Abstract
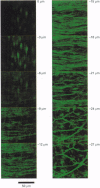
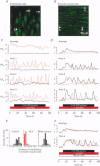
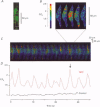
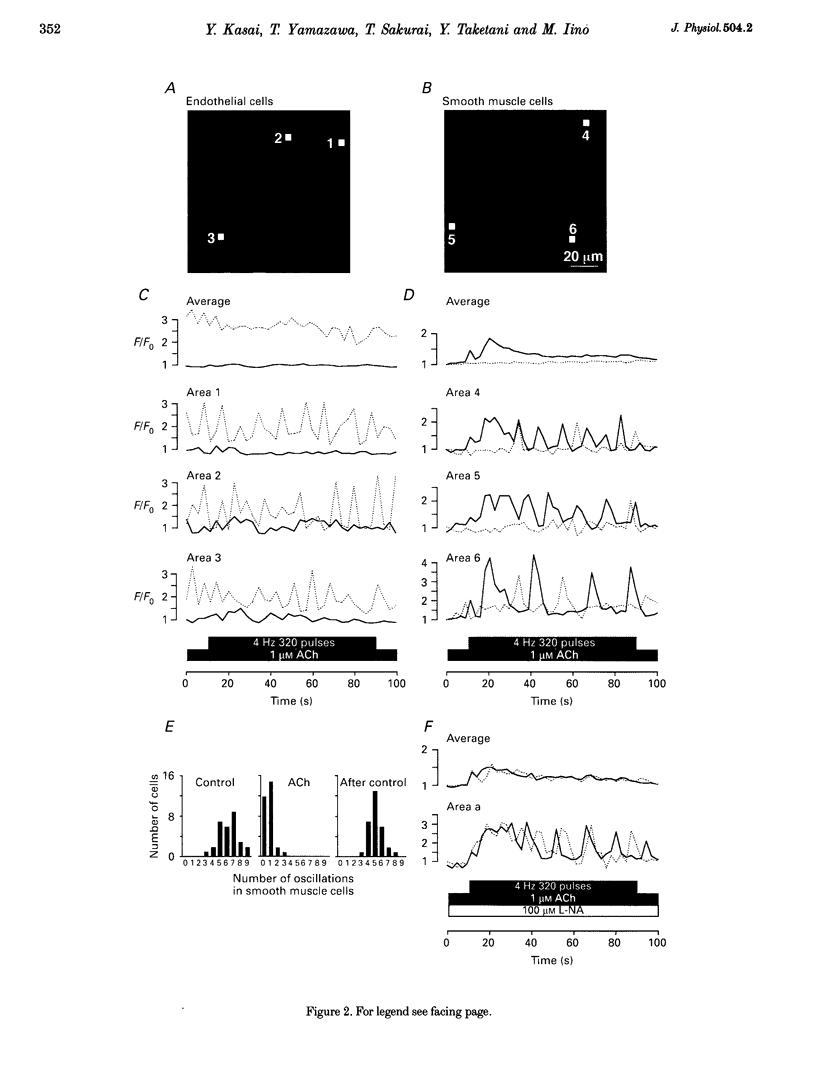
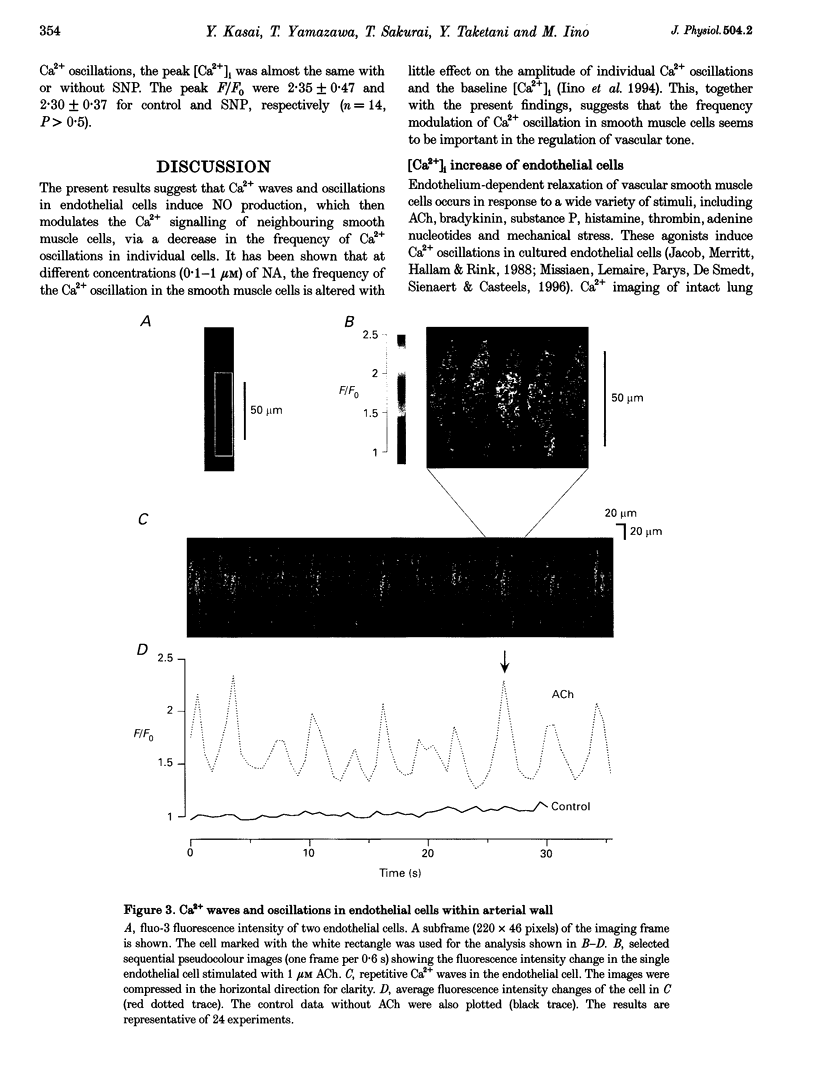
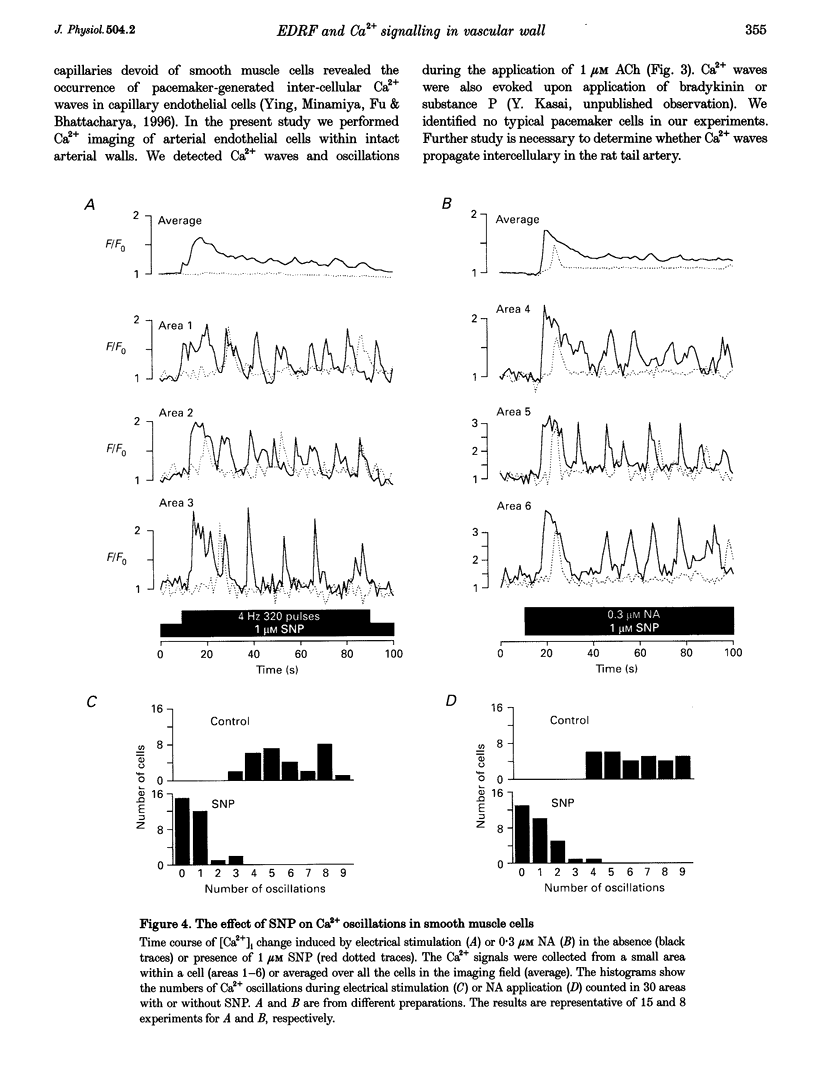
1. We visualized intracellular Ca2+ concentration ([Ca2+]i) changes, using fluo-3 as an indicator, of individual vascular smooth muscle cells and endothelial cells within intact rat tail arteries by confocal microscopy. 2. Using a piezo-driven objective, we focused on endothelial and smooth muscle cell layers alternately to obtain Ca2+ images of their cells. In the presence of 1 microM acetylcholine (ACh), individual endothelial cells responded with intermittent increases in the [Ca2+]i (Ca2+ oscillations). At the same time, the frequency of Ca2+ oscillations in smooth muscle cells induced by electrical stimulation of the perivascular sympathetic nerve was greatly decreased. 3. A [Ca2+]i rise during the oscillations in the endothelial cells propagated in the form of a wave along the long axis of the cells. 4. In the presence of a NO synthase inhibitor, no significant inhibitory effect of ACh on the Ca2+ signalling in the vascular smooth muscle cells was detected, although the Ca2+ oscillations in the endothelial cells persisted. 5. The inhibitory effect of ACh on the frequency of Ca2+ oscillations in the vascular smooth muscle cells was mimicked by 1 microM sodium nitroprusside, a NO donor. 