including gingival bleeding, purpura, petechiae, rash, epistaxis, hemoptysis, genital bleeding, hematuria, petechiae, buccal hematoma, and hemorrhagic blisters. Furthermore, most patients were treated with corticosteroids, intravenous immunoglobulin (IVIG), or a combination of both. One patient had a platelet count >100 × 109/L, and no treatment was administered. The overall outcome of all patients was favorable, with no reported deaths. The detailed findings are outlined in Table 1 and Table 2.
Table 1.
Characteristics of the included case reports.
| Study | Age (Years) | Sex | Vaccine Dose | Platelet Count | Time from | ||
|---|---|---|---|---|---|---|---|
| Before Vaccination | After Vaccination | Vaccination to Diagnosis | Recovery from ITP | ||||
| Shlamovitz 2013 [25] | 50 | M | 1 | NA | <5 × 103/μL | 4 d | 6 d |
| Mantadakis 2010 [26] | 3 | M | 2 | NA | 11 × 103/μL | 26 d | 1 d |
| Asiimwe 2023 [27] | 70 | M | NA | Normal | 2 × 103/μL | 6 d | 3 d |
| Almohammadi 2019 [28] | 68 | M | 3 | 210–267 × 103/μL | 0/μL | 2 d | 10 d |
| Cummins 1998 [29] | 67 | F | NA | NA | 111 × 109/L | 21 d | 14 d |
| Ikegame 2006 [30] | 19 | F | 1 | >180 × 109/L | 10 × 109/L | 14 d | 19 d |
| Tishler 2006 [31] | 68 | M | NA | NA | 3000/mm3 | 14 d | 5 d |
| Nagasaki 2016 [32] | 81 | F | NA | 184 × 103/μL | 39 × 103/μL | 28 d | Within 5 months |
| 75 | F | NA | 251 × 103/μL | 5 × 103/μL | 35 d | 28 d | |
| 87 | F | NA | NA | 2 × 103/μL | 14 d | NA | |
| Hamiel 2016 [33] | 4.5 | M | 3 | >200 × 103/μL | 17 × 103/μL | 6 d | 10 d |
| Ohta 2022 [34] | 88 | M | NA | 150 × 103/μL | 1 × 103/μL | 4 d | NA |
| Mamori 2008 [35] | 75 | F | NA | 164 × 103/μL | 5 × 103/μL | 7 d | 16 d |
| Wan Jamaludin 2018 [36] | 31 | F | 1 | 203 × 109/L | 3 × 109/L | 7 d | 1 year |
| Kelton 1981 [37] | 38 | M | NA | Normal | 20 × 103/μL | 14 d | NA |
| Mateos 2007 [38] | 83 | F | NA | NA | 1 × 109/L | 10 d | 5 d |
M, Male; F, Female; NA, Not Available; d, day.
Table 2.
Clinical characteristics of the patients in the case reports.
| Study | Medical History | Presentation | Treatment | Outcome |
|---|---|---|---|---|
| Shlamovitz 2013 [25] | None | Bleeding gums | Prednisone and IVIG | Recovery |
| Mantadakis 2010 [26] | None | Diffuse petechiae | IVIG | Recovery |
| Asiimwe 2023 [27] | HP infection | Mixed petechial rash, oral bleeding | Methylprednisolone, IVIG, oral dexamethasone | Recovery |
| Almohammadi 2019 [28] | Hepatitis C, prediabetes, hypertriglyceridemia | Epistaxis, hematuria, and bleeding gums | Platelet transfusion, IVIG, dexamethasone, and oral prednisone | Recovery |
| Cummins 1998 [29] | Chronic angina | Abnormal blood count | No treatment | Recovery |
| Ikegame 2006 [30] | Acute lymphoblastic leukemia | Transient nasal bleeding | Immunoglobulin, and prednisolone | Recovery |
| Tishler 2006 [31] | Hypertension | Rash and melena | Intravenous gamma globulins, corticosteroids, red blood cells, and proton pump inhibitors | Recovery |
| Nagasaki 2016 [32] | None | None | HP eradication | Recovery |
| None | Gingival and nasal hemorrhaging | HP eradication, prednisolone | Recovery | |
| None | genital bleeding, purpura | Prednisolone | Recovery | |
| Hamiel 2016 [33] | None | Cutaneous and mucosal bleeding | IVIG | Recovery |
| Ohta 2022 [34] | Brain stroke, hypertension, and dyslipidemia | Oral bleeding, bleeding blisters, and systemic petechiae | Prednisolone | Recovery |
| Mamori 2008 [35] | Primary biliary cirrhosis | Epistaxis, petechiae | Prednisone | Recovery |
| Wan Jamaludin 2018 [36] | Hodgkin Lymphoma | Spontaneous bruises, petechiae, and buccal hematoma | Corticosteroids with a slow tapering course of prednisolone | Recovery |
| Kelton 1981 [37] | Chronic obstructive pulmonary disease, bronchiectasis | Purpura and hemoptysis | Corticosteroid | Recovery |
| Mateos 2007 [38] | Chronic lymphocytic leukemia | Petechiae, bruising, and hemorrhagic bullae in oral mucosa | Methylprednisolone | Recovery |
HP, Helicobacter pylori; IVIG, Intravenous immunoglobulin.
