Abstract
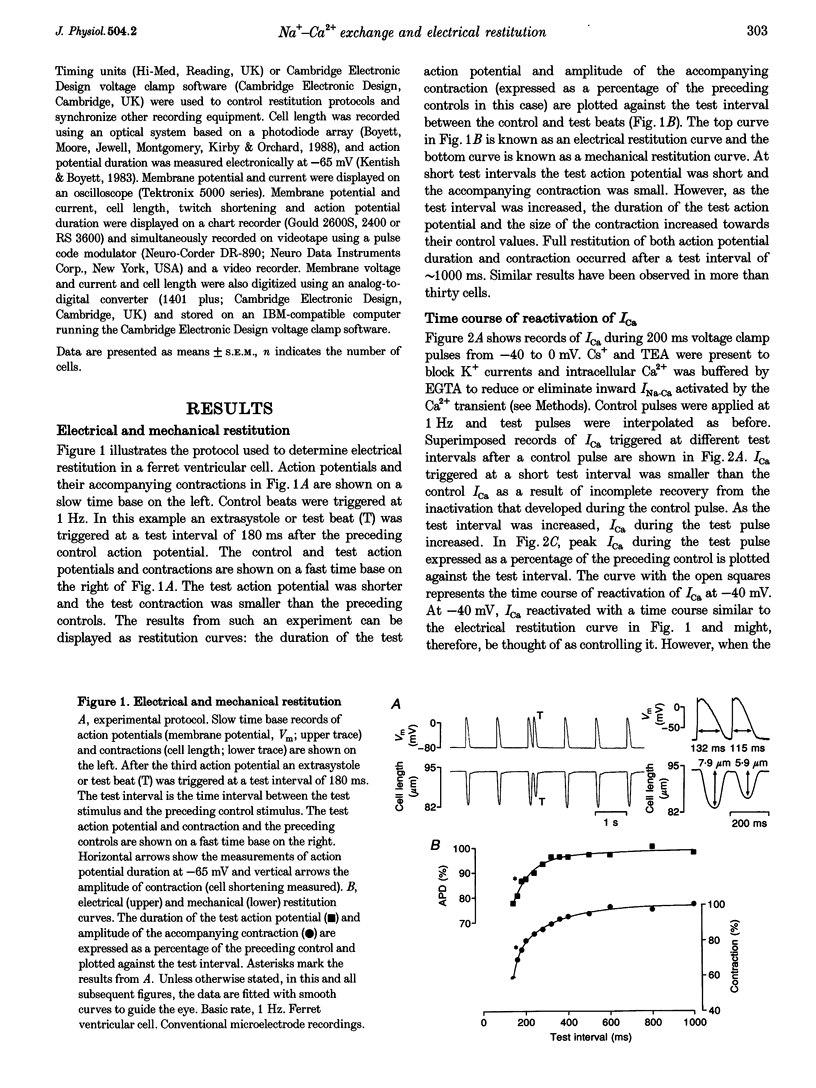
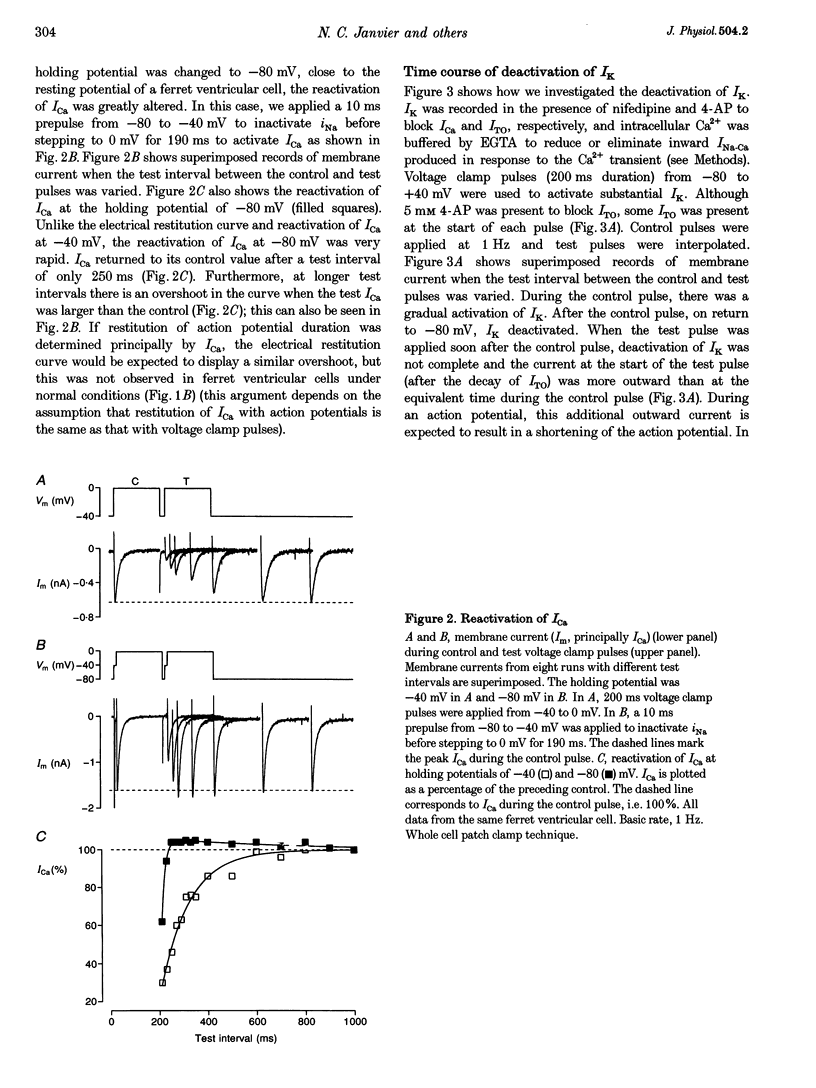
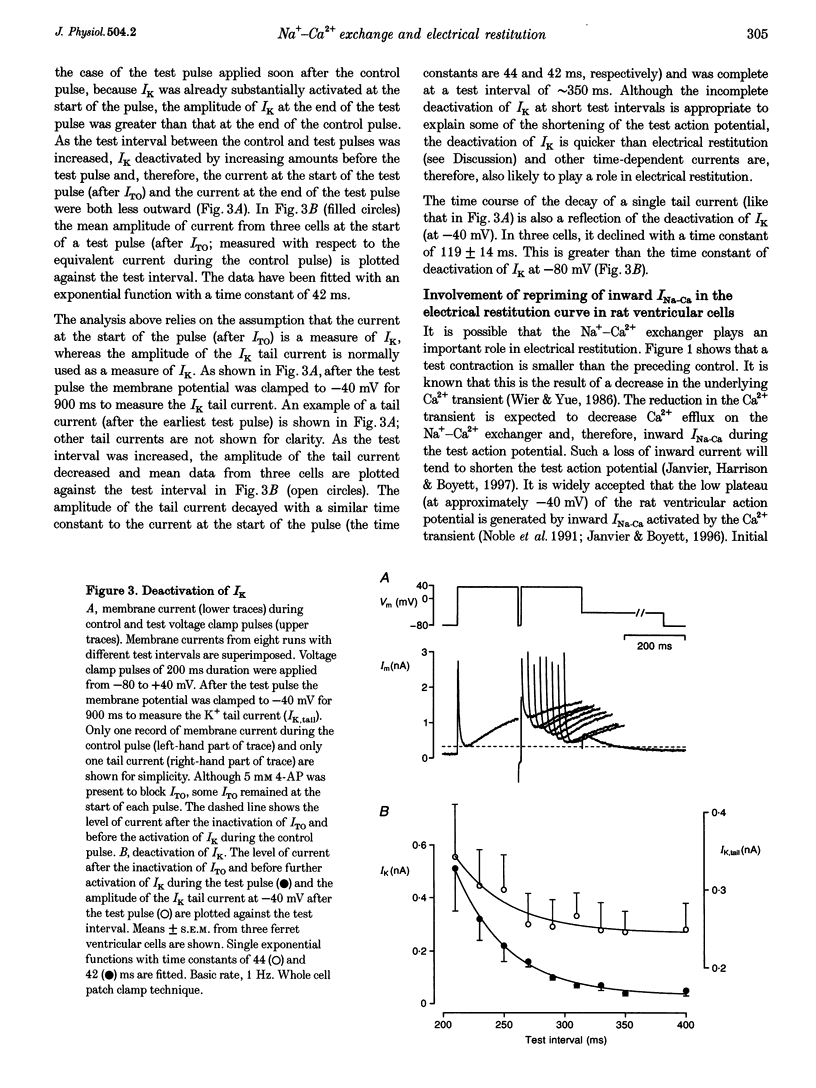
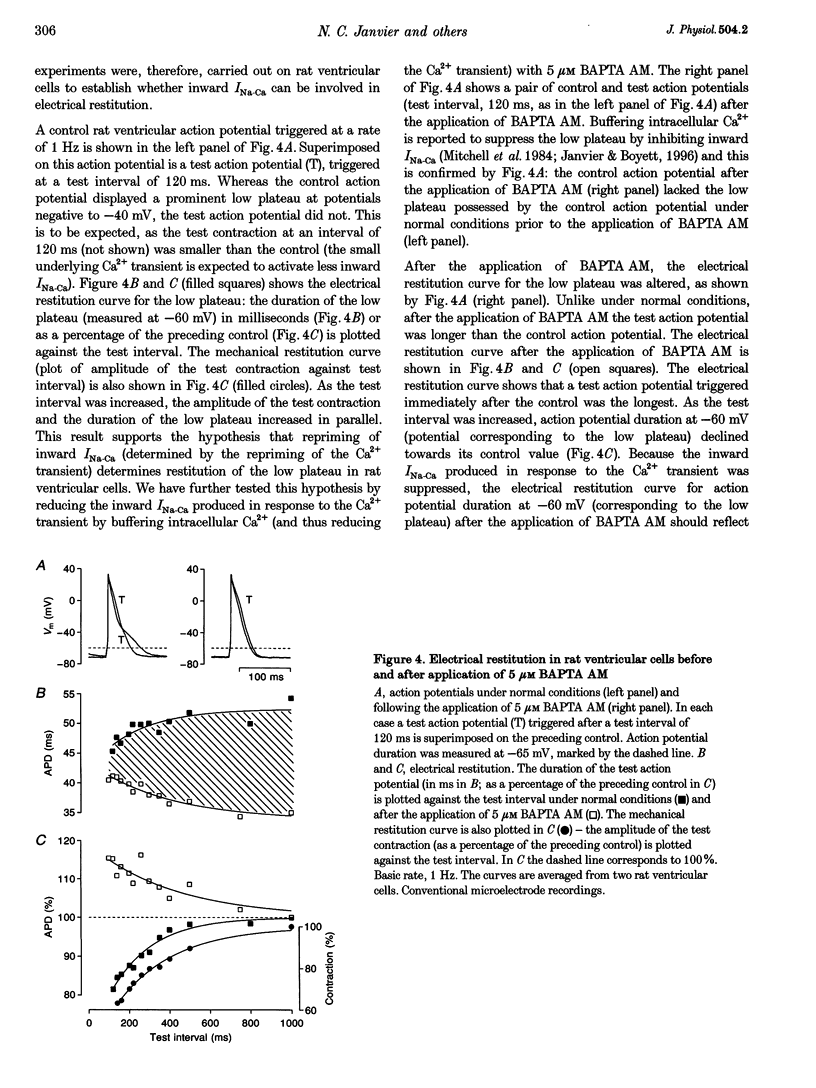
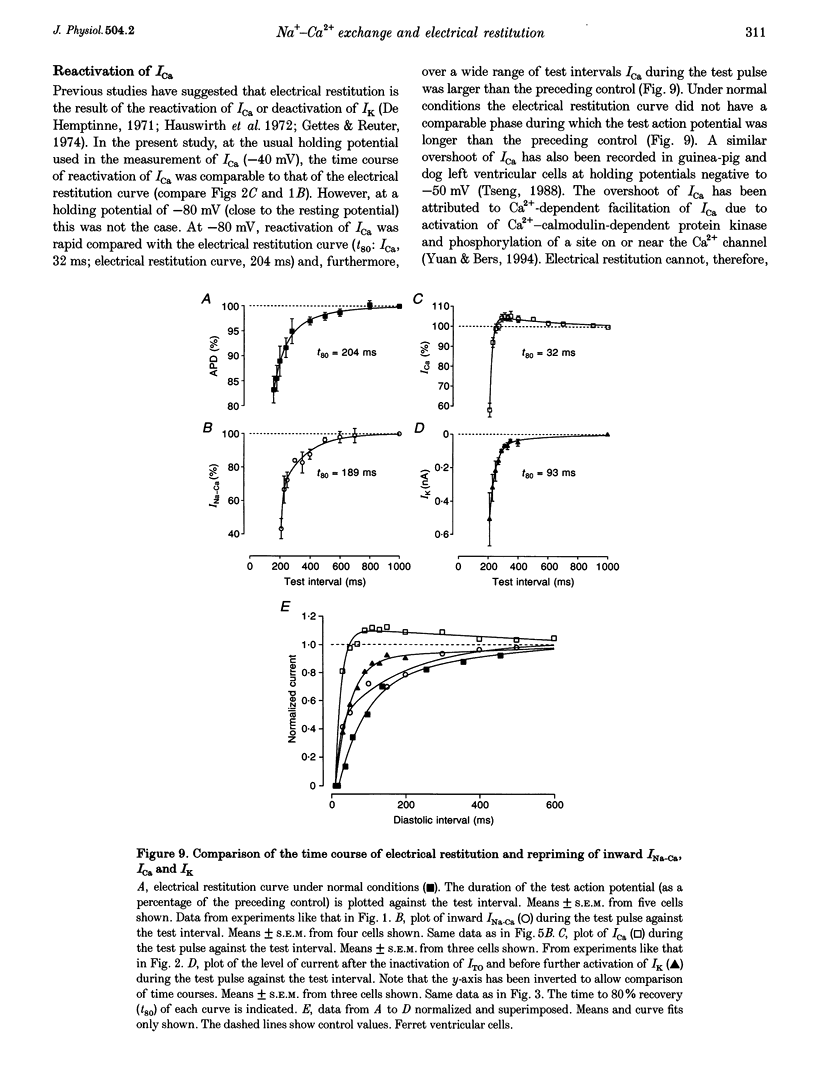
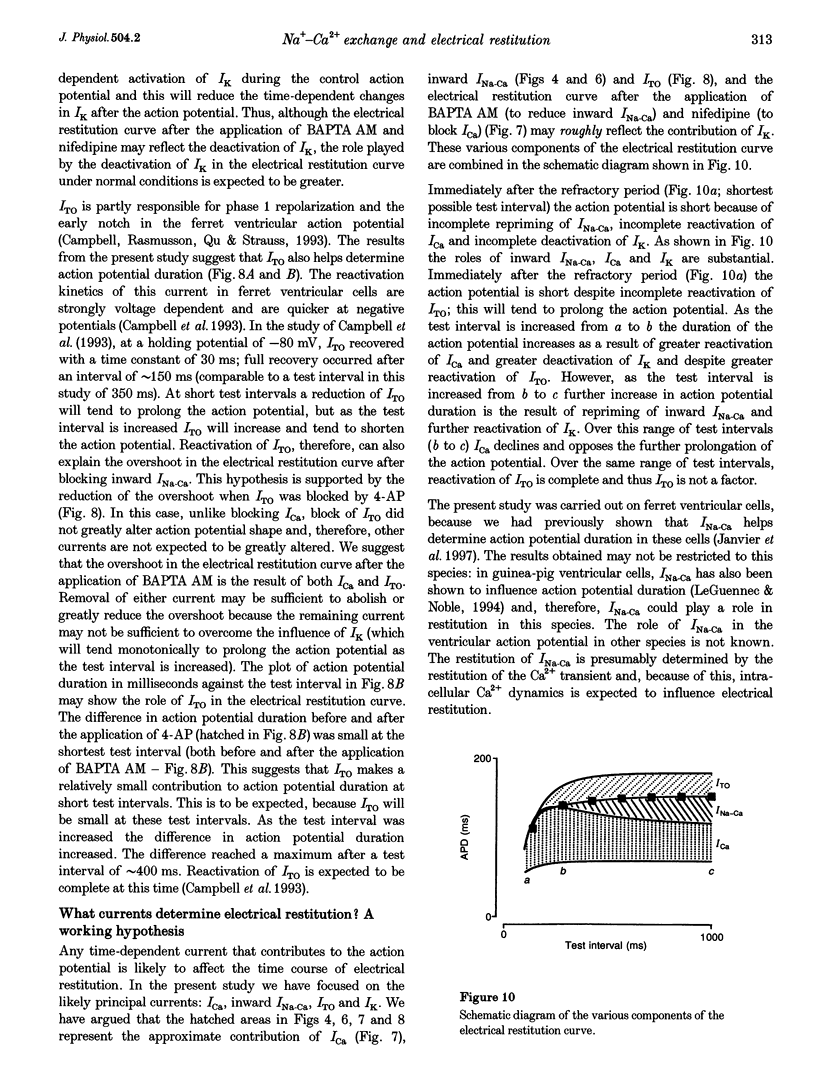
1. The mechanisms underlying electrical restitution (recovery of action potential duration after a preceding beat) were investigated in ferret ventricular cells. The time to 80% recovery (t80) of action potential duration was approximately 204 ms. 2. At a holding potential of -80 mV, the Ca2+ current (ICa) reactivated and the delayed rectifier K+ current (IK) deactivated very rapidly (t80: approximately 32 and approximately 93 ms, respectively). The kinetics of both currents are too fast to account for electrical restitution alone. 3. The putative inward Na(+)-Ca2+ exchange current (INa-Ca) produced by the Na(+)-Ca2+ exchanger in response to the intracellular Ca2+ transient reprimed (t80: 189 ms) with the same time course as mechanical restitution (recovery of contraction) and with a similar time course to electrical restitution. 