Abstract
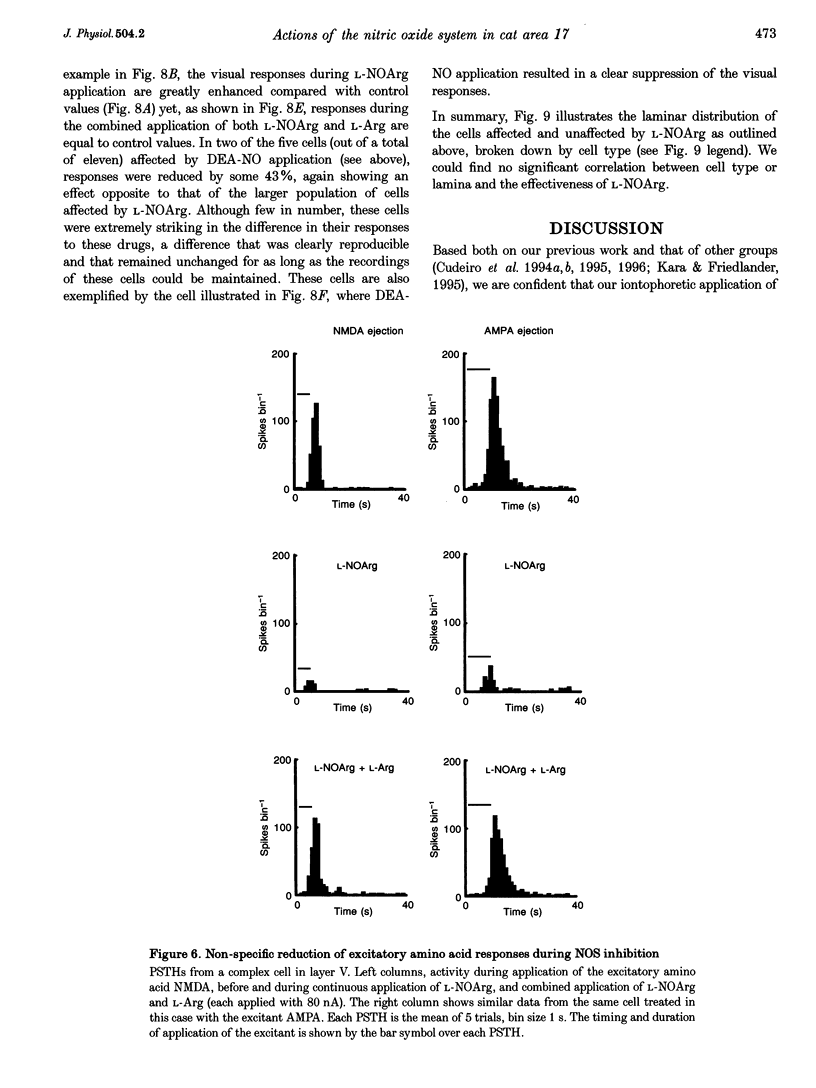
1. We iontophoretically applied NG-nitro-L-arginine (L-NOArg), an inhibitor of nitric oxide synthase (NOS), to cells (n = 77) in area 17 of anaesthetized and paralysed cats while recording single-unit activity extracellularly. In twenty-nine out of seventy-seven cells (38%), compounds altering NO levels affected visual responses. 2. In twenty-five out of twenty-nine cells, L-NOArg non-selectively reduced visually elicited responses and spontaneous activity. These effects were reversed by co-application of L-arginine (L-Arg), which was without effect when applied alone. Application of the NO donor diethylamine-nitric oxide (DEA-NO) produced excitation in three out of eleven cells, all three cells showing suppression by L-NOArg. In ten cells the effect of the soluble analogue of cGMP, 8-bromo-cGMP, was tested. In three of those in which L-NOArg application reduced firing, 8-bromo-cGMP had an excitatory effect. In six out of fifteen cells tested, L-NOArg non-selectively reduced responses to NMDA and alpha-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA). Again, co-application of L-Arg reversed this effect, without enhancing activity beyond control values. 3. In a further subpopulation of ten cells, L-NOArg decreased responses to ACh in five. 4. In four out of twenty-nine cells L-NOArg produced the opposite effect and increased visual responses. This was reversed by co-application of L-Arg. Some cells were also affected by 8-bromo-cGMP and DEA-NO in ways opposite to those described above. It is possible that the variety of effects seen here could also reflect trans-synaptic activation, or changes in local circuit activity. However, the most parsimonious explanation for our data is that NO differentially affects the activity of two populations of cortical cells, in the main causing a non-specific excitation.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaulieu C., Somogyi P. Enrichment of cholinergic synaptic terminals on GABAergic neurons and coexistence of immunoreactive GABA and choline acetyltransferase in the same synaptic terminals in the striate cortex of the cat. J Comp Neurol. 1991 Feb 22;304(4):666–680. doi: 10.1002/cne.903040412. [DOI] [PubMed] [Google Scholar]
- Bickford M. E., Günlük A. E., Guido W., Sherman S. M. Evidence that cholinergic axons from the parabrachial region of the brainstem are the exclusive source of nitric oxide in the lateral geniculate nucleus of the cat. J Comp Neurol. 1993 Aug 15;334(3):410–430. doi: 10.1002/cne.903340307. [DOI] [PubMed] [Google Scholar]
