Abstract
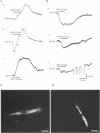
1. The resting and agonist-stimulated properties of endothelial cells and electrical communication between the endothelium and smooth muscle were investigated in open segments of guinea-pig mesenteric lymphatic vessels using intracellular microelectrodes. 2. Endothelial cells had a mean resting membrane potential (RMP) of -71.5 +/- 0.5 mV (n = 100) which was significantly different from the value of -60.8 +/- 1.1 mV (n = 75) recorded in smooth muscle. 3. Acetylcholine (ACh, 5-10 microM) generally evoked an initial hyperpolarization followed by depolarization (mean 3.4 +/- 0.5 mV and 15.4 +/- 1.0 mV, respectively, n = 75). 4. Ca(2+)-activated K+ channels were likely to underlie the ACh-induced hyperpolarization as this response exhibited an increased in membrane conductance, was larger in 0.5 mM K+ solution and was blocked by charybdotoxin (50 nM). 5. The endothelium did not exhibit a response to nitric oxide (NO) as the NO-donor sodium nitroprusside did not alter the RMP and the electrical responses to ACh were not affected by the NO-synthase inhibitor N omega-nitro L-arginine at a concentration which markedly inhibited smooth muscle hyperpolarization. 6. Electrical coupling between the endothelium and smooth muscle was not functional as there was extremely limited electrical continuity (1 in 12, endothelial/smooth muscle cell simultaneous recordings) and bradykinin, noradrenaline and isoprenaline caused different electrical responses in the two cell types. 7. These results provide the first description of RMP and electrical responses to various agonists in the lymphatic endothelium and its lack of functional electrical coupling with the smooth muscle.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brunet P. C., Bény J. L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26(4):228–234. doi: 10.1159/000158770. [DOI] [PubMed] [Google Scholar]
- Busse R., Fichtner H., Lückhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1988 Oct;255(4 Pt 2):H965–H969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- Bény J. L. Endothelial and smooth muscle cells hyperpolarized by bradykinin are not dye coupled. Am J Physiol. 1990 Mar;258(3 Pt 2):H836–H841. doi: 10.1152/ajpheart.1990.258.3.H836. [DOI] [PubMed] [Google Scholar]
- Bény J. L., Pacicca C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am J Physiol. 1994 Apr;266(4 Pt 2):H1465–H1472. doi: 10.1152/ajpheart.1994.266.4.H1465. [DOI] [PubMed] [Google Scholar]
- Chen G. F., Cheung D. W. Characterization of acetylcholine-induced membrane hyperpolarization in endothelial cells. Circ Res. 1992 Feb;70(2):257–263. doi: 10.1161/01.res.70.2.257. [DOI] [PubMed] [Google Scholar]
- Ferguson M. K. Modulation of lymphatic smooth muscle contractile responses by the endothelium. J Surg Res. 1992 Apr;52(4):359–363. doi: 10.1016/0022-4804(92)90116-h. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Edwards F. R. Sympathetic neuroeffector transmission in arteries and arterioles. Physiol Rev. 1989 Apr;69(2):546–604. doi: 10.1152/physrev.1989.69.2.546. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., van Helden D. F. Ionic basis of the resting potential of submucosal arterioles in the ileum of the guinea-pig. J Physiol. 1982 Dec;333:53–67. doi: 10.1113/jphysiol.1982.sp014438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little T. L., Xia J., Duling B. R. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995 Mar;76(3):498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Calcium-activated potassium channels in the endothelium of intact rat aorta. J Physiol. 1996 Apr 1;492(Pt 1):53–60. doi: 10.1113/jphysiol.1996.sp021288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J Physiol. 1993 Mar;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko S. M., Sage S. O. Smooth muscle cells affect endothelial membrane potential in rat aorta. Am J Physiol. 1994 Aug;267(2 Pt 2):H804–H811. doi: 10.1152/ajpheart.1994.267.2.H804. [DOI] [PubMed] [Google Scholar]