4. Substantial reduction of inward INa-Ca, by buffering intracellular Ca2+ with the acetyl methyl ester form of BAPTA, shortened the action potential and greatly altered the electrical restitution curve. Subsequent addition of nifedipine (to block ICa) or 4-aminopyridine (4-AP) (to block the transient outward current, ITO) further altered the electrical restitution curve. 5. Any time-dependent current that contributes to the action potential is likely to affect the time course of electrical restitution. Although ICa, IK and ITO were previously thought to be the only currents involved in electrical restitution, we conclude that inward INa-Ca also plays an important role.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyett M. R., Moore M., Jewell B. R., Montgomery R. A., Kirby M. S., Orchard C. H. An improved apparatus for the optical recording of contraction of single heart cells. Pflugers Arch. 1988 Dec;413(2):197–205. doi: 10.1007/BF00582531. [DOI] [PubMed] [Google Scholar]
- Campbell D. L., Rasmusson R. L., Qu Y., Strauss H. C. The calcium-independent transient outward potassium current in isolated ferret right ventricular myocytes. I. Basic characterization and kinetic analysis. J Gen Physiol. 1993 Apr;101(4):571–601. doi: 10.1085/jgp.101.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell M. B., Lederer W. J. A novel experimental chamber for single-cell voltage-clamp and patch-clamp applications with low electrical noise and excellent temperature and flow control. Pflugers Arch. 1986 May;406(5):536–539. doi: 10.1007/BF00583378. [DOI] [PubMed] [Google Scholar]
- De Hemptinne A. The frequency dependence of outward current in frog auricular fibres. An experimental and theoretical study. Pflugers Arch. 1971;329(4):332–340. doi: 10.1007/BF00588004. [DOI] [PubMed] [Google Scholar]