Of the observational studies included, three each were conducted in France and the United States, two in Italy, and one each in Berlin, Japan, and Taiwan. A case–control analysis was additionally performed within one of the three self-controlled case series studies, and one study included three different age groups. Ten studies were conducted between 2000 and 2018. Based on the methodological quality score, all 10 studies had NOS scores ≥ 7 and were deemed high quality. The details are presented in Table 3.
Table 3.
Characteristics of observational studies.
| Study | Study Design (Period) | Area | Age | Vaccine Type | OR | 95%CI | NOS |
|---|---|---|---|---|---|---|---|
| Grimaldi-Bensouda 2012 [22] | Case-control (2008–2011) | France | 18–79 | All | 0.9 | 0.4–2.1 | 9 |
| Garbe 2012 [23] | Case-control (2000–2009) | Berlin | ≥18 | All | 4.0 | 1.5–9.6 | 9 |
| Yokomichi 2020 [39] | Case-control (2015–2017) | Japan | NR | All | 1.56 | 0.50–4.85 | 8 |
| Lafaurie1 2022 [40] | Case-control (2009–2018) | France | ≥65 | All | 0.85 | 0.71–1.03 | 7 |
| Moro 2013 [41] | Cohort (2009–1010) | Italy | All | MIV | 1 | 0.1–16.0 | 7 |
| Nordin 2013 [42] | Cohort (2002–2009) | US | NR | TIV | 0.9 | 0.68–1.19 | 7 |
| Nordin 2014 [43] | Cohort (2009–2010) | US | NR | MIV | 1.22 | 0.65–2.28 | 7 |
| Villa 2013 [44] | Cohort (2006–2009) | Italy | ≥65 | TIV | 3.63 | 0.75–10.62 | 7 |
| Lafaurie2 2022 [40] | Self-controlled case series (2009–2018) | France | ≥65 | All | 0.91 | 0.79–1.05 | 7 |
| Huang 2013 [45] | Self-controlled case series (2009–2010) | Taiwan | All | MIV | 1.09 | 0.65–1.85 | 7 |
| O’Leary1 2012 [46] | Self-controlled case series (2000–2009) | US | 6–23 months | TIV | 2.69 | 0.81–8.88 | |
| O’Leary2 2012 [46] | 2–6 | TIV | 1.86 | 0.41–8.38 | 7 | ||
| O’Leary3 2012 [46] | 7–17 | TIV | 5.95 | 0.54–65.96 |
NR, No Reported; MIV, Monovalent Influenza Vaccines; TIV, Trivalent Influenza Vaccines.
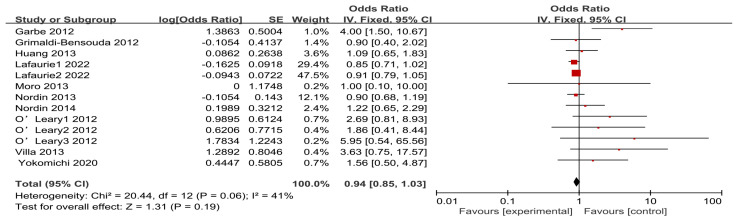
3.3. Results of Meta-Analysis
The studies included in the analysis exhibited low heterogeneity and were pooled using a fixed-effects model. The meta-analysis found no evidence of an association between influenza vaccination and ITP, with a pooled OR of 0.94 (95% CI: 0.85–1.03). The results of the meta-analysis are presented in Figure 2. To further determine whether there were differences between the study designs, vaccine types, and ITP occurrence, we conducted subgroup analyses. No significant associations were observed across different study designs. The ORs for case–control, cohort, and self-controlled case series studies were 0.91 (95% CI: 0.76–1.07), 0.98 (95% CI: 0.76–1.26), and 0.94 (95% CI: 0.83–1.08), respectively. When studies were grouped by type of influenza vaccine, no significant correlations were identified between monovalent (OR = 1.14, 95% CI: 0.77–1.68) and trivalent (OR = 1.03, 95% CI: 0.79–1.34) influenza vaccines. The outcomes of the subgroup analysis are presented in Table 4.
Figure 2.
Forest plot of the association between influenza vaccination and ITP. Black diamonds: overall effect size and its 95% confidence interval. Red graph: effect size of each study and its weight.
Table 4.
