Abstract
Thrombin-induced platelet microbicidal protein 1 (tPMP-1) is a small, cationic peptide generated from rabbit platelets when they are exposed to thrombin in vitro. It has potent microbicidal activity against a broad spectrum of bacterial and fungal pathogens, including Staphylococcus aureus. Previous in vitro studies involving whole staphylococcal cells and planar lipid bilayers (as artificial bacterial membrane models) suggested that membrane permeabilization by tPMP-1 is voltage dependent (S.-P. Koo, M. R. Yeaman, and A. S. Bayer, Infect. Immun. 64:3758–3764, 1996; M. R. Yeaman, A. S. Bayer, S. P. Koo, W. Foss, and P. M. Sullam, J. Clin. Investig. 101:178–187, 1998). Thus, the aims of the present study were to specifically characterize the electrophysiological events associated with membrane permeabilization by tPMP-1 by using artificial planar lipid bilayer membranes. We assessed the influence of transmembrane voltage polarity and magnitude on the initiation and modulation of tPMP-1 membrane permeabilization at various concentrations of tPMP-1 (range, 1 to 100 ng/ml) added to the cis side of the membranes. The incidence of membrane permeabilization induced by tPMP-1 at all of the concentrations tested was more frequent at −90 mV than at +90 mV. It is noteworthy that membrane permeabilization due to 1-ng/ml tPMP-1 was successfully initiated at −90 mV but not at +90 mV. Further, the mean onset times of induction of tPMP-1 activity were comparable under the various conditions. Modulation of ongoing membrane permeabilization was dependent on voltage and tPMP-1 concentration. Membrane permeabilization at a low tPMP-1 concentration (1 ng/ml) was directly correlated with trans-negative voltages, while a higher tPMP-1 concentration (100 ng/ml) induced conductance which was more dependent on trans-positive voltages. Collectively, these data indicate that the mechanism of tPMP-1 microbicidal activity at the bacterial cytoplasmic membrane may involve distinct induction and propagation stages of membrane permeabilization which, in turn, are modulated by transmembrane potential, as well as peptide concentration.
Mammalian platelets are believed to be integral components of host defense against blood-borne microbial pathogens. This function likely occurs through platelet-released antimicrobial peptides at sites of endovascular damage or infection (17–22). These antimicrobial peptides have been termed platelet microbicidal proteins or PMPs (19). Thrombin-induced PMP-1 (tPMP-1) is the predominant peptide isolated from thrombin-stimulated rabbit platelets in vitro (20, 22). It is small (molecular mass, 8,036 Da, as determined by mass spectroscopy) and has an amino acid composition indicative of a cationic peptide with a relatively high abundance of basic residues (Arg, Lys, and His; 23.8% of the total mass) (22). tPMP-1 exerts potent antimicrobial activities against common bloodstream pathogens, including Staphylococcus aureus, S. epidermidis, viridans group streptococci, Candida albicans, and Cryptococcus neoformans (17–22). In vitro, tPMP-1 microbicidal activity is most active at pH 7.2 and under conditions of low ionicity (9, 23).
Recent data from our laboratories support the hypothesis that tPMP-1 targets the bacterial cytoplasmic membrane to initiate its microbicidal effects (8–10, 23). In addition, preliminary investigations using planar lipid bilayer systems to simulate microbial membranes indicated that tPMP-1 directly permeabilizes such membranes, increasing conductance in a voltage-dependent manner (10). In prior studies, the initiation of tPMP-1 membrane permeabilization appeared to be voltage dependent, while persistence of a membrane effect due to tPMP-1 was not voltage driven. Using genetically related parent-mutant S. aureus strain pairs which differ in transmembrane electrical potential (ΔΨ), we previously demonstrated that a lowered ΔΨ in the mutants was directly related to a decrease in membrane permeabilization and a decrease in tPMP-1 susceptibility (8, 23). Collectively, these observations led us to hypothesize that ΔΨ influences tPMP-1 membrane activity at two possible levels, i.e., (i) induction of membrane permeabilization and (ii) ongoing modulation of membrane perturbation, leading to eventual membrane disruption. The present study aimed to further characterize these electrophysiologic events in vitro by using artificial planar lipid bilayers as model bacterial cytoplasmic membranes.
MATERIALS AND METHODS
Platelet microbicidal proteins.