- Mehrke G., Daut J. The electrical response of cultured guinea-pig coronary endothelial cells to endothelium-dependent vasodilators. J Physiol. 1990 Nov;430:251–272. doi: 10.1113/jphysiol.1990.sp018290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehrke G., Pohl U., Daut J. Effects of vasoactive agonists on the membrane potential of cultured bovine aortic and guinea-pig coronary endothelium. J Physiol. 1991 Aug;439:277–299. doi: 10.1113/jphysiol.1991.sp018667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B., Viana F., Droogmans G. Ion channels in vascular endothelium. Annu Rev Physiol. 1997;59:145–170. doi: 10.1146/annurev.physiol.59.1.145. [DOI] [PubMed] [Google Scholar]
- Ohhashi T., Takahashi N. Acetylcholine-induced release of endothelium-derived relaxing factor from lymphatic endothelial cells. Am J Physiol. 1991 Apr;260(4 Pt 2):H1172–H1178. doi: 10.1152/ajpheart.1991.260.4.H1172. [DOI] [PubMed] [Google Scholar]
- Reeder L. B., Yang L. H., Ferguson M. K. Modulation of lymphatic spontaneous contractions by EDRF. J Surg Res. 1994 Jun;56(6):620–625. doi: 10.1006/jsre.1994.1098. [DOI] [PubMed] [Google Scholar]
- Rubanyi G. M. Endothelium-derived relaxing and contracting factors. J Cell Biochem. 1991 May;46(1):27–36. doi: 10.1002/jcb.240460106. [DOI] [PubMed] [Google Scholar]
- Sakai T. Acetylcholine induces Ca-dependent K currents in rabbit endothelial cells. Jpn J Pharmacol. 1990 Jun;53(2):235–246. doi: 10.1254/jjp.53.235. [DOI] [PubMed] [Google Scholar]
- Segal S. S., Bény J. L. Intracellular recording and dye transfer in arterioles during blood flow control. Am J Physiol. 1992 Jul;263(1 Pt 2):H1–H7. doi: 10.1152/ajpheart.1992.263.1.H1. [DOI] [PubMed] [Google Scholar]
- Spagnoli L. G., Villaschi S., Neri L., Palmieri G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia. 1982 Jan 15;38(1):124–125. doi: 10.1007/BF01944566. [DOI] [PubMed] [Google Scholar]
- Tracey W. R., Peach M. J. Differential muscarinic receptor mRNA expression by freshly isolated and cultured bovine aortic endothelial cells. Circ Res. 1992 Feb;70(2):234–240. doi: 10.1161/01.res.70.2.234. [DOI] [PubMed] [Google Scholar]
- Vaca L., Licea A., Possani L. D. Modulation of cell membrane potential in cultured vascular endothelium. Am J Physiol. 1996 Mar;270(3 Pt 1):C819–C824. doi: 10.1152/ajpcell.1996.270.3.C819. [DOI] [PubMed] [Google Scholar]
- Van Helden D. F. Pacemaker potentials in lymphatic smooth muscle of the guinea-pig mesentery. J Physiol. 1993 Nov;471:465–479. doi: 10.1113/jphysiol.1993.sp019910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von der Weid P. Y., Van Helden D. F. Beta-adrenoceptor-mediated hyperpolarization in lymphatic smooth muscle of guinea pig mesentery. Am J Physiol. 1996 May;270(5 Pt 2):H1687–H1695. doi: 10.1152/ajpheart.1996.270.5.H1687. [DOI] [PubMed] [Google Scholar]
- Xia J., Duling B. R. Electromechanical coupling and the conducted vasomotor response. Am J Physiol. 1995 Dec;269(6 Pt 2):H2022–H2030. doi: 10.1152/ajpheart.1995.269.6.H2022. [DOI] [PubMed] [Google Scholar]
- Yokoyama S., Ohhashi T. Effects of acetylcholine on spontaneous contractions in isolated bovine mesenteric lymphatics. Am J Physiol. 1993 May;264(5 Pt 2):H1460–H1464. doi: 10.1152/ajpheart.1993.264.5.H1460. [DOI] [PubMed] [Google Scholar]
- von der Weid P. Y., Bény J. L. Simultaneous oscillations in the membrane potential of pig coronary artery endothelial and smooth muscle cells. J Physiol. 1993 Nov;471:13–24. doi: 10.1113/jphysiol.1993.sp019888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P. Y., Crowe M. J., Van Helden D. F. Endothelium-dependent modulation of pacemaking in lymphatic vessels of the guinea-pig mesentery. J Physiol. 1996 Jun 1;493(Pt 2):563–575. doi: 10.1113/jphysiol.1996.sp021404. [DOI] [PMC free article] [PubMed] [Google Scholar]