Abstract
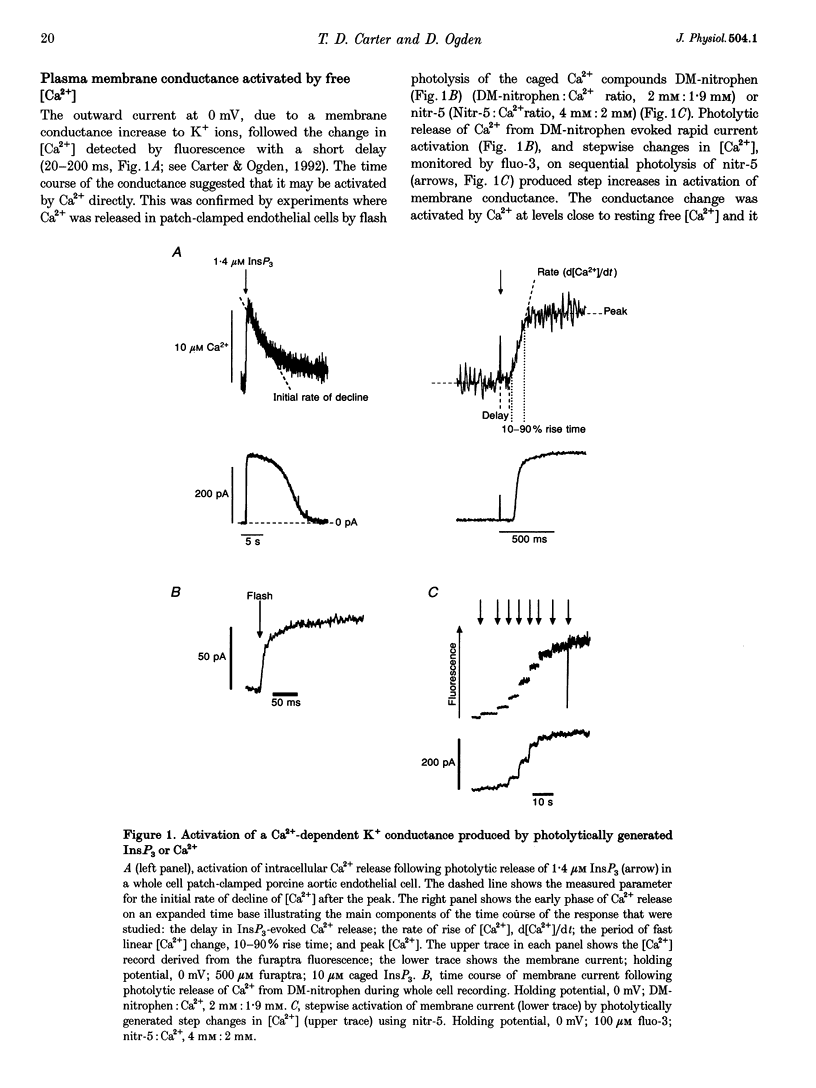
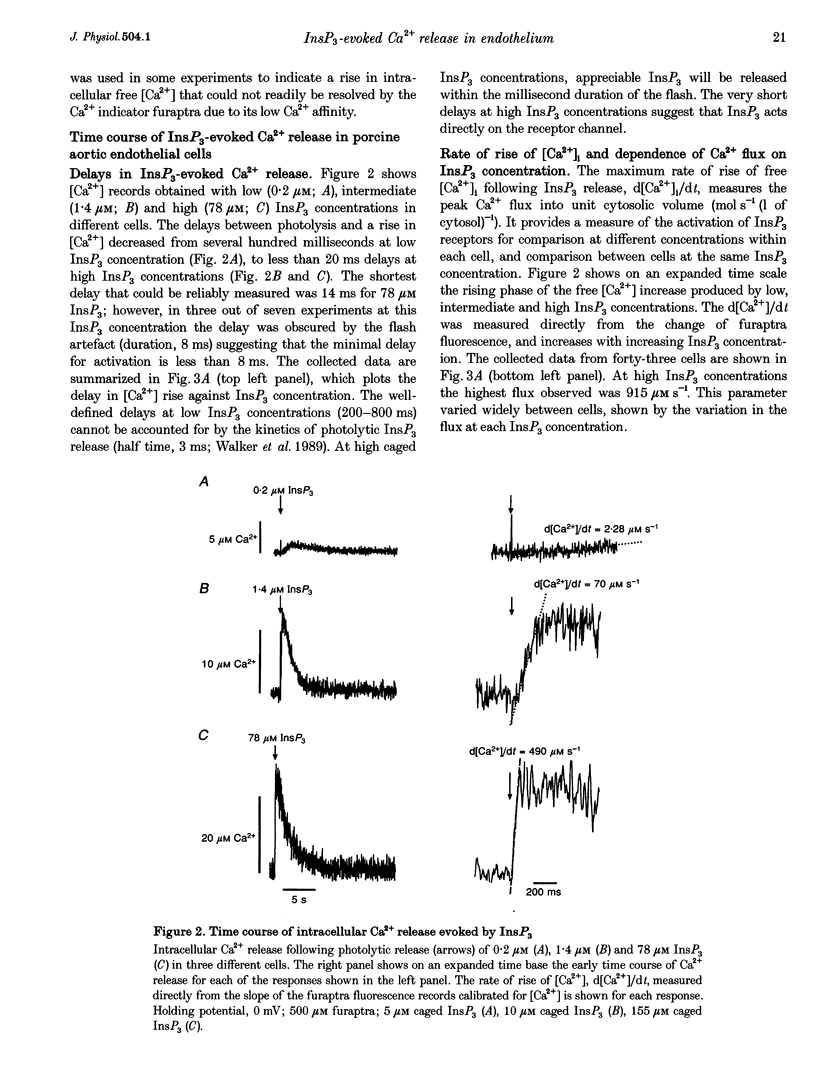
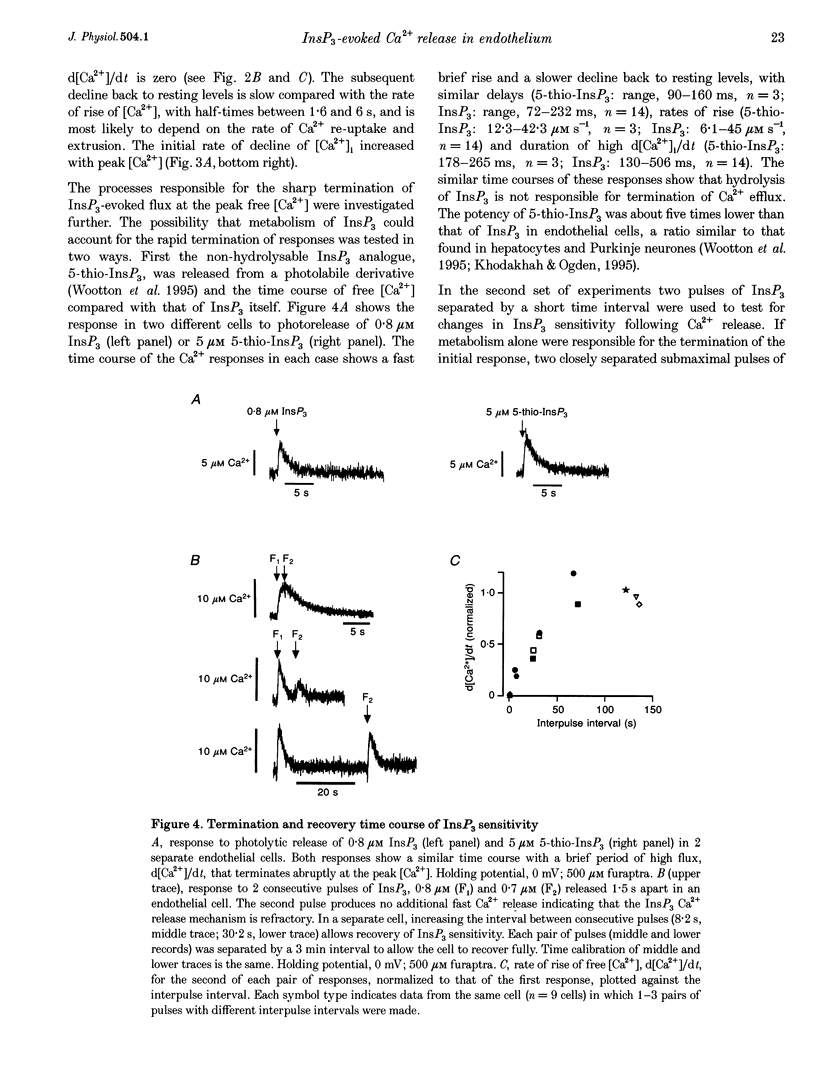
1. The role of the InsP3 receptor and its interaction with Ca2+ in shaping endothelial Ca2+ spikes was investigated by comparing InsP3-evoked intracellular Ca2+ release with hormonally evoked Ca2+ spikes in single endothelial cells. 2. InsP3 was generated by flash photolysis of intracellular caged InsP3. InsP3 at 0.2 microM or higher released Ca2+ from stores with a time course comprising a well-defined delay, a fast rise of free [Ca2+] to a peak where net flux into the cystosol is zero, and a slow decline to preflash levels. InsP3-evoked Ca2+ flux into unit cytosolic volume was measured as the rate of change of free [Ca2+]i during the fast rise, d[Ca2+]i/dt (mol s-1 l-1). 3. The mean delay decreased from 433 ms at 0.2 microM to 30 ms at 5 microM. At very high InsP3 concentrations, 78 microM, the delay was shorter, < 10 ms. At low InsP3 concentration the delay was reduced by approximately 30% by prior elevation of free [Ca2+]i, supporting a co-operative action of free [Ca2+] and InsP3 in activation. 4. Both Ca2+ flux and peak free [Ca2+]i increased with InsP3 concentration within each cell. Maximal activation was at > 5 microM, 50% maximum Ca2+ flux was at 1.6 microM InsP3 and the Hill coefficient was between 3.6 and 4.3. A large variation of Ca2+ flux and peak [Ca2+]i was found from cell to cell at the same InsP3 concentration. 