Fresh rabbit platelets were isolated and stimulated with bovine thrombin in glutamine-free Eagle’s minimal essential medium (pH 7.4; Irvine Scientific), yielding tPMP-1-rich supernatant, as described elsewhere (19). Previous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE) and high-pressure liquid chromatography (HPLC) analyses have indicated that tPMP-1 is the predominant cationic staphylocidal peptide present therein (19, 22). tPMP-1 was then homogeneously purified from the respective platelet supernatants by gel filtration and analytical reversed-phase HPLC (22). The purity of the peptide was confirmed by acid-urea PAGE, sodium dodecyl sulfate-PAGE, and reversed-phase HPLC (22). Retention of microbicidal activity by purified tPMP-1 was confirmed by 100% killing of 103 CFU of Bacillus subtilis ATCC 6633, an indicator organism highly susceptible to the peptide, per ml at 37°C within 30 min as described elsewhere (19, 22). Pilot studies indicated that tPMP-1 solubilization in 10% (vol/vol) dimethyl sulfoxide for 5 min at room temperature (before dilution and use) was optimal for measurement of activity on artificial planar lipid bilayers (data not shown).
Planar lipid membrane apparatus.
Planar lipid bilayer membranes were formed across the end of a Teflon tube (diameter, 0.5 mm) which was tightly fitted into a chamber containing a small central conduit (volume capacity, ∼100 μl) as described elsewhere (14). The tube was coated with a lipid solution consisting of 1.5% (wt/vol) 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol (POPG) (7:3 [wt/wt]; Avanti Polar Lipids Inc., Birmingham, Ala.) in n-heptane and allowed to dry. The tube was then positioned such that the membrane, when formed, would lie in the central flow of the conduit filled with an ionic buffer. Our previous microbicidal assays and flow cytometry studies have shown that tPMP-1 is functional at neutral pH. Therefore, a buffer consisting of 0.1 M KCl containing 10 mM Tris-HCl (pH 7.4) was used to bathe the membrane (22, 23). The anionic planar lipid bilayers were formed by aeration of the POPC-POPG lipid mixture across the tubing. A voltage potential was generated across the membrane by using a pair of Ag-AgCl electrodes, one of which was linked to a battery voltage source. The complementary electrode was connected to a measuring circuit consisting of a current amplifier and a chart recorder. Voltage polarity was defined by the addition of HPLC-purified tPMP-1 to the designated cis side of membranes. A trans-negative or trans-positive potential (indicated by a minus or plus sign, respectively) was generated by applying a negative or positive potential, respectively, to the compartment opposite the peptide-containing cis side (i.e., the trans compartment). The chamber was constructed such that the test peptide (diluted in buffer) could be added within seconds to rapidly displace peptide-free buffer in the cis compartment.
Voltage-dependent membrane permeabilization studies.
All experiments were performed at room temperature. All studies represent at least two independent experiments performed under the same conditions. Membrane permeabilization was recorded over time as an increase in transmembrane current and expressed in nanoamperes. Membrane conductance (g; expressed in nanosiemens) was calculated from Ohm’s law as follows: g = I/V, where I is current and V is voltage. Only membranes with a baseline conductance of less than 10 pS were used in these studies. Further, the stability of each membrane was tested by holding membranes at −90 mV for 20 to 30 min in buffer alone. The influence of differing transmembrane voltages on tPMP-1 membrane activity was studied by evaluating two events, i.e., initiation of planar membrane permeabilization by tPMP-1 and modulation of ongoing membrane permeabilization.
(i) Initiation of planar membrane permeabilization by tPMP-1.
The initiation of tPMP-1 membrane activity was studied by varying the polarity of transmembrane voltages between −90 and +90 mV and tPMP-1 concentrations from 1 to 100 ng/ml. Rapidity of tPMP-1 membrane permeabilization was determined by measuring the time to onset of activity from the point of addition of tPMP-1 to planar lipid bilayers to the initial increase in membrane current. Times to onset inversely relate to the ability of tPMP-1 to initiate permeabilization of membranes under selected electrophysiologic conditions. Previous studies of other cationic peptides (e.g., defensins), as well as tPMP-1, suggested that they permeabilize negatively charged lipid bilayers (5, 10). Furthermore, previous experiments using similar artificial planar lipid bilayer systems with tPMP-1 or other peptides indicated that membrane activity, if present, generally occurs within 10 min of peptide addition (data not shown). Thus, membranes in these experiments were observed for up to 10 min after the addition of tPMP-1. In selected studies, the mean magnitude of membrane conductance within the first 20 s of permeabilization was determined at the various trans-positive and trans-negative voltages and tPMP-1 concentrations.
(ii) Modulation of ongoing membrane permeabilization.
Modulation of ongoing membrane permeabilization by voltage was studied for tPMP-1 by monitoring membrane current while alternating between trans-positive and trans-negative voltages of increasing magnitude (range, 30 to 100 mV). Mean membrane current was then calculated for an interval of 5 min at selected voltages in each experiment, beginning from the time when membrane potential was initiated. Spontaneous reversibility of tPMP-1 membrane permeabilization was investigated by gently and continuously flushing the cis side of the planar lipid bilayer chamber with excess buffer during permeabilization events (i.e., while membrane current was increasing or decreasing, respectively). During such procedures, tPMP-1-containing buffer on the cis side of membranes was replaced with peptide-free buffer.