Results of the subgroup analysis.
| Subgroups | Numbers of Studies | I2 (%) | OR (95% CI) | p-Value |
|---|---|---|---|---|
| Study design | ||||
| Case-control | 4 | 71 | 0.91 (0.76–1.07) | 0.26 |
| Cohort | 4 | 14 | 0.98 (0.76–1.26) | 0.87 |
| Self-controlled case series | 5 | 39 | 0.94 (0.83–1.08) | 0.41 |
| Vaccine type | ||||
| All | 5 | 61 | 0.91 (0.81–1.01) | 0.08 |
| TIV | 5 | 53 | 1.03 (0.79–1.34) | 0.82 |
| MIV | 3 | 0 | 1.14 (0.77–1.68) | 0.52 |
MIV, Monovalent Influenza Vaccines; TIV, Trivalent Influenza Vaccines.
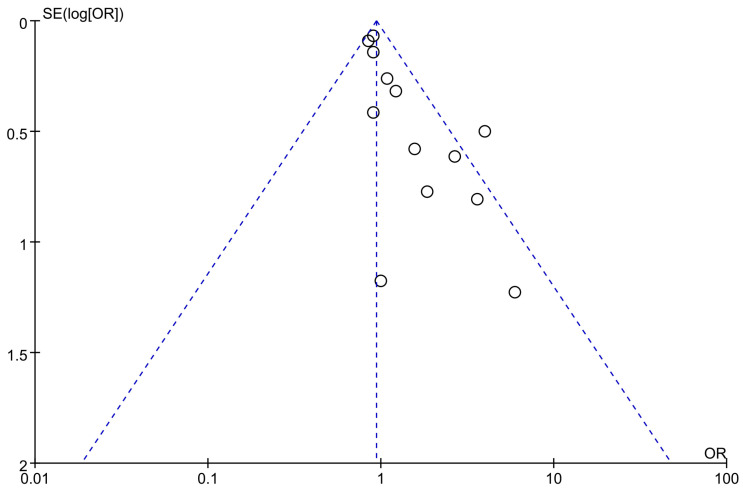
3.4. Publication Bias
Figure 3 shows the funnel plot generated for this study. An examination of the plot reveals asymmetry, which suggests the potential presence of publication bias. This finding is further supported by the results of Egger’s and Begg’s tests, both of which indicated a statistical indication of publication bias within the included studies.
Figure 3.
Funnel plot for the assessment of publication bias.
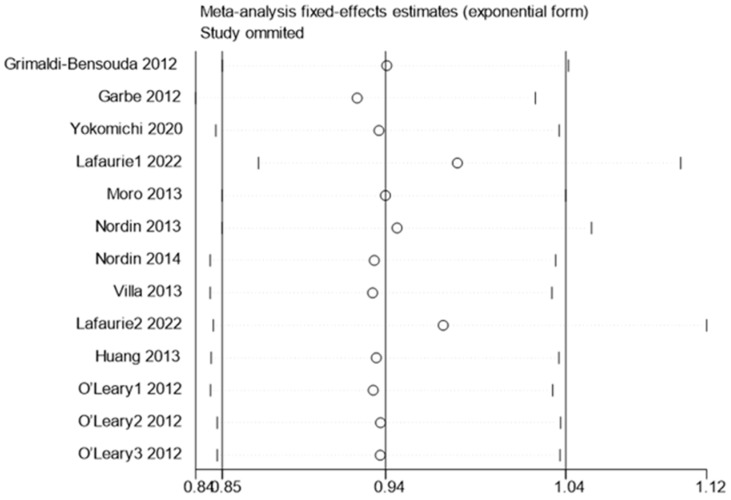
3.5. Sensitivity Analysis
The sensitivity analysis results showed no significant change in the combined effect values before and after the study-by-study exclusion, indicating good robustness and reliability of the findings. This is illustrated in Figure 4.
Figure 4.
Sensitivity analysis of influenza vaccination and the risk of ITP. 0.85–1.04 represents the 95% confidence interval for the pooled effect size. The circle represents the effect size after removing the study, and the two short vertical lines in the same row as the circle represent the 95% confidence interval of the effect size after removing the study.
4. Discussion
Whether ITP development is associated with influenza vaccination remains a topic of debate. Therefore, conducting a systematic review and meta-analysis of pertinent studies is necessary to thoroughly evaluate this relationship. Our study identified 16 patients who developed ITP after influenza vaccination. However, the aggregated findings from the meta-analysis did not reveal a statistically significant association between influenza vaccination and an increased risk of ITP development. These results suggest that although individual cases have been reported, the overall evidence fails to support a causal link.
