Abstract
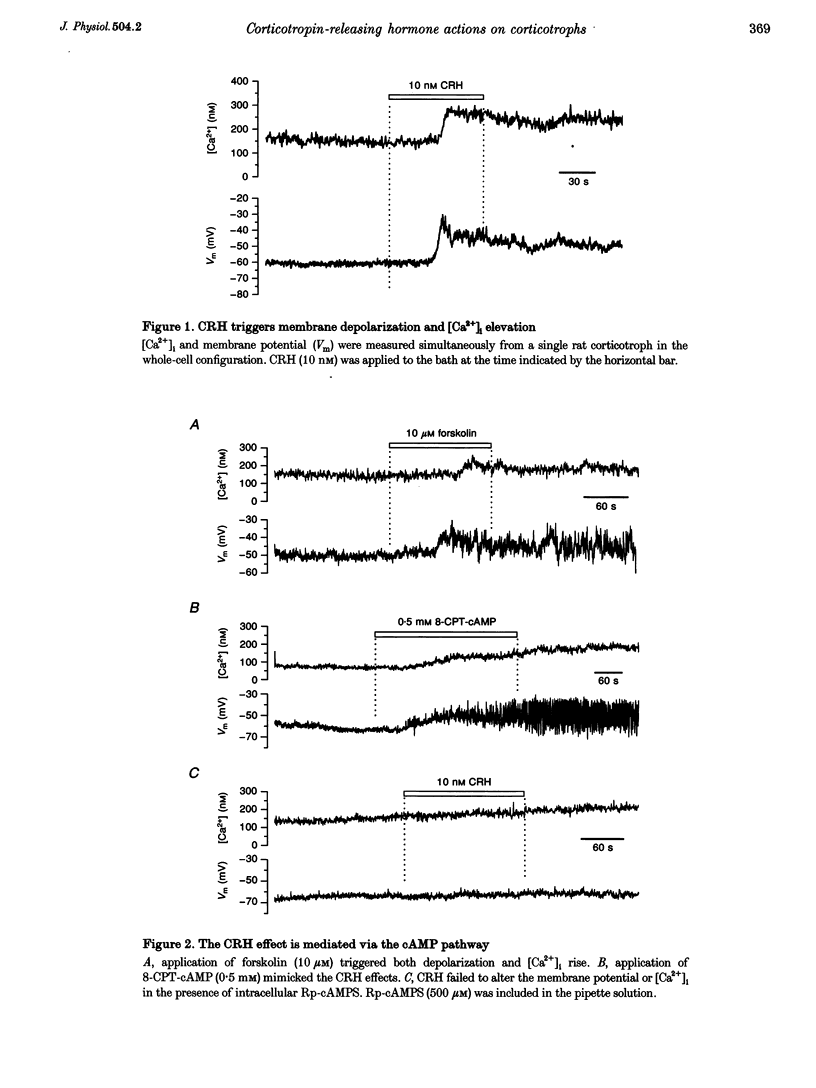
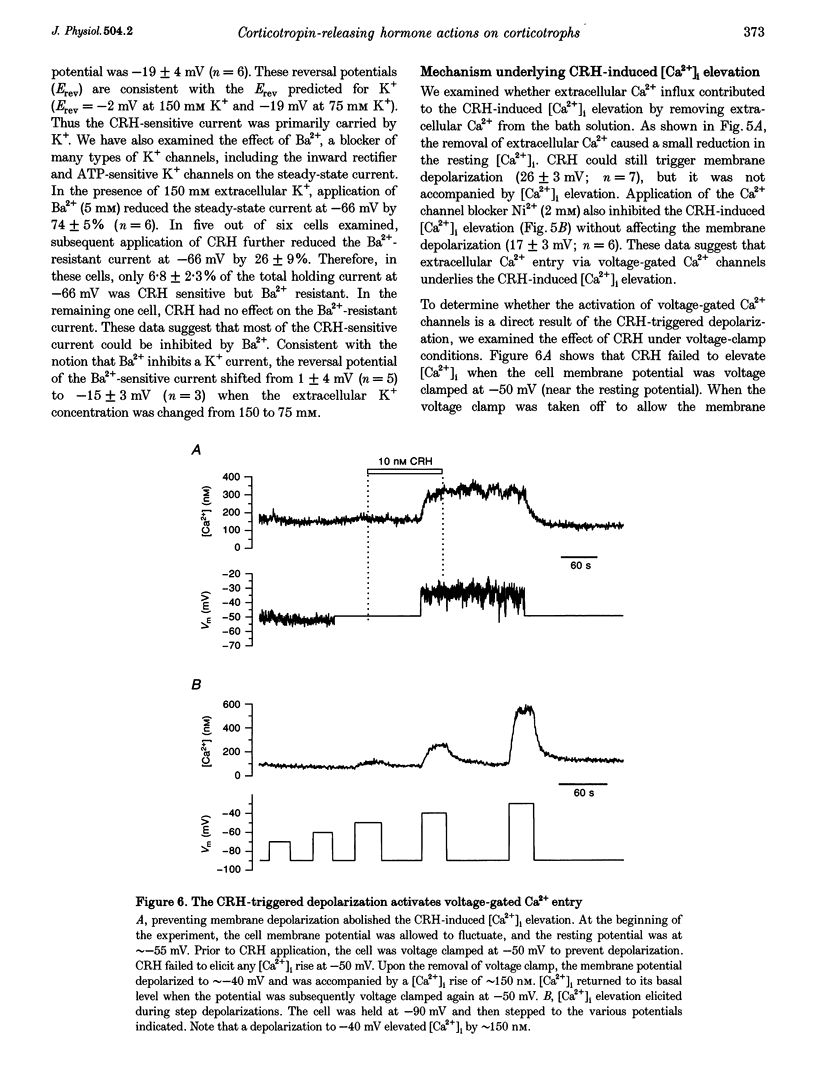
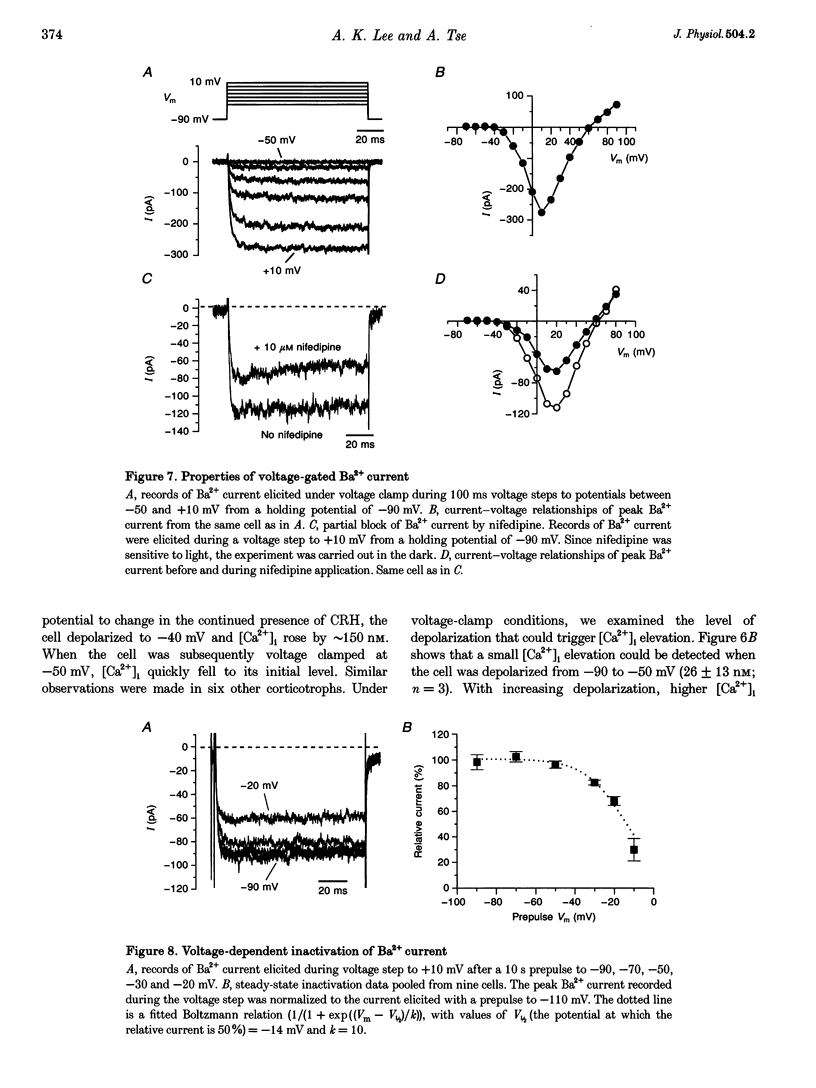
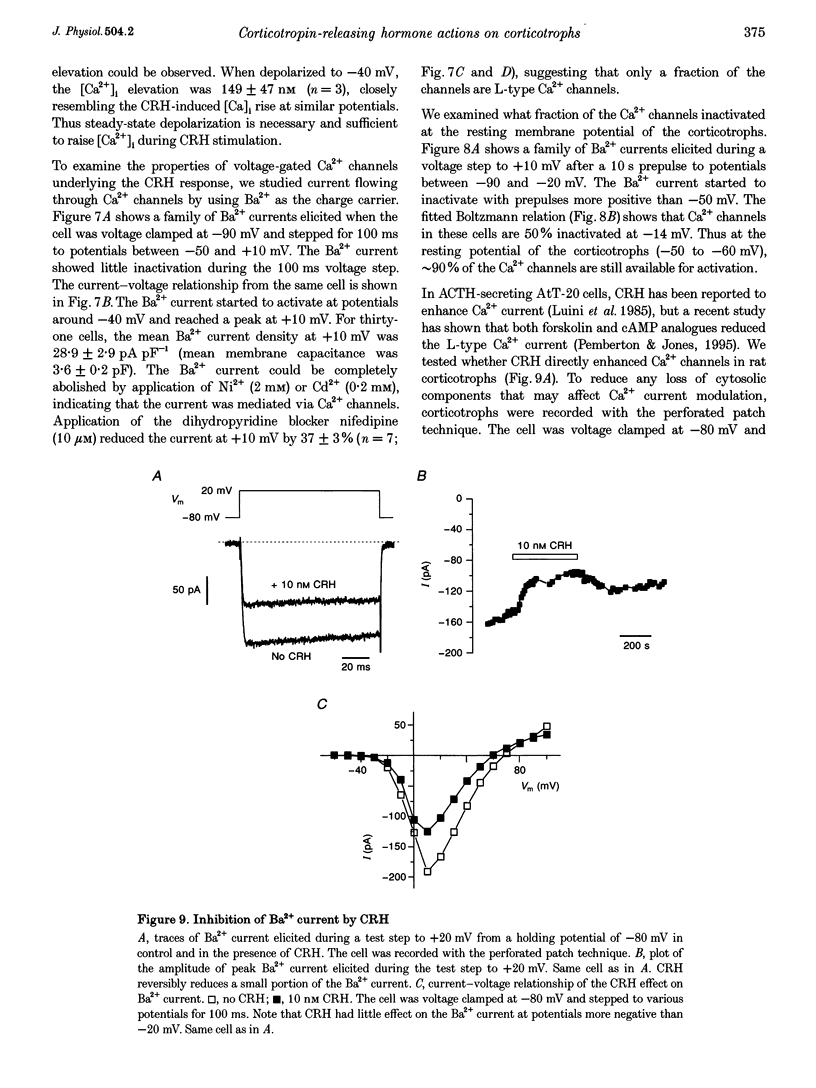
1. The patch-clamp technique was used in conjunction with the fluorescent Ca2+ indicator indo-1 to measure simultaneously cytosolic Ca2+ concentration ([Ca2+]i) and membrane potential in single rat corticotrophs identified with the reverse haemolytic plaque assay. 2. Application of the adrenocorticotropin (ACTH) secretagogue, corticotropin-releasing hormone (CRH), triggered a sustained [Ca2+]i elevation and membrane depolarization. 3. The CRH action was mediated via the cAMP-dependent protein kinase cascade. Both the CRH-induced depolarization and [Ca2+]i elevation could be mimicked by extracellular application of the adenylate cyclase activator forskolin or the membrane-permeable cAMP analogue, 8-(4-chlorophenylthio)-adenosine-3',5'-cyclic monophosphate (8-CPT-cAMP). Intracellular adenosine cyclic 3',5'-(Rp)-phosphothioate (Rp-cAMPS), a protein kinase A inhibitor, abolished the CRH effects. 4. Voltage-clamp studies suggest that the CRH-triggered depolarization was due to the reduction of background K+ conductances. The CRH-sensitive current was Ca2+ independent and was insensitive to the K+ channel blockers tetraethylammonium (TEA) or 4-aminopyridine (4-AP), but could be partially inhibited by Ba2+. 