5. Strong inhibition of InsP3-evoked flux was produced by an immediately preceding response, with complete inhibition at peak free [Ca2+]i due to the first pulse. InsP3 sensitivity returned over 1-2 min, with 50% recovery at approximately 25 s. The recovery of InsP3 sensitivity may determine the minimum interval between hormonally evoked spikes. 6. Ca2+ flux due to a pulse of InsP3 terminated rapidly, in the continued presence of InsP3, producing a well-defined peak [Ca2+]. A reciprocal relation was found between the duration and the rate of Ca2+ flux, such that high Ca2+ flux was of brief duration. The rate of termination of flux measured as the reciprocal of the 10-90% rise time of free [Ca2+]i showed a linear correlation with Ca2+ flux over a large range in all cells. A systematic deviation from linearity at low InsP3 concentration showed a greater rate of termination at low InsP3 concentration than at high for the same flux. 7. Elevating cytosolic free [Ca2+] by 0.1-2.5 microM strongly inhibited Ca2+ release by InsP3, and buffering free [Ca2+] to low levels greatly prolonged Ca2+ release. Both results support the idea that Ca2+ flux quickly produces locally high free [Ca2+] which inhibits the receptor and terminates Ca2+ release. 8. Hormonally evoked Ca2+ spikes showed a similar reciprocal relation between rise time and Ca2+ flux, seen in the initial Ca2+ spike evoked by extracellular ATP in porcine aortic endothelial cells and by acetylcholine in rat aortic endothelial cells in situ, supporting the idea that the same mechanism of cytosolic Ca2+ inhibition determines the duration of hormonally and InsP3-evoked Ca2+ spikes.
Full text
PDF


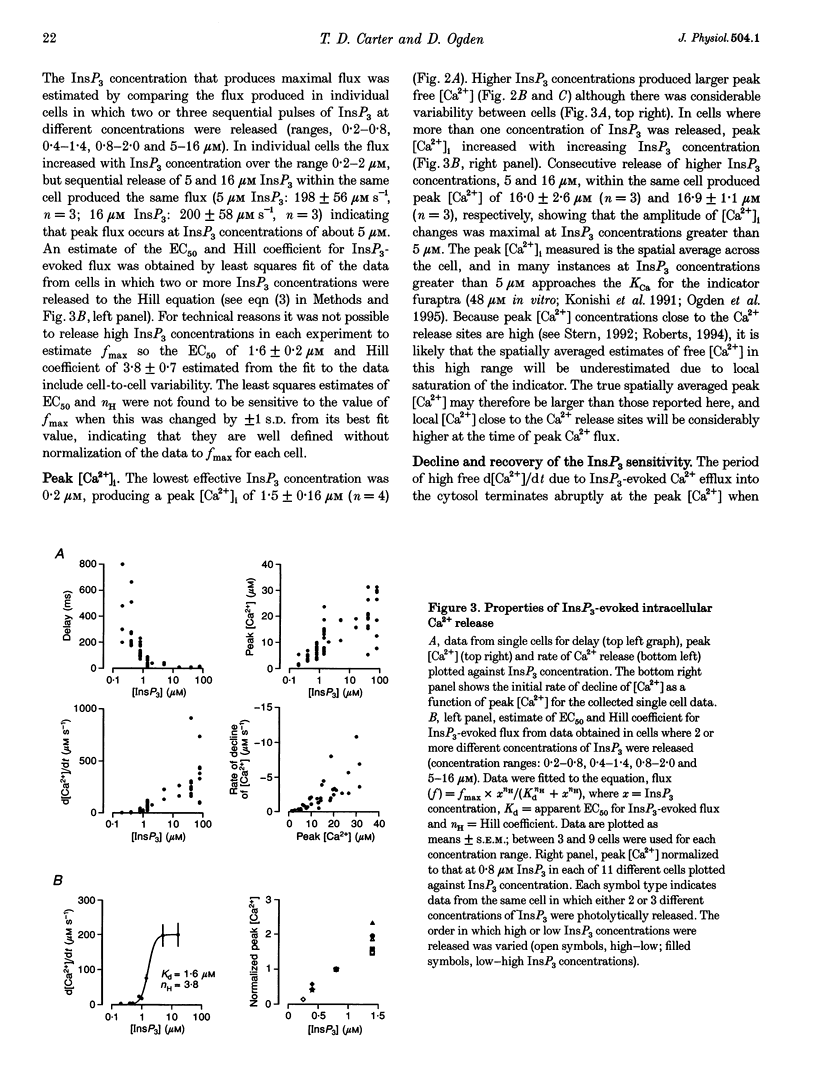





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
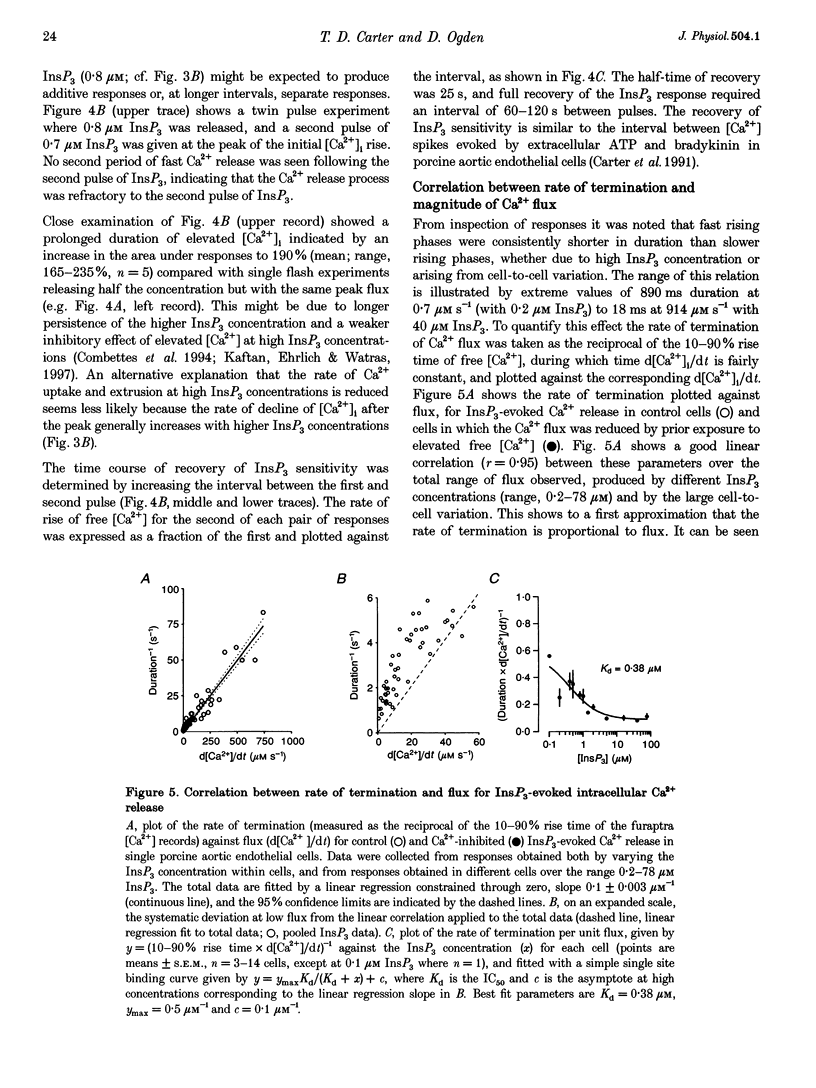