The neurotoxic fragment of the β-amyloid peptide, Αβ(25-35), is a voltage-dependent ionic-channel former that exhibits an increase in membrane conductance (open state) at trans-negative voltages and a decrease (closed state) at trans-positive voltages (13). Thus, this peptide (10 μg/ml) was used as a positive control for voltage magnitude and polarity in the present study.
Statistical analysis.
Differences in the means (± the standard deviation) of multiple groups were compared by using Kruskal-Wallis analysis of variance, with Tukey post hoc analysis for multiple comparisons of nonparametric data. P ≤ 0.05 was considered significant.
RESULTS
tPMP-1-induced membrane permeabilization.
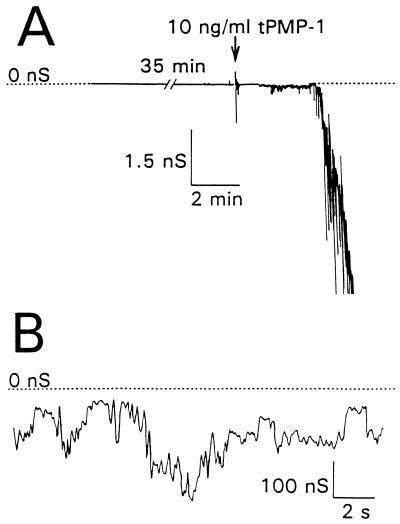
Unmodified lipid bilayer membranes exhibited low membrane conductance which was indistinguishable from the background (∼3 pS). Dimethyl sulfoxide (final concentration, 0.01 to 0.2%, vol/vol) or KCl buffer alone did not alter membrane permeabilization (conductance) when added to the cis side of planar lipid bilayer membranes over a 20- to 40-min observation period (data not shown). However, tPMP-1 at concentrations as low as 1 ng/ml induced an increase in membrane permeability of 103- to 105-fold within minutes of peptide addition (Fig. 1A). Conductance induced by tPMP-1 fluctuated on a millisecond time scale, with no apparent single-channel characteristics, consistent with previous pilot studies (Fig. 1B) (10). Once membrane permeabilization was initiated, one of two subsequent effects on membrane conductance was noted as the transmembrane voltage was held constant. First, membrane conductance increased to one or more apparent steady-state levels, with moderate fluctuations persisting throughout an observation period of more than 60 min (Fig. 1B). Alternatively, membrane conductance increased rapidly, within a few seconds, leading to rupture of the membrane. Both trans-negative and trans-positive voltages of −90 and +90 mV, respectively, induced tPMP-1 membrane permeabilization, although their frequencies varied (Table 1; also see below).
FIG. 1.
Membrane conductance induced by tPMP-1 in planar lipid bilayer membranes. Membrane conductance is shown as a function of time with reference to the scales indicated. A planar lipid bilayer membrane was bathed in a 0.1 M KCl salt solution containing 10 mM Tris-HCl (pH 7.4) and maintained at a membrane potential of −90 mV throughout the experiment. For panel A, the resting membrane was held at rest for 35 min before the addition of tPMP-1 (arrow). An increase in membrane permeabilization was noted within 60 s of peptide addition. In panel B, the ongoing membrane conductance induced in panel A is displayed on a different scale.
TABLE 1.
Frequency and time to onset of tPMP-1 activity
| Transmembrane voltage (mV) | tPMP-1 concn:
|
|||||
|---|---|---|---|---|---|---|
| 1 ng/ml
|
10 ng/ml
|
100 ng/ml
|
||||
| Frequencya | Timeb | Frequency | Time | Frequency | Time | |
| +90 | 0/5 (0) | >10 | 1/6 (17) | 3.8 | 4/10 (40) | 3.0 ± 4.4 |
| −90 | 2/5 (40) | 0.8 ± 0.2 | 4/7 (57) | 2.0 ± 1.6 | 10/12 (83) | 2.0 ± 3.2 |
Frequency of positive membrane permeabilization due to tPMP-1 is expressed as the ratio of the number of successful events to the total number of events and the percentage of successful events (in parentheses). Absence of membrane activity during the 10-min observation time after the addition of tPMP-1 was noted as an unsuccessful event.
Mean onset time (± the standard deviation) between the addition of tPMP-1 and the first observed increase in membrane conductance is shown. Unsuccessful events were recorded as having a time to onset of >10 min.
Voltage-dependent initiation of tPMP-1 activity.