5. The CRH-triggered steady-state depolarization stimulated extracellular Ca2+ entry via voltage-gated Ca2+ channels and raised [Ca2+]i. CRH failed to stimulate [Ca2+]i rise in cells that were voltage clamped at their resting potential. Removal of extracellular Ca2+ or inhibition of Ca2+ channels by Ni2+ abolished the [Ca2+]i rise. 6. Voltage-clamp studies of voltage-gated Ca2+ channels using Ba2+ as charge carrier show that approximately 90% of the channels were available for activation at the resting potential. CRH did not enhance the voltage-gated Ca2+ channels.
Full text
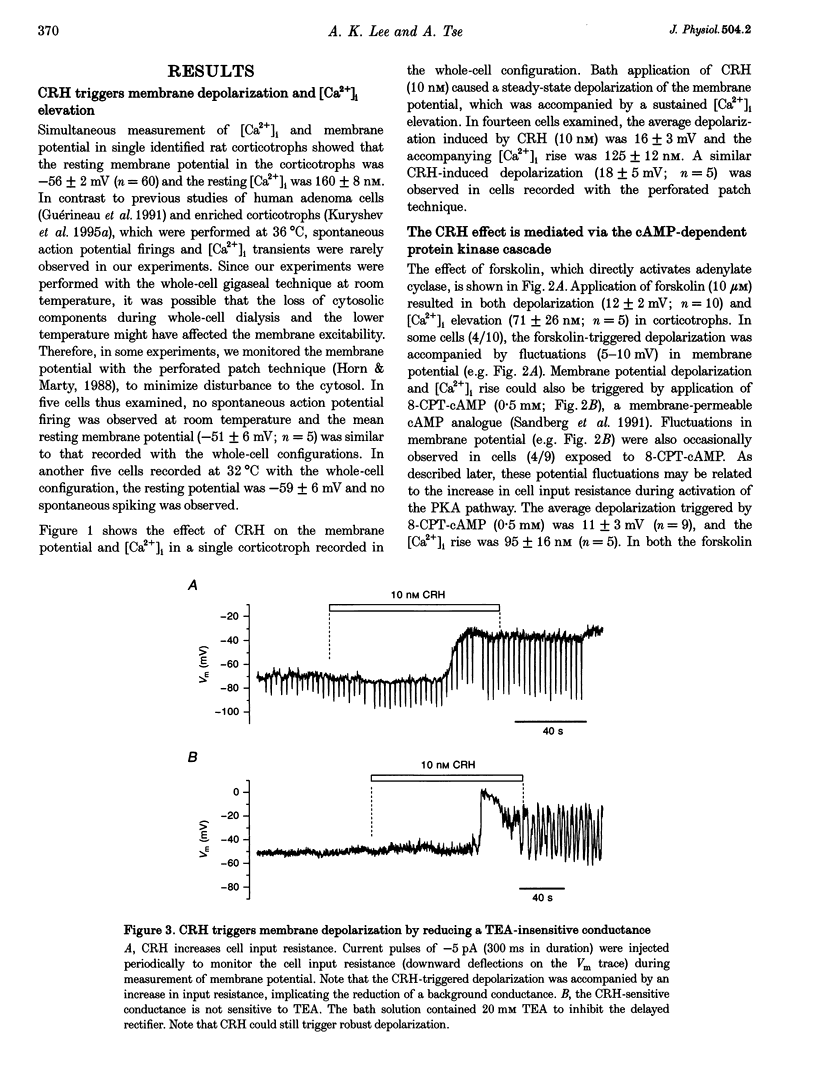
PDF

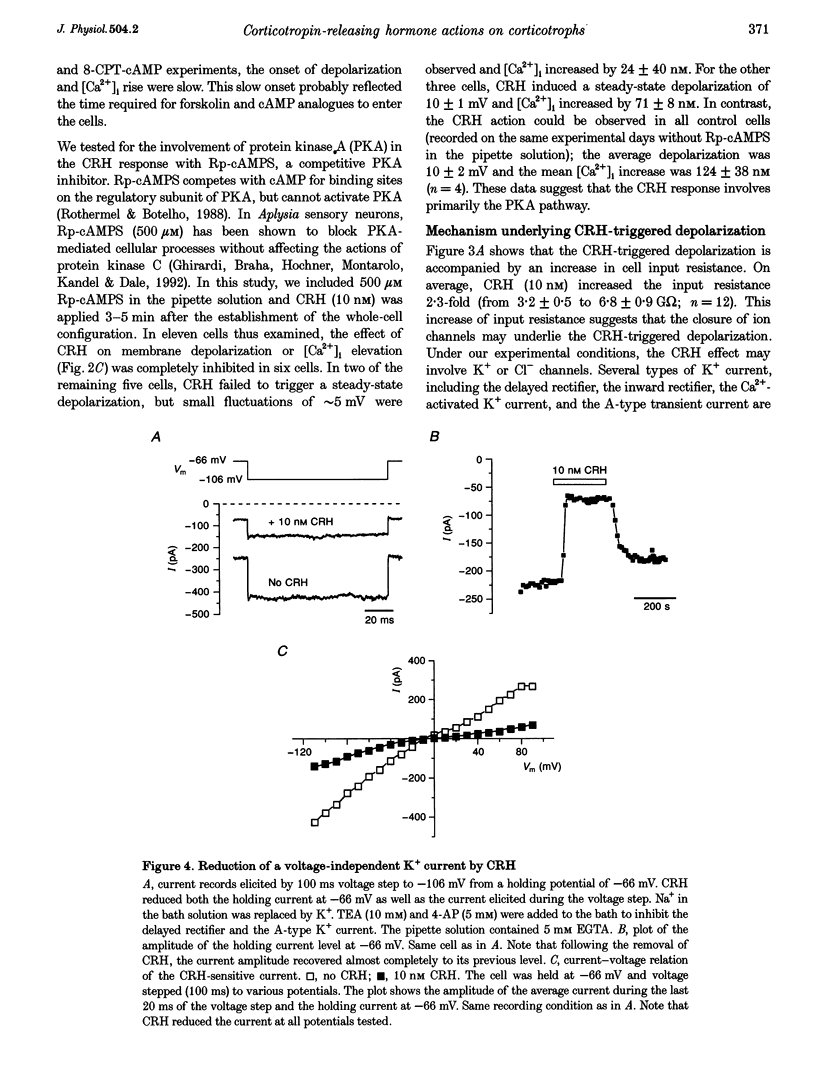



Selected References
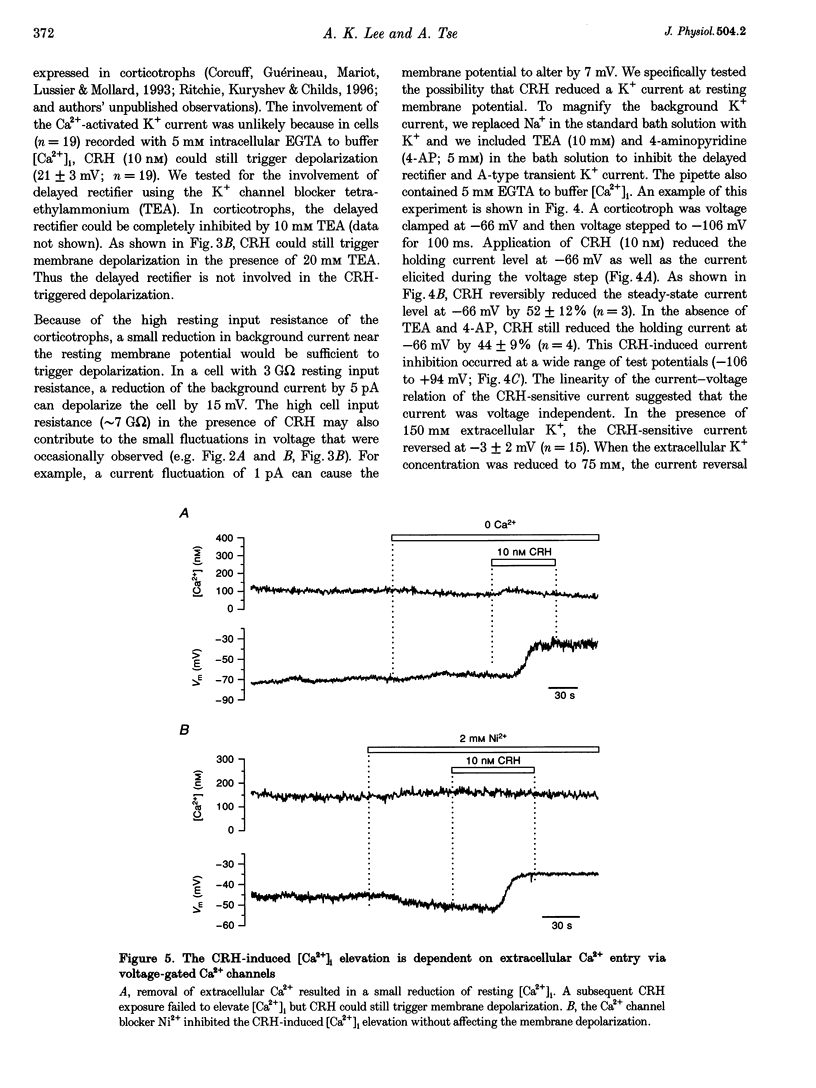
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Ashcroft F. M., Rorsman P. Calcium-independent potentiation of insulin release by cyclic AMP in single beta-cells. Nature. 1993 May 27;363(6427):356–358. doi: 10.1038/363356a0. [DOI] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinks J. R., Wier W. G., Hess P., Prendergast F. G. Measurement of Ca2+ concentrations in living cells. Prog Biophys Mol Biol. 1982;40(1-2):1–114. doi: 10.1016/0079-6107(82)90011-6. [DOI] [PubMed] [Google Scholar]