- Adams D. J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989 Oct;3(12):2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
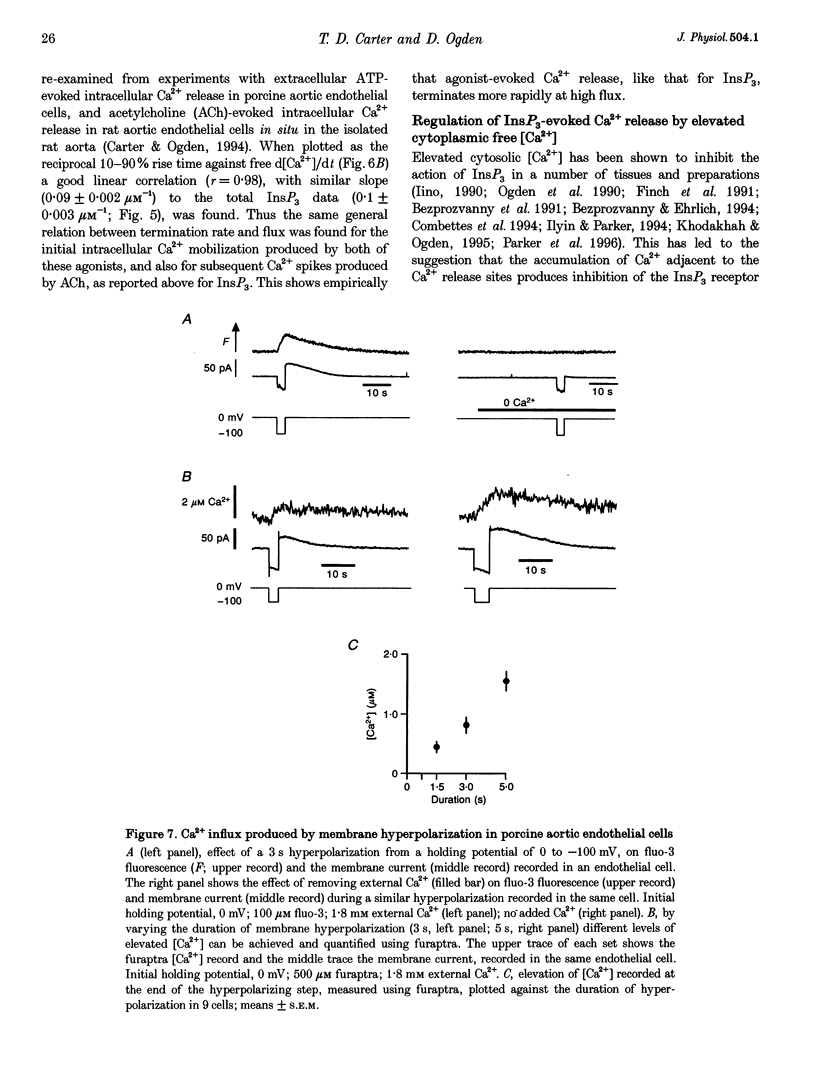
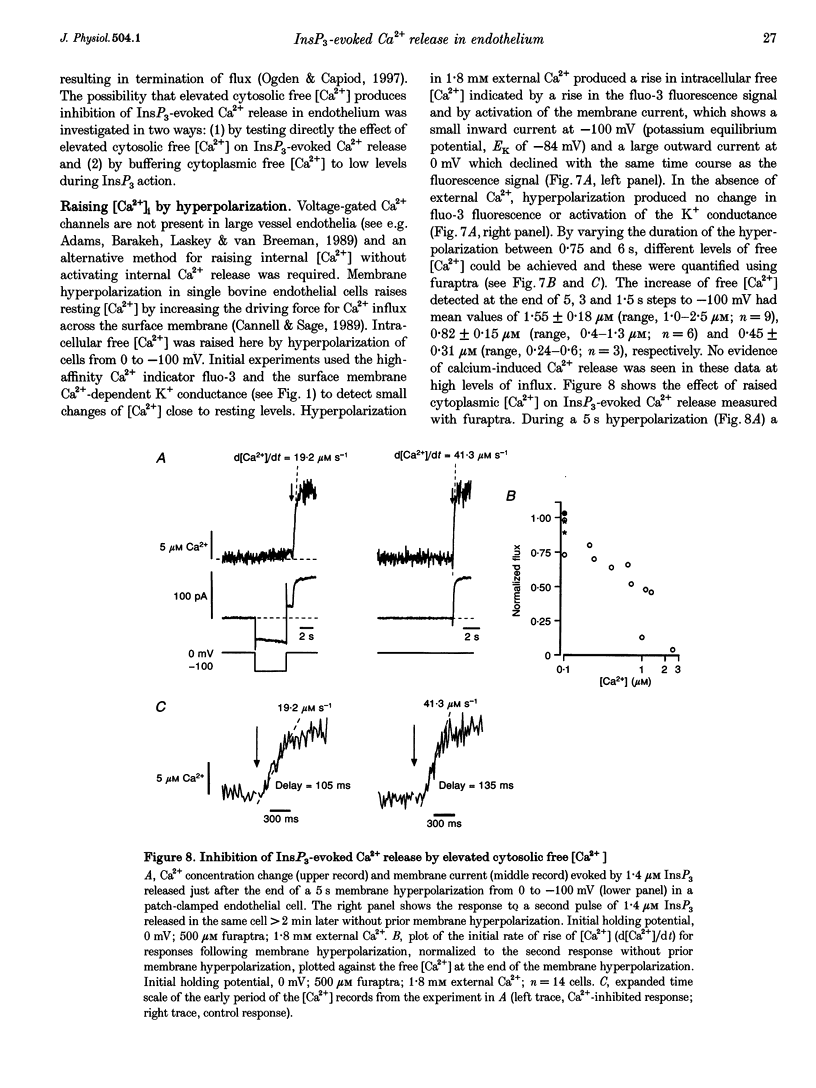
- Baylor S. M., Hollingworth S. Fura-2 calcium transients in frog skeletal muscle fibres. J Physiol. 1988 Sep;403:151–192. doi: 10.1113/jphysiol.1988.sp017244. [DOI] [PMC free article] [PubMed] [Google Scholar]
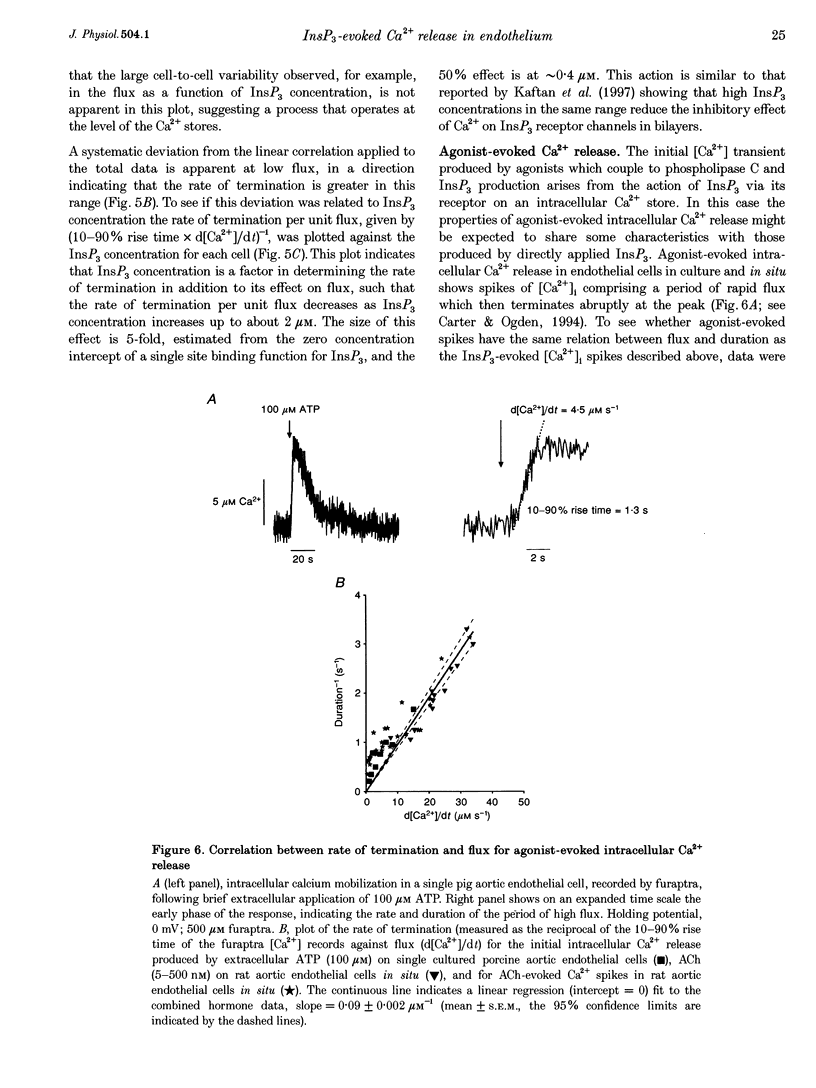
- Berridge M. J. Inositol trisphosphate-induced membrane potential oscillations in Xenopus oocytes. J Physiol. 1988 Sep;403:589–599. doi: 10.1113/jphysiol.1988.sp017266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Ehrlich B. E. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol. 1994 Nov;104(5):821–856. doi: 10.1085/jgp.104.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezprozvanny I., Watras J., Ehrlich B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991 Jun 27;351(6329):751–754. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- Cannell M. B., Sage S. O. Bradykinin-evoked changes in cytosolic calcium and membrane currents in cultured bovine pulmonary artery endothelial cells. J Physiol. 1989 Dec;419:555–568. doi: 10.1113/jphysiol.1989.sp017886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Bogle R. G., Bjaaland T. Spiking of intracellular calcium ion concentration in single cultured pig aortic endothelial cells stimulated with ATP or bradykinin. Biochem J. 1991 Sep 15;278(Pt 3):697–704. doi: 10.1042/bj2780697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter T. D., Ogden D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflugers Arch. 1994 Oct;428(5-6):476–484. doi: 10.1007/BF00374568. [DOI] [PubMed] [Google Scholar]