The mean times to onset of membrane permeabilization (minutes) for 1-, 10-, or 100-ng/ml tPMP-1 at −90 or +90 mV are shown in Table 1. When membrane permeabilization was not observed within 10 min of tPMP-1 addition, Αβ(25-35) was added to the same membrane to confirm the sensitivity of the membrane to permeabilization. Αβ(25-35) (10 μg/ml) was ∼100% effective at inducing membrane permeabilization within 1 min of peptide addition to membranes (data not shown). At 1-ng/ml tPMP-1, membrane permeabilization was induced at −90 mV but not at +90 mV. At the 1-, 10-, and 100-ng/ml tPMP-1 concentrations tested, membrane permeabilization was consistently induced at higher incidences (40, 57, and 83%, respectively) at a trans-negative voltage (−90 mV) than at the equivalent trans-positive voltage (+90 mV; 0, 17, and 40%, respectively; Table 1). The times of onset of induced membrane permeabilization observed at −90 versus +90 mV (range, 0.8 to 3.8 min) were not statistically significantly different at the various tPMP-1 concentrations used. In selected experiments, the mean initial magnitude of membrane conductance induced by tPMP-1 within the first 20 s of activity was determined at both −90 and +90 mV for the various tPMP-1 concentrations. The data reveal no significant influence of either voltage polarity or tPMP-1 concentration on the magnitude of membrane conductance (data not shown).
Voltage-dependent modulation of tPMP-1 activity.
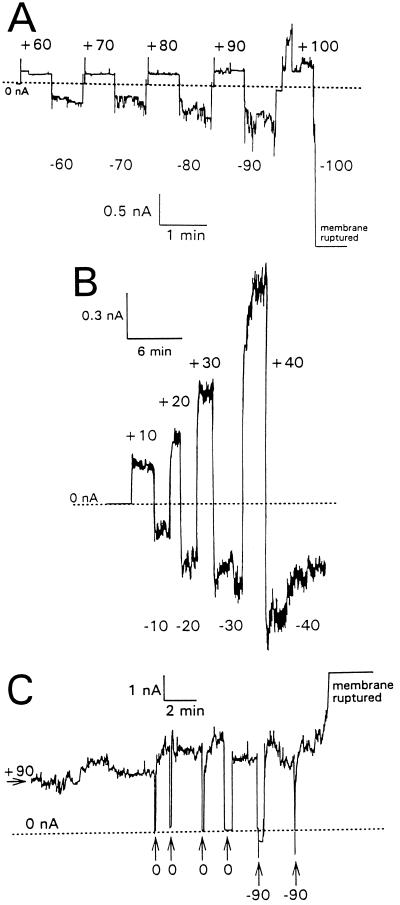
Once tPMP-1 membrane permeabilization was initiated, the modulation of this activity was influenced by both voltage magnitude and polarity, as well as by tPMP-1 concentration. For example, Fig. 2A to C shows strong but differing patterns of influence of voltage on tPMP-1 permeabilization at 1-, 40-, and 100-ng/ml tPMP-1, respectively. At 1-ng/ml tPMP-1, membrane permeabilization was directly related to trans-negative voltages, while membrane permeabilization was much lower and nearly constant at corresponding trans-positive voltages (Fig. 2A). As the voltage was increased from −60 to −100 mV, membrane permeabilization and current fluctuations became greater, finally rupturing the membrane at −100 mV (Fig. 2A). In contrast, membrane permeabilization induced at 40-ng/ml tPMP-1 increased at trans-positive voltages, while trans-negative voltages had the opposite effect (Fig. 2B). An increase in conductance was noted at +40 mV or above, while a decrease in membrane conductance occurred at −40 mV or lower (Fig. 2B). At 100-ng/ml tPMP-1, membrane permeabilization showed strong dependence on voltage polarity, with membrane conductance at +90 mV being ∼10-fold greater than at −90 mV (Fig. 2C). The membrane was eventually ruptured at +90 mV (Fig. 2C). Attempts to reverse tPMP-1 membrane permeabilization by flushing the planar lipid bilayer chamber with excess buffer were routinely unsuccessful during either opening or closing events of membrane permeabilization.
FIG. 2.
Voltage modulation of tPMP-1 membrane permeabilization. Membrane current is shown as a function of time with reference to the scales indicated. Base level membrane currents (which were near zero) are indicated by dotted lines. The values are membrane potentials in millivolts. Planar lipid bilayer membranes were formed in 0.1 M KCl containing 10 mM Tris-HCl (pH 7.4) and held at −90 mV. The following concentrations of tPMP-1 were then added to stable membranes held at the indicated membrane potentials: A, 1-ng/ml tPMP-1 at −90 mV; B, 40-ng/ml tPMP-1 at −90 mV; C, 100-ng/ml tPMP-1 at +90 mV. After tPMP-1-induced membrane permeabilization had been observed, the membrane potential was switched to 0 mV and the voltage was increased stepwise to the indicated values. For panel C, the membrane was kept at a potential of +90 mV and pulsed briefly with either 0 or −90 mV, as indicated.
Transmembrane current versus voltage.