The diagnosis of ITP was exclusionary and involved a detailed history, physical examination, and peripheral blood smear [47]. All the above cases were caused by the influenza vaccine after ruling out other potential causes. In this study, the mean age of patients with ITP secondary to influenza vaccination was found to be 56.7 years, similar to that observed in a case–control surveillance study in Berlin, where the median age of patients with drug-induced ITP was 56 years [23]. Other epidemiologic studies have also shown that the disease peaks in older patients [48]. In addition to the increased incidence of ITP with age, this occurrence may be attributed to the fact that influenza vaccination is recommended to the older population, and they are more likely to have bleeding manifestations. However, we were unable to determine sex differences in patients with influenza vaccine-associated ITP because of the insufficient number of cases. Recent findings reported a higher prevalence in females, correlating with an increased incidence of systemic autoimmune diseases in women [49]. Of note, one patient reported by Hamiel et al. had been hospitalized twice for ITP, with both instances occurring within one week of influenza vaccination [33]. This seems to reinforce the causal relationship between the influenza vaccine and ITP, but some studies have proposed that the influenza vaccine may trigger and worsen pre-existing thrombocytopenia [50]. Diabetes mellitus, hypertension, thyroid disease, and gastrointestinal disorders are the most common comorbidities in adult patients with ITP [51]. In our study, it was similarly found that some patients had diabetes mellitus, hypertension, and chronic angina prior to vaccination, suggesting that the development of ITP is not associated with chronic diseases. Only two patients took longer than one month from diagnosis to recovery, which may have been caused by their treatment strategies and physical conditions. Corticosteroids and IVIG are the first-line treatment options for ITP, and a combination of the two can accelerate outcomes [52]. In this study, most of the patients showed improvement in platelet counts and bleeding manifestations after using corticosteroids and IVIG. Although IVIG is less effective in older adults and has potentially serious side effects, it remains the best treatment in emergency situations [53]. It is important to note that most ITP cases are asymptomatic, leading to other potential undetected occurrences.
The current meta-analysis found no association between influenza vaccination and ITP risk. Subgroup analyses of different study designs and vaccine types yielded similar results. However, this finding contradicts the results of a previous meta-analysis. In Elsaid et al.’s study, the IRR of ITP 42 days after influenza vaccination was 1.85 (95% CI: 1.03–3.32) [54]. This result was derived by combining three different age subgroups from one study; however, our study included more studies with different designs and vaccination times. The results of our study showed that neither monovalent nor trivalent influenza vaccines increased the risk of ITP. In a comparative study assessing the safety of MF59 adjuvanted trivalent influenza vaccines in older adults, Villa et al. reported a 4.52-fold increased risk of ITP after adjuvanted trivalent influenza vaccination compared to the non-adjuvanted influenza vaccine. Therefore, further studies are required to confirm whether adjuvants affect the development of ITP. In addition, Yokomichi et al. explored the risk of ITP after inactivated, live, and concurrent vaccinations and found no significant risk of ITP after vaccination alone or concurrently in any age group.
The pathogenic mechanisms underlying vaccine-related ITP remain unclear. Current hypotheses primarily include molecular mimicry, bystander activation, epitope spreading, and polyclonal activation [55]. Among these, molecular mimicry is the most widely supported theory. This posits that due to structural similarities between epitopes, antibodies targeting antigens from infectious agents may cross-react with antigens on the platelet surface. Hemagglutinin is the main antigen of the influenza vaccine and shares structural similarities with platelet antigens [56]. Consequently, ITP triggered by the influenza vaccine may arise from Hemagglutinin interacting with platelets through certain receptors, promoting platelet lysis. Additionally, adjuvants, yeast proteins, and preservatives present in the influenza vaccine could activate polyclonal B cells, enhancing autoimmune responses and potentially inducing ITP or other autoimmune diseases. Some studies have shown that directly adding the H3N2 virus to platelets induces platelet aggregation in humans and animals, suggesting that the influenza virus may also directly affect platelets [57].
Our study has some limitations. First, we searched three databases and included only English literature, which may have led to the omission of some important studies. Future studies could expand the search and inclusion criteria to obtain more relevant data. Second, owing to the limited data in the articles included in the study, we were unable to analyze subgroups according to age and vaccination duration. Third, two of the studies included pregnant women as participants; therefore, our findings did not allow for an accurate assessment of the risk of ITP in the whole population. Fourth, most patients with ITP are asymptomatic, leading to underreporting; therefore, in this study, we may have missed this subset of cases. Finally, our study had a publication bias, which may affect the reliability of our findings, but we deem that it was not caused by the sample size or quality of the study. Several abstracts and conference papers with relevant data were found in the literature search, but we did not include them in the study. It is unclear whether including these studies would reduce publication bias. Additionally, including non-English studies and unpublished data may also address this bias. Thus, the findings of this study should be approached and interpreted with caution.