- Carter T. D., Ogden D. Kinetics of intracellular calcium release by inositol 1,4,5-trisphosphate and extracellular ATP in porcine cultured aortic endothelial cells. Proc Biol Sci. 1992 Dec 22;250(1329):235–241. doi: 10.1098/rspb.1992.0154. [DOI] [PubMed] [Google Scholar]
- Champeil P., Combettes L., Berthon B., Doucet E., Orlowski S., Claret M. Fast kinetics of calcium release induced by myo-inositol trisphosphate in permeabilized rat hepatocytes. J Biol Chem. 1989 Oct 25;264(30):17665–17673. [PubMed] [Google Scholar]
- Combettes L., Hannaert-Merah Z., Coquil J. F., Rousseau C., Claret M., Swillens S., Champeil P. Rapid filtration studies of the effect of cytosolic Ca2+ on inositol 1,4,5-trisphosphate-induced 45Ca2+ release from cerebellar microsomes. J Biol Chem. 1994 Jul 1;269(26):17561–17571. [PubMed] [Google Scholar]
- Davies P. F., Mundel T., Barbee K. A. A mechanism for heterogeneous endothelial responses to flow in vivo and in vitro. J Biomech. 1995 Dec;28(12):1553–1560. doi: 10.1016/0021-9290(95)00102-6. [DOI] [PubMed] [Google Scholar]
- Finch E. A., Turner T. J., Goldin S. M. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991 Apr 19;252(5004):443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Frearson J. A., Harrison P., Scrutton M. C., Pearson J. D. Differential regulation of von Willebrand factor exocytosis and prostacyclin synthesis in electropermeabilized endothelial cell monolayers. Biochem J. 1995 Jul 15;309(Pt 2):473–479. doi: 