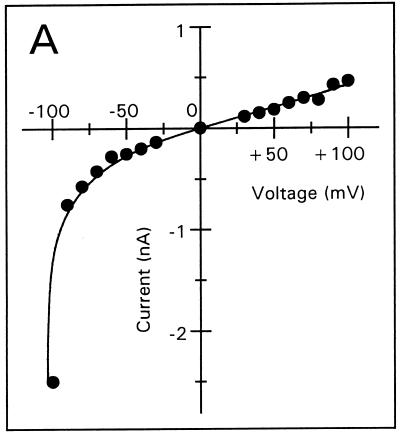
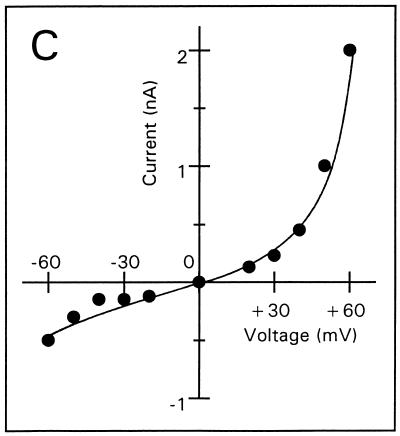
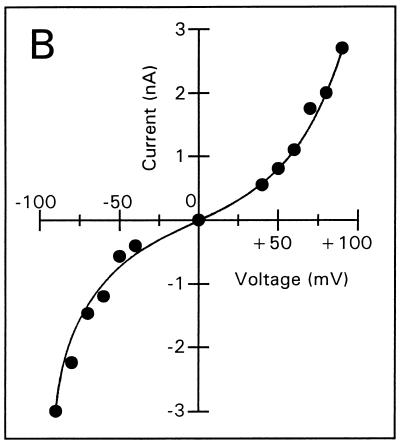
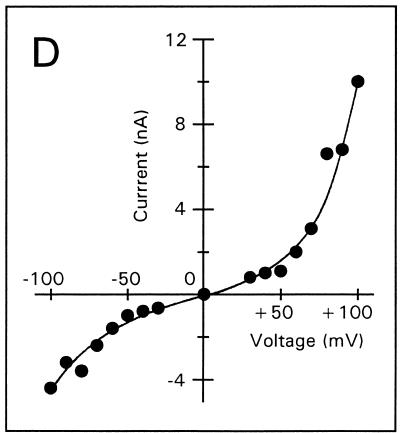
Voltage-dependent membrane permeabilization induced by tPMP-1 or Αβ(25-35) was further analyzed by calculating the mean membrane current from an interval of 5 min after each voltage change, as described in Materials and Methods (Fig. 3A to E). At 1-ng/ml tPMP-1, substantial voltage dependence of membrane permeabilization was observed, particularly at trans-negative voltages below −90 mV (Fig. 3A). For example, the absolute membrane current induced at −100 mV was 2.5 nA, compared to 0.5 nA at +100 mV. At 10-ng/ml tPMP-1, membrane current was equally influenced by both trans-negative and trans-positive voltages (Fig. 3B). It is interesting that membrane permeabilization at 40- or 200-ng/ml tPMP-1 was modulated in a manner opposite to that observed at 1-ng/ml tPMP-1 (Fig. 3C and D). A strong, nonlinear increase in membrane current was observed at trans-positive voltages above +40 and +60 mV for 40- and 200-ng/ml tPMP-1, respectively, while trans-negative voltages only induced linear (ohmic) increases at these tPMP-1 concentrations (Fig. 3C and D). Although membrane current at trans-negative voltages was at least 2.5-fold lower than at trans-positive voltages in both cases, the increase in membrane permeabilization of the former from the baseline was still substantial (∼103-fold). Comparison of the membrane current levels induced at trans-negative voltages for 1-ng/ml tPMP-1 (Fig. 3A) versus 200-ng/ml tPMP-1 (Fig. 3D) indicates that they are comparable. It is noteworthy that membrane permeabilization for 40- or 200-ng/ml tPMP-1 was induced at −90 or −150 mV, respectively. Despite differences in induction voltage and peptide concentration, the overall effects of voltage on membrane permeabilization appear to be comparable (Fig. 3C and D). As expected, Αβ(25-35) exhibited strong voltage-dependent opening at trans-negative voltages and closing at trans-positive voltages, consistent with previous observations (Fig. 3E) (13).
FIG. 3.
Membrane current versus voltage. Each set of data was obtained from one of several repeat experiments. Membrane current represents the mean current measured during a 5-min interval after the voltage had been changed to the respective value, against which the current was plotted. Membrane permeabilization was initiated by the following concentrations of tPMP-1 in membranes induced at the following membrane potentials: A, 1-ng/ml tPMP-1 at −90 mV; B, 10-ng/ml tPMP-1 at +90 mV; C, 40-ng/ml tPMP-1 at −90 mV; D, 200-ng/ml tPMP-1 at −150 mV. For panel E, voltage-dependent membrane permeabilization was induced by 10-μg/ml Αβ(25-35) at −110 mV.