5. Conclusions
In this systematic review and meta-analysis containing 24 studies, we found a low probability of developing ITP following influenza vaccination, along with generally favorable prognostic outcomes. This study provides no evidence to support a causal relationship between influenza vaccination and immune thrombocytopenia. Therefore, while maintaining the current influenza vaccination strategies, the monitoring of abnormal vaccination reactions should be further enhanced. Furthermore, larger observational studies are required to more accurately assess the potential relationship between ITP and influenza vaccination. More longitudinal and diverse research across varied communities is needed to investigate the long-term consequences and potential demographic disparities in immunological responses. Future research should look into the function of adjuvants or other components in vaccinations and their possible impact on immune response and autoimmune consequences. In addition, the development of ITP after other vaccinations has been reported in several studies, and it is equally interesting to ponder whether there is an association between them. This area of research warrants further investigation to enhance our understanding of vaccine-related adverse events and inform vaccination practices.
Author Contributions
Data extraction and paper writing, Z.L. (Zhicai Liu) and J.W.; data checking, J.D. and J.H.; data analysis, Z.L. (Zhaojun Lu) and X.Z.; paper guidance and revision, Y.X. and Y.L.; Z.L. and J.W. contributed equally to this work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Hangzhou Center for Disease Control and Prevention (protocol code 2023-KS10 and 8 December 2023).
Data Availability Statement
All studies we included were searchable on Web of Science, Embase, or PubMed databases.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Medical Science and Technology Project of Zhejiang Province (grant number 2024KY233).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO Overview. [(accessed on 13 October 2024)]. Available online: https://www.who.int/health-topics/influenza-seasonal#tab=tab_1.
- 2.Iuliano A.D., Roguski K.M., Chang H.H., Muscatello D.J., Palekar R., Tempia S., Cohen C., Gran J.M., Schanzer D., Cowling B.J., et al. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018;391:1285–1300. doi: 10.1016/S0140-6736(17)33293-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bekkat-Berkani R., Wilkinson T., Buchy P., Dos Santos G., Stefanidis D., Devaster J.M., Meyer N. Seasonal influenza vaccination in patients with COPD: A systematic literature review. BMC Pulm. Med. 2017;17:79. doi: 10.1186/s12890-017-0420-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohseni H., Kiran A., Khorshidi R., Rahimi K. Influenza vaccination and risk of hospitalization in patients with heart failure: A self-controlled case series study. Eur. Heart J. 2017;38:326–333. doi: 10.1093/eurheartj/ehw411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sekiya T., Ohno M., Nomura N., Handabile C., Shingai M., Jackson D.C., Brown L.E., Kida H. Selecting and Using the Appropriate Influenza Vaccine for Each Individual. Viruses. 2021;13:973. doi: 10.3390/v13060971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley M.Z., Halsey N.A., Omer S.B., Orenstein W.A., O’Leary S.T., Limaye R.J., Salmon D.A. The state of vaccine safety science: Systematic reviews of the evidence. Lancet Infect. Dis. 2020;20:e80–e89. doi: 10.1016/S1473-3099(20)30130-4. [DOI] [PubMed] [Google Scholar]
- 7.DeStefano F., Bodenstab H.M., Offit P.A. Principal Controversies in Vaccine Safety in the United States. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2019;69:726–731. doi: 10.1093/cid/ciz135. [DOI] [PubMed] [Google Scholar]
- 8.Olivieri B., Betterle C., Zanoni G. Vaccinations and Autoimmune Diseases. Vaccines. 2021;9:815. doi: 10.3390/vaccines9080815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter K. Influenza Vaccine. JAMA. 2020;324:1476. doi: 10.1001/jama.2020.16846. [DOI] [PubMed] [Google Scholar]