- Chang C. P., Pearse R. V., 2nd, O'Connell S., Rosenfeld M. G. Identification of a seven transmembrane helix receptor for corticotropin-releasing factor and sauvagine in mammalian brain. Neuron. 1993 Dec;11(6):1187–1195. doi: 10.1016/0896-6273(93)90230-o. [DOI] [PubMed] [Google Scholar]
- Childs G. V. Structure-function correlates in the corticotropes of the anterior pituitary. Front Neuroendocrinol. 1992 Jul;13(3):271–317. [PubMed] [Google Scholar]
- Corcuff J. B., Guérineau N. C., Mariot P., Lussier B. T., Mollard P. Multiple cytosolic calcium signals and membrane electrical events evoked in single arginine vasopressin-stimulated corticotrophs. J Biol Chem. 1993 Oct 25;268(30):22313–22321. [PubMed] [Google Scholar]
- Ghirardi M., Braha O., Hochner B., Montarolo P. G., Kandel E. R., Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992 Sep;9(3):479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guérineau N., Corcuff J. B., Tabarin A., Mollard P. Spontaneous and corticotropin-releasing factor-induced cytosolic calcium transients in corticotrophs. Endocrinology. 1991 Jul;129(1):409–420. doi: 10.1210/endo-129-1-409. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Horn R., Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. J Gen Physiol. 