6. These results indicate that Ca2+ waves and oscillations in vascular endothelial cells regulate NO production, which modulates vascular tone by decreasing the frequency of Ca2+ oscillations in smooth muscle cells activated by sympathetic agonists.
Full text
PDF

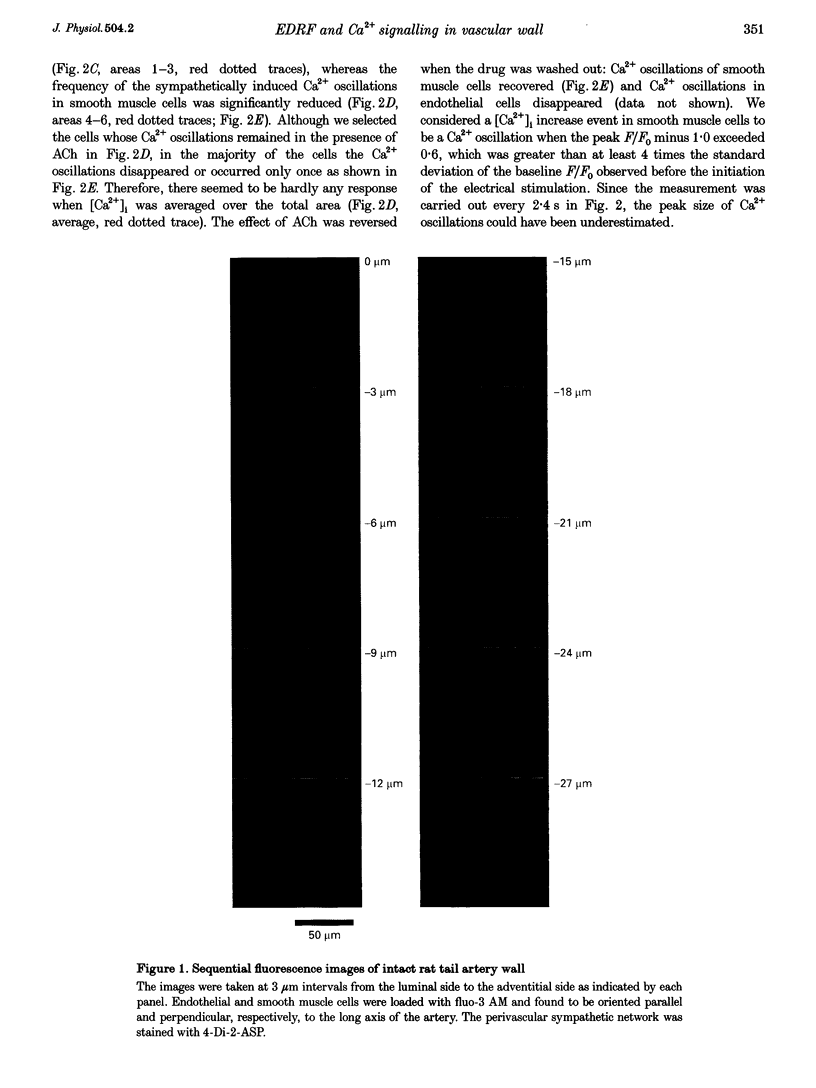



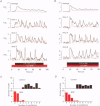
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andriantsitohaina R., Lagaud G. J., Andre A., Muller B., Stoclet J. C. Effects of cGMP on calcium handling in ATP-stimulated rat resistance arteries. Am J Physiol. 1995 Mar;268(3 Pt 2):H1223–H1231. doi: 10.1152/ajpheart.1995.268.3.H1223. [DOI] [PubMed] [Google Scholar]
- Bonev A., Isenberg G. Arginine-vasopressin induces mode-2 gating in L-type Ca2+ channels (smooth muscle cells of the urinary bladder of the guinea-pig). Pflugers Arch. 1992 Feb;420(2):219–222. doi: 10.1007/BF00374994. [DOI] [PubMed] [Google Scholar]
- Camacho P., Lechleiter J. D. Increased frequency of calcium waves in Xenopus laevis oocytes that express a calcium-ATPase. Science. 1993 Apr 9;260(5105):226–229. doi: 10.1126/science.8385800. [DOI] [PubMed] [Google Scholar]
- Clapp L. H., Gurney A. M. Modulation of calcium movements by nitroprusside in isolated vascular smooth muscle cells. Pflugers Arch. 1991 Jun;418(5):462–470. doi: 10.1007/BF00497774. [DOI] [PubMed] [Google Scholar]