- 10.Martín Arias L.H., Sanz R., Sáinz M., Treceño C., Carvajal A. Guillain-Barré syndrome and influenza vaccines: A meta-analysis. Vaccine. 2015;33:3773–3778. doi: 10.1016/j.vaccine.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Haber P., Moro P.L., Cano M., Lewis P., Stewart B., Shimabukuro T.T. Post-licensure surveillance of quadrivalent live attenuated influenza vaccine United States, Vaccine Adverse Event Reporting System (VAERS), July 2013–June 2014. Vaccine. 2015;33:1987–1992. doi: 10.1016/j.vaccine.2015.01.080. [DOI] [PubMed] [Google Scholar]
- 12.Cooper N., Ghanima W. Immune Thrombocytopenia. N. Engl. J. Med. 2019;381:945–955. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 13.Schoonen W.M., Kucera G., Coalson J., Li L., Rutstein M., Mowat F., Fryzek J., Kaye J.A. Epidemiology of immune thrombocytopenic purpura in the General Practice Research Database. Br. J. Haematol. 2009;145:235–244. doi: 10.1111/j.1365-2141.2009.07615.x. [DOI] [PubMed] [Google Scholar]
- 14.Neylon A.J., Saunders P.W., Howard M.R., Proctor S.J., Taylor P.R. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: A prospective study of a population-based cohort of 245 patients. Br. J. Haematol. 2003;122:966–974. doi: 10.1046/j.1365-2141.2003.04547.x. [DOI] [PubMed] [Google Scholar]
- 15.David P., Shoenfeld Y. ITP following vaccination. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;99:243–244. doi: 10.1016/j.ijid.2020.07.085. [DOI] [PubMed] [Google Scholar]
- 16.Moulis G., Sommet A., Sailler L., Lapeyre-Mestre M., Montastruc J.L. Drug-induced immune thrombocytopenia: A descriptive survey in the French PharmacoVigilance database. Platelets. 2012;23:490–494. doi: 10.3109/09537104.2011.633179. [DOI] [PubMed] [Google Scholar]
- 17.Black C., Kaye J.A., Jick H. MMR vaccine and idiopathic thrombocytopaenic purpura. Br. J. Clin. Pharmacol. 2003;55:107–111. doi: 10.1046/j.1365-2125.2003.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woo E.J., Wise R.P., Menschik D., Shadomy S.V., Iskander J., Beeler J., Varricchio F., Ball R. Thrombocytopenia after vaccination: Case reports to the US Vaccine Adverse Event Reporting System, 1990–2008. Vaccine. 2011;29:1319–1323. doi: 10.1016/j.vaccine.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 19.Jadavji T., Scheifele D., Halperin S. Thrombocytopenia after immunization of Canadian children, 1992 to 2001. Pediatr. Infect. Dis. J. 2003;22:119–122. doi: 10.1097/01.inf.0000048961.08486.d1. [DOI] [PubMed] [Google Scholar]
- 20.Polat A., Akca H., Dagdeviren E. Severe thrombocytopenia after hepatitis B vaccine in an infant from Turkey. Vaccine. 2008;26:6495–6496. doi: 10.1016/j.vaccine.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 21.Arya L.S., Ghai O.P., Saraya A.K. Thrombocytopenic purpura following DPT vaccination. Pediatr. Hematol. Oncol. 1993;10:381–383. doi: 10.3109/08880019309029521. [DOI] [PubMed] [Google Scholar]
- 22.Grimaldi-Bensouda L., Michel M., Aubrun E., Leighton P., Viallard J.F., Adoue D., Magy-Bertrand N., Tisserand G., Khellaf M., Durand J.M., et al. A case-control study to assess the risk of immune thrombocytopenia associated with vaccines. Blood. 2012;120:4938–4944. doi: 10.1182/blood-2012-05-431098. [DOI] [PubMed] [Google Scholar]
- 23.Garbe E., Andersohn F., Bronder E., Salama A., Klimpel A., Thomae M., Schrezenmeier H., Hildebrandt M., Späth-Schwalbe E., Grüneisen A., et al. Drug-induced immune thrombocytopaenia: Results from the Berlin Case-Control Surveillance Study. Eur. J. Clin. Pharmacol. 2012;68:821–832. doi: 10.1007/s00228-011-1184-3. [DOI] [PubMed] [Google Scholar]
- 24.Karafillakis E., Larson H.J. The benefit of the doubt or doubts over benefits? A systematic literature review of perceived risks of vaccines in European populations. Vaccine. 2017;35:4840–4850. doi: 10.1016/j.vaccine.2017.07.061. [DOI] [PubMed] [Google Scholar]
- 25.Shlamovitz G.Z., Johar S. A case of Evans’ syndrome following influenza vaccine. J. Emerg. Med. 2013;44:e149–e151. doi: 10.1016/j.jemermed.2012.01.060. [DOI] [PubMed] [Google Scholar]
- 26.Mantadakis E., Farmaki E., Thomaidis S., Tsalkidis A., Chatzimichael A. A case of immune thrombocytopenic purpura after influenza vaccination: Consequence or coincidence? J. Pediatr. Hematol./Oncol. 2010;32:e227–e229. doi: 10.1097/MPH.0b013e3181e33fe0. [DOI] [PubMed] [Google Scholar]