1988 Aug;92(2):145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L. G., Canny B. J., Orth D. N., Leong D. A. Distinct classes of corticotropes mediate corticotropin-releasing hormone- and arginine vasopressin-stimulated adrenocorticotropin release. Endocrinology. 1991 Jan;128(1):197–203. doi: 10.1210/endo-128-1-197. [DOI] [PubMed] [Google Scholar]
- Klein M., Camardo J., Kandel E. R. Serotonin modulates a specific potassium current in the sensory neurons that show presynaptic facilitation in Aplysia. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5713–5717. doi: 10.1073/pnas.79.18.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryshev Y. A., Childs G. V., Ritchie A. K. Corticotropin-releasing hormone stimulates Ca2+ entry through L- and P-type Ca2+ channels in rat corticotropes. Endocrinology. 1996 Jun;137(6):2269–2277. doi: 10.1210/endo.137.6.8641175. [DOI] [PubMed] [Google Scholar]
- Kuryshev Y. A., Childs G. V., Ritchie A. K. Corticotropin-releasing hormone stimulation of Ca2+ entry in corticotropes is partially dependent on protein kinase A. Endocrinology. 1995 Sep;136(9):3925–3935. doi: 10.1210/endo.136.9.7649101. [DOI] [PubMed] [Google Scholar]