10.1042/bj3090473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freay A., Johns A., Adams D. J., Ryan U. S., Van Breemen C. Bradykinin and inositol 1,4,5-trisphosphate-stimulated calcium release from intracellular stores in cultured bovine endothelial cells. Pflugers Arch. 1989 Aug;414(4):377–384. doi: 10.1007/BF00585046. [DOI] [PubMed] [Google Scholar]
- Hajnóczky G., Thomas A. P. The inositol trisphosphate calcium channel is inactivated by inositol trisphosphate. Nature. 1994 Aug 11;370(6489):474–477. doi: 10.1038/370474a0. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Iino M. Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J Gen Physiol. 1990 Jun;95(6):1103–1122. doi: 10.1085/jgp.95.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Endo M. Calcium-dependent immediate feedback control of inositol 1,4,5-triphosphate-induced Ca2+ release. Nature. 1992 Nov 5;360(6399):76–78. doi: 10.1038/360076a0. [DOI] [PubMed] [Google Scholar]
- Ilyin V., Parker I. Role of cytosolic Ca2+ in inhibition of InsP3-evoked Ca2+ release in Xenopus oocytes. J Physiol. 1994 Jun 15;477(Pt 3):503–509. doi: 10.1113/jphysiol.1994.sp020211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob R., Merritt J. E., Hallam T. J., Rink T. J. Repetitive spikes in cytoplasmic calcium evoked by histamine in human endothelial cells. Nature. 1988 Sep 1;335(6185):40–45. doi: 10.1038/335040a0. [DOI] [PubMed] [Google Scholar]
- Khodakhah K., Ogden D. Fast activation and inactivation of inositol trisphosphate-evoked Ca2+ release in rat cerebellar Purkinje neurones. J Physiol. 