- Garland C. J., Plane F., Kemp B. K., Cocks T. M. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995 Jan;16(1):23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Girard S., Clapham D. Acceleration of intracellular calcium waves in Xenopus oocytes by calcium influx. Science. 1993 Apr 9;260(5105):229–232. doi: 10.1126/science.8385801. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Stuehr D. J. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Hirata M., Kohse K. P., Chang C. H., Ikebe T., Murad F. Mechanism of cyclic GMP inhibition of inositol phosphate formation in rat aorta segments and cultured bovine aortic smooth muscle cells. J Biol Chem. 1990 Jan 25;265(3):1268–1273. [PubMed] [Google Scholar]
- Iino M., Kasai H., Yamazawa T. Visualization of neural control of intracellular Ca2+ concentration in single vascular smooth muscle cells in situ. EMBO J. 1994 Nov 1;13(21):5026–5031. doi: 10.1002/j.1460-2075.1994.tb06831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Koga T., Yoshida Y., Cai J. Q., Islam M. O., Imai S. Purification and characterization of 240-kDa cGMP-dependent protein kinase substrate of vascular smooth muscle. Close resemblance to inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1994 Apr 15;269(15):11640–11647. [PubMed] [Google Scholar]
- Komalavilas P., Lincoln T. M. Phosphorylation of the inositol 1,4,5-trisphosphate receptor by cyclic GMP-dependent protein kinase. J Biol Chem. 1994 Mar 25;269(12):8701–8707. [PubMed] [Google Scholar]
- Lincoln T. M., Cornwell T. L. Intracellular cyclic GMP receptor proteins. FASEB J. 1993 Feb 1;7(2):328–338. doi: 10.1096/fasebj.7.2.7680013. [DOI] [PubMed] [Google Scholar]
- Missiaen L., Lemaire F. X., Parys J. B., De Smedt H., Sienaert I., Casteels R. Initiation sites for Ca2+ signals in endothelial cells. Pflugers Arch. 1996 Jan;431(3):318–324. doi: 10.1007/BF02207268. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Rooney T. A., Joseph S. K., Queen C., Thomas A. P. Cyclic GMP induces oscillatory calcium signals in rat hepatocytes. J Biol Chem. 1996 Aug 16;271(33):19817–19825. doi: 10.1074/jbc.271.33.19817. [DOI] [PubMed] [Google Scholar]
- Saito S. Y., Hori M., Ozaki H., Karaki H. Cytochalasin D inhibits smooth muscle contraction by directly inhibiting contractile apparatus. J Smooth Muscle Res. 1996 Apr;32(2):51–60. doi: 10.1540/jsmr.32.51. [DOI] [PubMed] [Google Scholar]
- Ying X., Minamiya Y., Fu C., Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circ Res. 1996 Oct;79(4):898–908. doi: 10.1161/01.res.79.4.898. [DOI] [PubMed] [Google Scholar]
- Zhou X. B., Ruth P., Schlossmann J., Hofmann F., Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J Biol Chem. 1996 Aug 16;271(33):19760–19767. doi: 10.1074/jbc.271.33.19760. [DOI] [PubMed] [Google Scholar]