- Kuryshev Y. A., Childs G. V., Ritchie A. K. Three high threshold calcium channel subtypes in rat corticotropes. Endocrinology. 1995 Sep;136(9):3916–3924. doi: 10.1210/endo.136.9.7649100. [DOI] [PubMed] [Google Scholar]
- Labrie F., Veilleux R., Lefevre G., Coy D. H., Sueiras-Diaz J., Schally A. V. Corticotropin-releasing factor stimulates accumulation of adenosine 3', 5'-monophosphate in rat pituitary corticotrophs. Science. 1982 May 28;216(4549):1007–1008. doi: 10.1126/science.6281886. [DOI] [PubMed] [Google Scholar]
- Lee A. K. Dopamine (D2) receptor regulation of intracellular calcium and membrane capacitance changes in rat melanotrophs. J Physiol. 1996 Sep 15;495(Pt 3):627–640. doi: 10.1113/jphysiol.1996.sp021621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong D. A. A complex mechanism of facilitation in pituitary ACTH cells: recent single-cell studies. J Exp Biol. 1988 Sep;139:151–168. doi: 10.1242/jeb.139.1.151. [DOI] [PubMed] [Google Scholar]
- Loechner K. J., Kream R. M., Dunlap K. Calcium currents in a pituitary cell line (AtT-20): differential roles in stimulus-secretion coupling. Endocrinology. 1996 Apr;137(4):1429–1437. doi: 10.1210/endo.137.4.8625921. [DOI] [PubMed] [Google Scholar]