1995 Sep 1;487(Pt 2):343–358. doi: 10.1113/jphysiol.1995.sp020884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodakhah K., Ogden D. Functional heterogeneity of calcium release by inositol trisphosphate in single Purkinje neurones, cultured cerebellar astrocytes, and peripheral tissues. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):4976–4980. doi: 10.1073/pnas.90.11.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi M., Hollingworth S., Harkins A. B., Baylor S. M. Myoplasmic calcium transients in intact frog skeletal muscle fibers monitored with the fluorescent indicator furaptra. J Gen Physiol. 1991 Feb;97(2):271–301. doi: 10.1085/jgp.97.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T., Holowka D., Stryer L. Highly cooperative opening of calcium channels by inositol 1,4,5-trisphosphate. Science. 1988 Apr 29;240(4852):653–656. doi: 10.1126/science.2452482. [DOI] [PubMed] [Google Scholar]
- Missiaen L., De Smedt H., Droogmans G., Casteels R. Ca2+ release induced by inositol 1,4,5-trisphosphate is a steady-state phenomenon controlled by luminal Ca2+ in permeabilized cells. Nature. 1992 Jun 18;357(6379):599–602. doi: 10.1038/357599a0. [DOI] [PubMed] [Google Scholar]
- Oancea E., Meyer T. Reversible desensitization of inositol trisphosphate-induced calcium release provides a mechanism for repetitive calcium spikes. J Biol Chem. 1996 Jul 19;271(29):17253–17260. doi: 10.1074/jbc.271.29.17253. [DOI] [PubMed] [Google Scholar]
- Ogden D. C., Capiod T., Walker J. W., Trentham D. R. Kinetics of the conductance evoked by noradrenaline, inositol trisphosphate or Ca2+ in guinea-pig isolated hepatocytes. J Physiol. 