- Bickford M. E., Günlük A. E., Van Horn S. C., Sherman S. M. GABAergic projection from the basal forebrain to the visual sector of the thalamic reticular nucleus in the cat. J Comp Neurol. 1994 Oct 22;348(4):481–510. doi: 10.1002/cne.903480402. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Bickford R. G., Ponomareff G., Thal L. J., Mandel R., Gage F. H. Nucleus basalis and thalamic control of neocortical activity in the freely moving rat. J Neurosci. 1988 Nov;8(11):4007–4026. doi: 10.1523/JNEUROSCI.08-11-04007.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudeiro J., Grieve K. L., Rivadulla C., Rodríguez R., Martínez-Conde S., Acuña C. The role of nitric oxide in the transformation of visual information within the dorsal lateral geniculate nucleus of the cat. Neuropharmacology. 1994 Nov;33(11):1413–1418. doi: 10.1016/0028-3908(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Cudeiro J., Rivadulla C., Rodriguez R., Martinez-Conde S., Acuña C., Alonso J. M. Modulatory influence of putative inhibitors of nitric oxide synthesis on visual processing in the cat lateral geniculate nucleus. J Neurophysiol. 1994 Jan;71(1):146–149. doi: 10.1152/jn.1994.71.1.146. [DOI] [PubMed] [Google Scholar]
- Cudeiro J., Rivadulla C., Rodriguez R., Martinez-Conde S., Martinez L., Grieve K. L., Acu-na C. Further observations on the role of nitric oxide in the feline lateral geniculate nucleus. Eur J Neurosci. 1996 Jan;8(1):144–152. doi: 10.1111/j.1460-9568.1996.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Cudeiro J., Sillito A. M. Spatial frequency tuning of orientation-discontinuity-sensitive corticofugal feedback to the cat lateral geniculate nucleus. J Physiol. 1996 Jan 15;490(Pt 2):481–492. doi: 10.1113/jphysiol.1996.sp021159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson T. M., Bredt D. S., Fotuhi M., Hwang P. M., Snyder S. H. Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eysel U. T., Wörgötter F., Pape H. C. Local cortical lesions abolish lateral inhibition at direction selective cells in cat visual cortex. Exp Brain Res. 1987;68(3):606–612. doi: 10.1007/BF00249803. [DOI] [PubMed] [Google Scholar]
- Furfine E. S., Harmon M. F., Paith J. E., Garvey E. P. Selective inhibition of constitutive nitric oxide synthase by L-NG-nitroarginine. Biochemistry. 1993 Aug 24;32(33):8512–8517. doi: 10.1021/bi00084a017. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991 Feb;14(2):60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- Henry G. H. Receptive field classes of cells in the striate cortex of the cat. Brain Res. 1977 Sep 9;133(1):1–28. doi: 10.1016/0006-8993(77)90045-2. [DOI] [PubMed] [Google Scholar]
- Horton J. C., Hubel D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature. 1981 Aug 20;292(5825):762–764. doi: 10.1038/292762a0. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Regulation of the cerebral microcirculation during neural activity: is nitric oxide the missing link? Trends Neurosci. 1993 Jun;16(6):206–214. doi: 10.1016/0166-2236(93)90156-g. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P., Schmidt K., Brunner F., Mayer B. Inhibitors of brain nitric oxide synthase. Binding kinetics, metabolism, and enzyme inactivation. J Biol Chem. 1994 Jan 21;269(3):1674–1680. [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchiiwa S., Kuchiiwa T., Mori S., Nakagawa S. NADPH diaphorase neurones are evenly distributed throughout cat neocortex irrespective of functional specialization of each region. Neuroreport. 1994 Aug 15;5(13):1662–1664. doi: 10.1097/00001756-199408150-00030. [DOI] [PubMed] [Google Scholar]
- Martin K. A., Whitteridge D. The relationship of receptive field properties to the dendritic shape of neurones in the cat striate cortex. J Physiol. 1984 Nov;356:291–302. doi: 10.1113/jphysiol.1984.sp015465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Montague P. R., Gancayco C. D., Winn M. J., Marchase R. B., Friedlander M. J. Role of NO production in NMDA receptor-mediated neurotransmitter release in cerebral cortex. Science. 1994 Feb 18;263(5149):973–977. doi: 10.1126/science.7508638. [DOI] [PubMed] [Google Scholar]
- Murphy P. C., Sillito A. M. Cholinergic enhancement of direction selectivity in the visual cortex of the cat. Neuroscience. 1991;40(1):13–20. doi: 10.1016/0306-4522(91)90170-s. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Ramoa A. S., Paradiso M. A., Freeman R. D. Blockade of intracortical inhibition in kitten striate cortex: effects on receptive field properties and associated loss of ocular dominance plasticity. Exp Brain Res. 1988;73(2):285–296. doi: 10.1007/BF00248220. [DOI] [PubMed] [Google Scholar]
- Rivadulla C., Rodriguez R., Martinez-Conde S., Acuña C., Cudeiro J. The influence of nitric oxide on perigeniculate GABAergic cell activity in the anaesthetized cat. Eur J Neurosci. 1996 Dec;8(12):2459–2466. doi: 10.1111/j.1460-9568.1996.tb01540.x. [DOI] [PubMed] [Google Scholar]
- Sandell J. H. NADPH diaphorase histochemistry in the macaque striate cortex. J Comp Neurol. 1986 Sep 15;251(3):388–397. doi: 10.1002/cne.902510309. [DOI] [PubMed] [Google Scholar]
- Schuman E. M., Madison D. V. Nitric oxide and synaptic function. Annu Rev Neurosci. 1994;17:153–183. doi: 10.1146/annurev.ne.17.030194.001101. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. Cholinergic modulation of the functional organization of the cat visual cortex. Brain Res. 1983 Dec 19;289(1-2):143–155. doi: 10.1016/0006-8993(83)90015-x. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder S. H., Bredt D. S. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991 Apr;12(4):125–128. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979 Jul 27;171(1):11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wood J., Garthwaite J. Models of the diffusional spread of nitric oxide: implications for neural nitric oxide signalling and its pharmacological properties. Neuropharmacology. 1994 Nov;33(11):1235–1244. doi: 10.1016/0028-3908(94)90022-1. [DOI] [PubMed] [Google Scholar]