- Luini A., Lewis D., Guild S., Corda D., Axelrod J. Hormone secretagogues increase cytosolic calcium by increasing cAMP in corticotropin-secreting cells. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8034–8038. doi: 10.1073/pnas.82.23.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pemberton K. E., Jones S. V. Enhancement of an L-type calcium current in AtT-20 cells; a novel effect of the m4 muscarinic receptor. Pflugers Arch. 1995 Mar;429(5):699–707. doi: 10.1007/BF00373991. [DOI] [PubMed] [Google Scholar]
- Ritchie A. K., Kuryshev Y. A., Childs G. V. Corticotropin-releasing hormone and calcium signaling in corticotropes. Trends Endocrinol Metab. 1996 Dec;7(10):365–369. doi: 10.1016/s1043-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- Rothermel J. D., Parker Botelho L. H. A mechanistic and kinetic analysis of the interactions of the diastereoisomers of adenosine 3',5'-(cyclic)phosphorothioate with purified cyclic AMP-dependent protein kinase. Biochem J. 1988 May 1;251(3):757–762. doi: 10.1042/bj2510757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Butt E., Nolte C., Fischer L., Halbrügge M., Beltman J., Jahnsen T., Genieser H. G., Jastorff B., Walter U. Characterization of Sp-5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole- 3',5'-monophosphorothioate (Sp-5,6-DCl-cBiMPS) as a potent and specific activator of cyclic-AMP-dependent