DISCUSSION
Our current hypothesis contends that tPMP-1 initially targets the bacterial cytoplasmic membrane, leading to membrane permeabilization as a principal mechanism of bactericidal action. This belief is supported by several previous observations, including (i) rapid permeabilization of cytoplasmic membranes of whole staphylococcal cells and protoplasts within seconds of tPMP-1 exposure (10, 23) and (ii) tPMP-1-induced increases in conductance across artificial planar lipid bilayers, indicating direct permeabilization of the membrane by tPMP-1 (10). In addition, previous evidence indicates that staphylocidal activity and membrane permeabilization due to tPMP-1 are voltage dependent, with activity being facilitated by a ΔΨ of −100 mV or a more negative voltage (8, 23). For example, two genetically distinct tPMP-1-resistant mutants (S. aureus JB-1 and 19R) exhibit substantially lower ΔΨ values (−98 and −102 mV, respectively) than their genetically related tPMP-1-susceptible parental strains (S. aureus 6850 and 19S, respectively) with intact ΔΨ values (−150 and −132 mV, respectively) (2, 8). Moreover, tPMP-1-induced membrane permeabilization of a parent-mutant strain pair (whole cells or protoplasts) was directly related to ΔΨ (10, 23). Furthermore, reconstitution of ΔΨ in the mutant strain is associated with enhanced killing and permeabilization by tPMP-1 to near-parental levels (8, 23). These findings are similar to the protection that membrane depolarization affords Escherichia coli against the voltage-dependent channel-forming colicins (12, 16). Lastly, preliminary studies using planar lipid bilayers and whole staphylococcal cells indicated that the induction of tPMP-1 membrane permeabilization is facilitated by a transmembrane potential of at least −90 mV (10, 23). Despite these important findings, the specific biophysical relationships among ΔΨ, tPMP-1 concentration, and tPMP-1-induced membrane permeabilization have not been fully delineated. Thus, the present study aimed to further test this hypothesis and to characterize the electrophysiologic consequences of tPMP-1 interactions with membranes.
The planar lipid bilayer membrane technique used in our current investigation has been utilized by numerous investigators to study membrane permeabilization by antimicrobial peptides (3, 4, 7, 11, 15). The characteristics of peptide-membrane interactions, which can be reliably determined by using this method, include the following parameters: (i) onset time to membrane permeabilization, (ii) extent of membrane permeabilization, (iii) voltage threshold for the induction of membrane permeabilization, and (iv) influence of voltage polarity and magnitude on ongoing membrane permeabilization. The lipid composition of planar lipid bilayer membranes used in the present study (i.e., a POPC/POPG ratio of 7:3 [wt/wt]) differs from that of bacterial membranes in that the latter contain cardiolipin and neutral lipids consisting mainly of phosphatidylethanolamine instead of phosphatidylcholine. The artificial membranes have a net negative surface charge due to the 30% content of the negatively charged lipid POPG with no cationic lipid. Thus, despite differences in lipid composition, the surface charge of the artificial membranes reflects the net anionic surface charge of bacterial membranes. This was important for the purpose of the current study, since it has been shown that cationic peptides which are similar to tPMP-1 (e.g., defensins) likely target anionic membranes (5, 7).
Membrane permeabilization was induced by tPMP-1 at concentrations as low as 1 ng/ml. Our data show that membrane conductance with variable fluctuations was induced by tPMP-1 with no clear indication of single-channel activity. The high frequency of membrane rupture, together with the high variability of membrane conductance induced by tPMP-1, indicates that membrane permeabilization most likely occurs by a generalized breakdown of the membrane structure. However, several aspects of our data might also suggest that pore formation participates in tPMP-1-induced activity: (i) it is known that stable, long-lived single-channel activities induced by channel-forming peptides such as defensins are difficult to detect due to the highly heterogeneous population of channel currents, even under stringent conditions (7); (ii) membrane conductance induced by tPMP-1, although showing a high degree of fluctuation, did reach one or more apparent steady-state levels under a given condition; (iii) rectification of conductance was also observed under most circumstances, whereby conductance induced by corresponding positive and negative voltages appear asymmetrical; and (iv) tPMP-1 activity is voltage dependent, similar to those of other pore-forming cationic peptides, such as defensins (7). The present data are not sufficient to precisely distinguish between the two possible mechanisms of tPMP-1 action, namely, generalized breakdown of the membrane [so-called “carpet effect”] versus discrete pore formation. Thus, the possibility of permeabilization of target membranes by the formation of porelike structures by tPMP-1 cannot be excluded.