- 27.Asiimwe E., Kahlon K.S. Acute Immune Thrombocytopenia Following Influenza Vaccination in a Patient With Untreated Helicobacter pylori Infection. Cureus. 2023;15:e43946. doi: 10.7759/cureus.43946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Almohammadi A., Lundin M.S., Abro C., Hrinczenko B. Epistaxis and gross haematuria with severe thrombocytopaenia associated with influenza vaccination. BMJ Case Rep. 2019;12:e229423. doi: 10.1136/bcr-2019-229423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cummins D., Wilson M.E., Foulger K.J., Dawson D., Hogarth A.M. Haematological changes associated with influenza vaccination in people aged over 65: Case report and prospective study. Clin. Lab. Haematol. 1998;20:285–287. doi: 10.1046/j.1365-2257.1998.00149.x. [DOI] [PubMed] [Google Scholar]
- 30.Ikegame K., Kaida K., Fujioka T., Kawakami M., Hasei H., Inoue T., Taniguchi Y., Yoshihara S., Hayashi S., Kurata Y., et al. Idiopathic thrombocytopenic purpura after influenza vaccination in a bone marrow transplantation recipient. Bone Marrow Transplant. 2006;38:323–324. doi: 10.1038/sj.bmt.1705442. author reply 324–325. [DOI] [PubMed] [Google Scholar]
- 31.Tishler M., Levy O., Amit-Vazina M. Immune thrombocytopenic purpura following influenza vaccination. Isr. Med. Assoc. J. IMAJ. 2006;8:322–323. [PubMed] [Google Scholar]
- 32.Nagasaki J., Manabe M., Ido K., Ichihara H., Aoyama Y., Ohta T., Furukawa Y., Mugitani A. Postinfluenza Vaccination Idiopathic Thrombocytopenic Purpura in Three Elderly Patients. Case Rep. Hematol. 2016;2016:7913092. doi: 10.1155/2016/7913092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamiel U., Kventsel I., Youngster I. Recurrent Immune Thrombocytopenia After Influenza Vaccination: A Case Report. Pediatrics. 2016;138:e20160124. doi: 10.1542/peds.2016-0124. [DOI] [PubMed] [Google Scholar]
- 34.Ohta R., Sano C. Severe Immune Thrombocytopenic Purpura Following Influenza Vaccination: A Case Report. Cureus. 2022;14:e21250. doi: 10.7759/cureus.21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mamori S., Amano K., Kijima H., Takagi I., Tajiri H. Thrombocytopenic purpura after the administration of an influenza vaccine in a patient with autoimmune liver disease. Digestion. 2008;77:159–160. doi: 10.1159/000140977. [DOI] [PubMed] [Google Scholar]
- 36.Wan Jamaludin W.F., Kok W.H., Loong L., Palaniappan S.K., Zakaria M.Z., Ong T.C., Abdul Wahid S.F. Vaccine-induced immune thrombocytopaenia purpura in autologous haematopoietic stem cell transplantation. Med. J. Malays. 2018;73:430–432. [PubMed] [Google Scholar]
- 37.Kelton J.G. Vaccination-Associated relapse of immune thrombocytopenia. JAMA. 1981;245:369–370. doi: 10.1001/jama.1981.03310290037020. [DOI] [PubMed] [Google Scholar]
- 38.Mateos M., Arguinano J.M., Ardaiz M.A., Burguete Y., Montoya M.C., Jarne V., Redondo A.M., Paloma M.J., Labaca M.A., Ezpeleta I., et al. Immune thrombocytopenia following influenza vaccination in a patient with chronic lymphocytic leukaemia. Haematol. -Hematol. J. 2007;92:478. [Google Scholar]
- 39.Yokomichi H., Tanaka-Taya K., Koshida R., Nakano T., Yasui Y., Mori M., Ando Y., Morino S., Okuno H., Satoh H., et al. Immune thrombocytopenic purpura risk by live, inactivated and simultaneous vaccinations among Japanese adults, children and infants: A matched case–control study. Int. J. Hematol. 2020;112:105–114. doi: 10.1007/s12185-020-02866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lafaurie M., Lapeyre-Mestre M., Sailler L., Sommet A., Moulis G. Risk of Immune Thrombocytopenia After Influenza Vaccine. JAMA Intern. Med. 2022;182:444–445. doi: 10.1001/jamainternmed.2021.8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moro M.L., Nobilio L., Voci C., Di Mario S., Candela S., Magrini N. A population based cohort study to assess the safety of pandemic influenza vaccine Focetria® in Emilia-Romagna region, Italy-Part Two. Vaccine. 2013;31:1438–1446. doi: 10.1016/j.vaccine.2012.07.090. [DOI] [PubMed] [Google Scholar]
- 42.Nordin J.D., Kharbanda E.O., Benitez G.V., Nichol K., Lipkind H., Naleway A., Lee G.M., Hambidge S., Shi W., Olsen A. Maternal Safety of Trivalent Inactivated Influenza Vaccine in Pregnant Women. Obstet. Gynecol. 2013;121:519–525. doi: 10.1097/AOG.0b013e3182831b83. [DOI] [PubMed] [Google Scholar]