1990 Mar;422:585–602. doi: 10.1113/jphysiol.1990.sp018002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D. C., Khodakhah K., Carter T. D., Gray P. T., Capiod T. Mechanisms of intracellular calcium release during hormone and neurotransmitter action investigated with flash photolysis. J Exp Biol. 1993 Nov;184:105–127. doi: 10.1242/jeb.184.1.105. [DOI] [PubMed] [Google Scholar]
- Ogden D., Capiod T. Regulation of Ca2+ release by InsP3 in single guinea pig hepatocytes and rat Purkinje neurons. J Gen Physiol. 1997 Jun;109(6):741–756. doi: 10.1085/jgp.109.6.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden D., Khodakhah K., Carter T., Thomas M., Capiod T. Analogue computation of transient changes of intracellular free Ca2+ concentration with the low affinity Ca2+ indicator furaptra during whole-cell patch-clamp recording. Pflugers Arch. 1995 Feb;429(4):587–591. doi: 10.1007/BF00704165. [DOI] [PubMed] [Google Scholar]
- Parker I., Ivorra I. Inhibition by Ca2+ of inositol trisphosphate-mediated Ca2+ liberation: a possible mechanism for oscillatory release of Ca2+. Proc Natl Acad Sci U S A. 1990 Jan;87(1):260–264. doi: 10.1073/pnas.87.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker I., Yao Y., Ilyin V. Fast kinetics of calcium liberation induced in Xenopus oocytes by photoreleased inositol trisphosphate. Biophys J. 1996 Jan;70(1):222–237. doi: 10.1016/S0006-3495(96)79565-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzuto R., Brini M., Murgia M., Pozzan T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science. 1993 Oct 29;262(5134):744–747. doi: 10.1126/science.8235595. [DOI] [PubMed] [Google Scholar]