Collectively, the present data demonstrate several important points regarding the mechanism of tPMP-1-induced membrane permeabilization. First, permeabilization of artificial membranes due to tPMP-1 was voltage dependent in terms of induction and propagation events. This observation supports our previous finding that bacterial ΔΨ (featuring a trans-negative voltage) appears to drive tPMP-1 permeabilization of the cytoplasmic membrane, a principal target of tPMP-1 activity (8, 10, 23). In the current study involving the induction of permeabilization of artificial membranes, a two- to fourfold higher incidence of permeabilization was observed at trans-negative than at trans-positive voltages. Further, current-versus-voltage plots of ongoing membrane permeabilization induced by tPMP-1 indicate a 103-fold increase in membrane permeabilization at a trans-negative voltage of −100 mV. Second, there appears to be an optimum threshold potential of approximately −100 mV for the interaction of tPMP-1 with artificial membranes, beyond which membrane activity progresses from permeabilization to disruption of the membrane. These data are supportive of our prior observation that a bacterial ΔΨ above −100 mV (e.g., −150 mV) is required for maximal tPMP-1 activity (8, 10, 23). Finally, failure to diminish tPMP-1 membrane permeabilization by flushing the cis side of the membranes with excess tPMP-1-free buffer suggests that association of tPMP-1 with membranes is not spontaneously reversible. Taken together with previous data (10, 23), our findings indicate that the mechanism of tPMP-1 activity involves ΔΨ-dependent permeabilization of the bacterial cytoplasmic membrane; this may lead to global membrane destabilization and ultimate rupture.
It should be emphasized that, in addition to the electrophysiologic state of the target cytoplasmic membrane, several other determinants have been identified which appear to influence tPMP-1 microbicidal activity. Previous studies indicate that logarithmic-phase staphylococcal cells and protoplasts are more resistant to killing and membrane disruption by tPMP-1, respectively, than are stationary-phase cells of the same strains (9, 10). Also, tPMP-1-resistant staphylococcal strains have membrane bioenergetics that differ (e.g., an increased basal rate of O2 consumption and ATP generation) from those of their genetically related tPMP-1-susceptible counterparts (2). Moreover, the cytoplasmic membrane composition of tPMP-1-resistant staphylococcal strains shows an increase in polyunsaturated fatty acids, with a resultant increase in membrane fluidity, compared to that of their tPMP-1-susceptible counterparts (1). Taken together, these data suggest that the microbial growth phase, the overall state of cellular bioenergetics, and the lipid composition of the cytoplasmic membrane profoundly influence tPMP-1 staphylocidal activity in vitro. The precise interrelationships among these parameters with respect to tPMP-1 susceptibility or resistance remain to be elucidated.
tPMP-1 exhibited an unusual concentration-dependent effect on voltage-dependent membrane permeabilization. At a low tPMP-1 concentration (1 ng/ml), membrane permeabilization was greater at trans-negative voltages. At higher concentrations (40 to 200 ng/ml), membrane permeabilization was greater at trans-positive voltages, while a moderate concentration (10 ng/ml) exhibited an intermediate pattern. Similar concentration-dependent electrophysiologic activities have been shown for the channel-forming bacterial peptide monazomycin, which undergoes altered voltage dependence as its concentration increases (6). Thus, the present phenomena may represent a novel aspect of the mechanism of action of tPMP-1 among mammalian endogenous antimicrobial peptides.
Concentrations of tPMP-1 necessary to induce membrane permeabilization in the present study (i.e., 1 to 100 ng/ml, corresponding to 0.125 to 12.5 nM, respectively) are consistent with those which exert microbicidal activities against S. aureus and B. subtilis in vitro (22). The functional concentration of tPMP-1 released from platelets at putative sites of action (i.e., sites of endothelial damage and microbial colonization) is not specifically known (19, 22). However, the relative abundance of tPMP-1 from thrombin-stimulated rabbit platelets in vitro is ∼15 μg/109 platelets, 100-fold higher than the highest concentration used in the current study (22). Assuming that only a fraction of the platelet population which is deposited at the site of infection is stimulated to release tPMP-1, it is highly likely that tPMP-1 concentrations encompassing those used in the current study exist in settings of infection in vivo. Thus, the present in vitro findings may also have implications for the mechanism of antimicrobial host defense of platelets in vivo.
ACKNOWLEDGMENTS
This work was supported in part by research grants from the National Institutes of Health (AI39108 to A.S.B. and M.R.Y., AI39001 to M.R.Y., and MH01174 to B.L.K.), the University of California AIDS Research Program (to B.L.K.), and the UCLA AIDS Institute (to B.L.K.).