- Gettes L. S., Reuter H. Slow recovery from inactivation of inward currents in mammalian myocardial fibres. J Physiol. 1974 Aug;240(3):703–724. doi: 10.1113/jphysiol.1974.sp010630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausworth O., Noble D., Tsien R. W. The dependence of plateau currents in cardiac Purkinje fibres on the interval between action potentials. J Physiol. 1972 Apr;222(1):27–49. doi: 10.1113/jphysiol.1972.sp009786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janse M. J., Wit A. L. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989 Oct;69(4):1049–1169. doi: 10.1152/physrev.1989.69.4.1049. [DOI] [PubMed] [Google Scholar]
- Janvier N. C., Boyett M. R. The role of Na-Ca exchange current in the cardiac action potential. Cardiovasc Res. 1996 Jul;32(1):69–84. [PubMed] [Google Scholar]
- Janvier N. C., Harrison S. M., Boyett M. R. The role of inward Na(+)-Ca2+ exchange current in the ferret ventricular action potential. J Physiol. 1997 Feb 1;498(Pt 3):611–625. doi: 10.1113/jphysiol.1997.sp021887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentish J. C., Boyett M. R. A simple electronic circuit for monitoring changes in the duration of the action potential. Pflugers Arch. 1983 Aug;398(3):233–235. doi: 10.1007/BF00657157. [DOI] [PubMed] [Google Scholar]
- Le Guennec J. V., Noble D. Effects of rapid changes of external Na+ concentration at different moments during the action potential in guinea-pig myocytes. J Physiol. 1994 Aug 1;478(Pt 3):493–504. doi: 10.1113/jphysiol.1994.sp020268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. R., Powell T., Terrar D. A., Twist V. W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br J Pharmacol. 1984 Mar;81(3):543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble D., Noble S. J., Bett G. C., Earm Y. E., Ho W. K., So I. K. The role of sodium-calcium exchange during the cardiac action potential. Ann N Y Acad Sci. 1991;639:334–353. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- Taggart P., Sutton P. M., Boyett M. R., Lab M., Swanton H. Human ventricular action potential duration during short and long cycles. Rapid modulation by ischemia. Circulation. 1996 Nov 15;94(10):2526–2534. doi: 10.1161/01.cir.94.10.2526. [DOI] [PubMed] [Google Scholar]
- Tseng G. N. Calcium current restitution in mammalian ventricular myocytes is modulated by intracellular calcium. Circ Res. 1988 Aug;63(2):468–482. doi: 10.1161/01.res.63.2.468. [DOI] [PubMed] [Google Scholar]
- Wier W. G., Yue D. T. Intracellular calcium transients underlying the short-term force-interval relationship in ferret ventricular myocardium. J Physiol. 1986 Jul;376:507–530. doi: 10.1113/jphysiol.1986.sp016167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W., Bers D. M. Ca-dependent facilitation of cardiac Ca current is due to Ca-calmodulin-dependent protein kinase. Am J Physiol. 1994 Sep;267(3 Pt 2):H982–H993. doi: 10.1152/ajpheart.1994.267.3.H982. [DOI] [PubMed] [Google Scholar]