- 43.Nordin J.D., Kharbanda E.O., Vazquez-Benitez G., Lipkind H., Lee G.M., Naleway A.L. Monovalent H1N1 influenza vaccine safety in pregnant women, risks for acute adverse events. Vaccine. 2014;32:4985–4992. doi: 10.1016/j.vaccine.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 44.Villa M., Black S., Groth N., Rothman K.J., Apolone G., Weiss N.S., Aquino I., Boldori L., Caramaschi F., Gattinoni A., et al. Safety of MF59-adjuvanted influenza vaccination in the elderly: Results of a comparative study of mf59-adjuvanted vaccine versus nonadjuvanted influenza vaccine in Northern Italy. Am. J. Epidemiol. 2013;178:1139–1145. doi: 10.1093/aje/kwt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W.T., Yang H.W., Liao T.L., Wu W.J., Yang S.E., Chih Y.C., Chuang J.H. Safety of Pandemic (H1N1) 2009 Monovalent Vaccines in Taiwan: A Self-Controlled Case Series Study. PLoS ONE. 2013;8:e58827. doi: 10.1371/journal.pone.0058827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O’Leary S.T., Glanz J.M., McClure D.L., Akhtar A., Daley M.F., Nakasato C., Baxter R., Davis R.L., Izurieta H.S., Lieu T.A., et al. The risk of immune thrombocytopenic purpura after vaccination in children and adolescents. Pediatrics. 2012;129:248–255. doi: 10.1542/peds.2011-1111. [DOI] [PubMed] [Google Scholar]
- 47.Sandal R., Mishra K., Jandial A., Sahu K.K., Siddiqui A.D. Update on diagnosis and treatment of immune thrombocytopenia. Expert Rev. Clin. Pharmacol. 2021;14:553–568. doi: 10.1080/17512433.2021.1903315. [DOI] [PubMed] [Google Scholar]
- 48.Moulis G., Palmaro A., Montastruc J.L., Godeau B., Lapeyre-Mestre M., Sailler L. Epidemiology of incident immune thrombocytopenia: A nationwide population-based study in France. Blood. 2014;124:3308–3315. doi: 10.1182/blood-2014-05-578336. [DOI] [PubMed] [Google Scholar]
- 49.Schifferli A., Holbro A., Chitlur M., Coslovsky M., Imbach P., Donato H., Elalfy M., Graciela E., Grainger J., Holzhauer S., et al. A comparative prospective observational study of children and adults with immune thrombocytopenia: 2-year follow-up. Am. J. Hematol. 2018;93:751–759. doi: 10.1002/ajh.25086. [DOI] [PubMed] [Google Scholar]
- 50.Yasuda H., Nagata M., Moriyama H., Kobayashi H., Akisaki T., Ueda H., Hara K., Yokono K. Development of fulminant Type 1 diabetes with thrombocytopenia after influenza vaccination: A case report. Diabet. Med. J. Br. Diabet. Assoc. 2012;29:88–89. doi: 10.1111/j.1464-5491.2011.03391.x. [DOI] [PubMed] [Google Scholar]
- 51.Kühne T., Berchtold W., Michaels L.A., Wu R., Donato H., Espina B., Tamary H., Rodeghiero F., Chitlur M., Rischewski J., et al. Newly diagnosed immune thrombocytopenia in children and adults: A comparative prospective observational registry of the Intercontinental Cooperative Immune Thrombocytopenia Study Group. Haematologica. 2011;96:1831–1837. doi: 10.3324/haematol.2011.050799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neunert C., Terrell D.R., Arnold D.M., Buchanan G., Cines D.B., Cooper N., Cuker A., Despotovic J.M., George J.N., Grace R.F., et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Michel M., Rauzy O.B., Thoraval F.R., Languille L., Khellaf M., Bierling P., Godeau B. Characteristics and outcome of immune thrombocytopenia in elderly: Results from a single center case-controlled study. Am. J. Hematol. 2011;86:980–984. doi: 10.1002/ajh.22170. [DOI] [PubMed] [Google Scholar]
- 54.Elsaid M., Nune A., Brakat A.M., Anand A., Alashwah M., Maher A., Lama N., Peñamante C.A.C. Immune thrombocytopenic purpura after influenza vaccine administration; a systematic review and meta-analysis. Trop. Dis. Travel Med. Vaccines. 2023;9:22. doi: 10.1186/s40794-023-00206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gan G., Liu H., Liang Z., Zhang G., Liu X., Ma L. Vaccine-associated thrombocytopenia. Thromb. Res. 2022;220:12–20. doi: 10.1016/j.thromres.2022.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Saudagar V., Patil S., Goh S., Pothiawala S. Vigilance regarding immune thrombocytopenic purpura after COVID-19 vaccine. Ir. J. Med. Sci. 2022;191:919–920. doi: 10.1007/s11845-021-02614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terada H., Baldini M., Ebbe S., Madoff M.A. Interaction of influenza virus with blood platelets. Blood. 1966;28:213–228. doi: 10.1182/blood.V28.2.213.213. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All studies we included were searchable on Web of Science, Embase, or PubMed databases.