REFERENCES
- 1.Bayer A S, Yeaman M R, Koo S-P, Prasad R. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Resistance to staphylocidal effects of thrombin-induced microbicidal protein is associated with alterations in membrane fluidity and lipid content, abstr. A-107; p. 19. [Google Scholar]
- 2.Bayer A S, Yeaman M R, Sahl H-G, Brar D, Proctor R A. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Relationship of phenotypic resistance to thrombin-induced platelet microbicidal protein (tPMP) and cytoplasmic membrane bioenergetics in Staphylococcus aureus (SA), abstr. A-106; p. 19. [Google Scholar]
- 3.Christensen B, Fink J, Merrifield R B, Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc Natl Acad Sci USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falla T J, Karunaratne D N, Hancock R E W. Mode of action of the antimicrobial peptide indolicidin. J Biol Chem. 1996;271:19298–19303. doi: 10.1074/jbc.271.32.19298. [DOI] [PubMed] [Google Scholar]
- 5.Fujii G, Selsted M E, Eisenberg D. Defensins promote fusion and lysis of negatively-charged membranes. Protein Sci. 1993;2:1301–1312. doi: 10.1002/pro.5560020813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heyer R J, Muller R U, Finkelstein A. Inactivation of monazomycin-induced voltage-dependent conductance in thin lipid membranes. II. Inactivation produced by monazomycin transport through the membrane. J Gen Physiol. 1976;67:731–748. doi: 10.1085/jgp.67.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koo S-P, Bayer A S, Sahl H-G, Proctor R A, Yeaman M R. Staphylocidal action of thrombin-induced platelet microbicidal protein is not solely dependent on transmembrane potential. Infect Immun. 1996;64:1070–1074. doi: 10.1128/iai.64.3.1070-1074.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koo S-P, Yeaman M R, Bayer A S. Staphylocidal action of thrombin-induced platelet microbicidal protein is influenced by microenvironment and target cell growth phase. Infect Immun. 1996;64:3758–3764. doi: 10.1128/iai.64.9.3758-3764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koo S-P, Yeaman M R, Nast C C, Bayer A S. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun. 1997;65:4795–4800. doi: 10.1128/iai.65.11.4795-4800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kordel M, Benz R, Sahl H-G. Mode of action of the staphylococcinlike peptide Pep5: voltage-dependent depolarization of bacterial and artificial membranes. J Bacteriol. 1988;170:84–88. doi: 10.1128/jb.170.1.84-88.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luria S E. Colicins and the energetics of cell membranes. Sci Am. 1975;233:30–37. doi: 10.1038/scientificamerican1275-30. [DOI] [PubMed] [Google Scholar]
- 13.Mirzabekov T A, Lin M-C, Yuan W-L, Marshall P J, Carman M, Tomaselli K, Lieberburg I, Kagan B L. Channel formation in planar lipid bilayers by a neurotoxic fragment of the beta-amyloid peptide. Biochem Biophys Res Commun. 1994;202:1142–1148. doi: 10.1006/bbrc.1994.2047. [DOI] [PubMed] [Google Scholar]
- 14.Mirzabekov T A, Silberstein A V, Kagan B L. Use of planar lipid bilayer membranes for rapid screening of membrane active compounds. Methods Enzymol. 1999;294:661–674. doi: 10.1016/s0076-6879(99)94038-7. [DOI] [PubMed] [Google Scholar]
- 15.Sahl H-G, Kordel M, Benz R. Voltage-dependent depolarization of bacterial membranes and artificial lipid bilayers by the peptide antibiotic nisin. Arch Microbiol. 1987;149:120–124. doi: 10.1007/BF00425076. [DOI] [PubMed] [Google Scholar]
- 16.Schein S J, Kagan B L, Finkelstein A. Colicin K acts by forming voltage-dependent channels in phospholipid bilayer membranes. Nature. 1978;276:159–163. doi: 10.1038/276159a0. [DOI] [PubMed] [Google Scholar]
- 17.Sullam P M, Frank U, Tauber M G, Yeaman M R, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Yeaman M R, Bayer A S. In vitro resistance to platelet microbicidal protein correlates with endocarditis source among staphylococcal isolates. Antimicrob Agents Chemother. 1994;38:729–732. doi: 10.1128/aac.38.4.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yeaman M R, Puentes S M, Norman D C, Bayer A S. Partial characterization and staphylocidal activity of thrombin-induced platelet microbicidal protein. Infect Immun. 1992;60:1202–1209. doi: 10.1128/iai.60.3.1202-1209.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yeaman M R, Ibrahim A S, Edwards J E, Jr, Bayer A S, Ghannoum M A. Thrombin-induced platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1992;37:546–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeaman M R, Soldan S S, Ghannoum M A, Edwards J E, Jr, Filler S G, Bayer A S. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeaman M R, Tang Y-Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yeaman M R, Bayer A S, Koo S-P, Foss W, Sullam P M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureus cytoplasmic membrane by distinct mechanisms of action. J Clin Investig. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]