- Roberts W. M. Localization of calcium signals by a mobile calcium buffer in frog saccular hair cells. J Neurosci. 1994 May;14(5 Pt 2):3246–3262. doi: 10.1523/JNEUROSCI.14-05-03246.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. W., Hallam T. J., Rink T. J. TMB-8 inhibits secretion evoked by phorbol ester at basal cytoplasmic free calcium in quin2-loaded platelets much more effectively than it inhibits thrombin-induced calcium mobilisation. FEBS Lett. 1984 Oct 15;176(1):139–143. doi: 10.1016/0014-5793(84)80928-x. [DOI] [PubMed] [Google Scholar]
- Somlyo A. V., Horiuti K., Trentham D. R., Kitazawa T., Somlyo A. P. Kinetics of Ca2+ release and contraction induced by photolysis of caged D-myo-inositol 1,4,5-trisphosphate in smooth muscle. The effects of heparin, procaine, and adenine nucleotides. J Biol Chem. 1992 Nov 5;267(31):22316–22322. [PubMed] [Google Scholar]
- Stern M. D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium. 1992 Mar;13(3):183–192. doi: 10.1016/0143-4160(92)90046-u. [DOI] [PubMed] [Google Scholar]
- Walker J. W., Feeney J., Trentham D. R. Photolabile precursors of inositol phosphates. Preparation and properties of 1-(2-nitrophenyl)ethyl esters of myo-inositol 1,4,5-trisphosphate. Biochemistry. 1989 Apr 18;28(8):3272–3280. doi: 10.1021/bi00434a023. [DOI] [PubMed] [Google Scholar]
- Wootton J. F., Corrie J. E., Capiod T., Feeney J., Trentham D. R., Ogden D. C. Kinetics of cytosolic Ca2+ concentration after photolytic release of 1-D-myo-inositol 1,4-bisphosphate 5-phosphorothioate from a caged derivative in guinea pig hepatocytes. Biophys J. 1995 Jun;68(6):2601–2607. doi: 10.1016/S0006-3495(95)80444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