protein kinase in cell extracts and intact cells. Biochem J. 1991 Oct 15;279(Pt 2):521–527. doi: 10.1042/bj2790521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipston M. J., Kelly J. S., Antoni F. A. Glucocorticoids block protein kinase A inhibition of calcium-activated potassium channels. J Biol Chem. 1996 Apr 19;271(16):9197–9200. doi: 10.1074/jbc.271.16.9197. [DOI] [PubMed] [Google Scholar]
- Shuster M. J., Camardo J. S., Siegelbaum S. A., Kandel E. R. Cyclic AMP-dependent protein kinase closes the serotonin-sensitive K+ channels of Aplysia sensory neurones in cell-free membrane patches. 1985 Jan 31-Feb 6Nature. 313(6001):392–395. doi: 10.1038/313392a0. [DOI] [PubMed] [Google Scholar]
- Sikdar S. K., Zorec R., Mason W. T. cAMP directly facilitates Ca-induced exocytosis in bovine lactotrophs. FEBS Lett. 1990 Oct 29;273(1-2):150–154. doi: 10.1016/0014-5793(90)81072-v. [DOI] [PubMed] [Google Scholar]
- Smith P. F., Frawley L. S., Neill J. D. Detection of LH release from individual pituitary cells by the reverse hemolytic plaque assay: estrogen increases the fraction of gonadotropes responding to GnRH. Endocrinology. 1984 Dec;115(6):2484–2486. doi: 10.1210/endo-115-6-2484. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. GnRH-induced Ca2+ oscillations and rhythmic hyperpolarizations of pituitary gonadotropes. Science. 1992 Jan 24;255(5043):462–464. doi: 10.1126/science.1734523. [DOI] [PubMed] [Google Scholar]
- Tse A., Hille B. Role of voltage-gated Na+ and Ca2+ channels in gonadotropin-releasing hormone-induced membrane potential changes in identified rat gonadotropes. Endocrinology. 1993 Apr;132(4):1475–1481. doi: 10.1210/endo.132.4.8384989. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Almers W., Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993 Apr 2;260(5104):82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Modulation of Ca2+ oscillation and apamin-sensitive, Ca2+-activated K+ current in rat gonadotropes. Pflugers Arch. 1995 Sep;430(5):645–652. doi: 10.1007/BF00386158. [DOI] [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B., Horstmann H., Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997 Jan;18(1):121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- Won J. G., Orth D. N. Roles of intracellular and extracellular calcium in the kinetic profile of adrenocorticotropin secretion by perifused rat anterior pituitary cells. I. Corticotropin-releasing factor stimulation. Endocrinology. 1990 Feb;126(2):849–857. doi: 10.1210/endo-126-2-849. [DOI] [PubMed] [Google Scholar]


