Abstract
Purpose
The optimal treatment of ypT1 rectal cancer after neoadjuvant chemoradiotherapy (nCRT) remains controversial. This study aimed to determine whether local excision is non-inferior to radical surgery and whether adjuvant chemotherapy (ACT) would improve survival in patients with ypT1 rectal cancer after nCRT.
Methods
We enrolled 1212 and 91 patients with ypT1 rectal cancer underwent nCRT followed by radical surgery from the SEER database (2004–2018) and the Zhejiang Cancer Hospital (ZJCH) (2010–2022), respectively. Another 62 patients underwent LE were also identified from SEER registries. Propensity score matching was performed to balance baseline characteristics between patients in different treatment groups.
Results
Regional nodal metastasis was histopathologically detected in 257 patients (20.7%) within the SEER cohort, showing a significant association with poor cancer-specific survival (CSS) and overall survival (OS). Consistent findings were also observed in the ZJCH cohort. After 1:1 propensity score matching (60 pairs), no significant differences were observed between the extended resection and local excision groups in CSS (hazard ratio [HR] 0.88, P = 0.785) and OS (HR 0.81, P = 0.450). Patients with regional nodal metastases were more likely to receive ACT, while no apparent survival benefit was observed with additional ACT after PSM adjusting (187 pairs). Notwithstanding, for individuals younger than 50 years, ACT might provide a survival benefit in CSS (HR 0.25, P = 0.033) and OS (HR 0.30, P = 0.022).
Conclusion
Although patients with ypT1 rectal cancer have a non-negligible risk for nodal metastasis, oncologic outcomes of local excision following nCRT seem to be comparable to radical surgery. ACT could not effectively improve prognosis in patients with ypT1 tumors, except for those younger than 50 years of age.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00384-024-04764-y.
Keywords: ypT1 rectal cancer, Local excision, Adjuvant chemotherapy, Survival outcome, SEER
Introduction
Colorectal cancer (CRC) is one of the most common fatal malignancies worldwide, with approximately one-third of these cases originating from the rectum [1]. Despite steady decline in the overall incidence of CRC in Western countries during the past three decades, the incidence of rectal cancer in younger adults has been exponentially increasing [2, 3]. Notably, the determination of an individual treatment strategy for patients with rectal cancer is a complex and multifaceted process. According to the National Comprehensive Cancer Network (NCCN) guideline, the current standard therapy for locally advanced rectal cancer (LARC) includes neoadjuvant chemoradiotherapy (nCRT), surgery, and adjuvant chemotherapy (ACT) [4]. nCRT was recommended with the goal of tumor downstaging, increasing organ preservation rate and decreasing locoregional relapse [5]. In the German CAO/ARO/AIO-94 trial, approximately 6% of patients with LARC were reported to downstage to the ypT1 stage after receiving nCRT, which maybe higher in the future because of the widespread promotion of total neoadjuvant therapy (TNT) [6, 7].
The presence of lymph node (LN) metastasis in rectal cancer portends a poor prognosis, which has been used to guide local and systemic treatment decisions. The rarity of ypT1 rectal cancer poses challenges in determining the risk of regional nodal metastasis and identifying optimal treatment strategies, which still remain inconclusive and controversial. For pT1 rectal cancer with low risk of LN metastasis, local excision is the preferred minimally invasive procedure, owing to a similar long-term survival but higher organ preservation rate compared with conventional radical surgery [4, 8]. However, these advantages from the local excision remain dubious for patients with ypT1 rectal cancer. Small retrospective studies included patients with ypT0-1 rectal cancer pointed out that local excision could yield similar oncologic outcomes compared with the total mesorectal excision, but a lower postoperative morbidity and better anal sphincter function [9, 10]. However, some investigators recommended against local excision for patients with ypT1 rectal cancer because of relatively high rates of LN metastasis and local recurrence [11]. Furthermore, the role of ACT for patients with ypT1 rectal cancer also continues to be controversial. The application of ACT is supported by the accumulating evidence from several landmark studies focused on colon cancer. However, up to now, all of completed randomized controlled trials (RCTs) failed to demonstrate any statistical efficacy of fluorouracil-based ACT in LARC after neoadjuvant chemoradiation and surgery [12–14]. Consequently, the clinical guidelines offer discordant recommendations for the postoperative treatment of LARC patients who respond well to nRCT [15]. Given the favorable therapeutic effects with standard nRCT among patients with ypT1 disease, there is insufficient evidence to support the routine use of ACT, even in the node-positive patients.
Here, we designed this first real-world study using a large national database to explore the risk of LN metastasis and the feasibility of local excision in patients with ypT1 rectal cancer after nCRT. Beyond that, particular attention was also paid to exploring the precise role of ACT in ypT1 rectal cancer.
Materials and methods
Data source and patient selection
The Surveillance, Epidemiology and End Results (SEER) program, a population-based cancer registry, is sponsored by the National Cancer Institute and aggregates cancer incidence and survival clinical data from 18 registries covering approximately 34.6% of the US population [16]. In this retrospective study, data on patients diagnosed with ypT1 rectal cancer after nCRT from 2004 to 2018 were obtained from the SEER 18 Registries using the SEER*Stat software (version 8.4.0). The selection criteria of eligible patients were set as follows: (1) the International Classification of Disease for Oncology, Third Edition code, ICD-O-3, topography code: C209; (2) ICD-O-3 histological type/behavior code: 9140/3, 9480/3,9490/3; (3) Rx Summary-Surgery/Radiation Sequence code: Radiation before surgery; and (4) all patients were restaged and ypT1 stage was defined according to the 8th American Joint Committee on Cancer (AJCC) TNM staging system. Patients with distant metastasis and those with unknown surgery status or TNM stage were excluded from this study. For each eligible patient, information pertaining to age at diagnosis, preoperative carcinoembryonic antigen (CEA) level, surgical procedures, LN harvest, LN metastasis, as well as ACT were determined. According to the surgical procedure codes, patients were classified into two treatment groups: underwent local excision only (local excision group, SEER site-specific surgery codes: 10–29) and underwent proctectomy plus regional LN dissection (extended resection group, SEER site-specific surgery codes: 30–80).
In addition, between 2010 and 2022, we enrolled another 91 consecutive ypT1 patients underwent nCRT followed by radical resection from the Zhejiang Cancer Hospital as the ZJCH cohort. For the adequate LN harvest, we focused on participants underwent extended resection in both SEER and ZJCH cohorts to facilitate further analyses of the frequency of LN metastasis.
Statistical analysis
Baseline demographics and clinical characteristics between nodal positive and nodal negative groups were described as percentages for categorical variables and compared with the Pearson chi-square test or Fisher’s exact test. A binomial logistic regression model was fitted to test the categorical associations between pathological LN metastasis and age at diagnosis, race, gender, year of diagnosis, number of LN examined, clinical N stage, and perineural invasion. Cancer-specific survival (CSS) and Overall survival (OS) were calculated from the date of diagnosis to the date of death from rectal cancer and any cause, respectively. Relapse-free survival (RFS) was calculated from the date of surgery to the date of recurrence or last follow-up. Survival outcomes were evaluated using the Kaplan–Meier method with the log-rank test for univariate comparisons. To improve the accuracy of our results, patients with unknown survival status and those suffering from at least two malignant tumors were excluded from survival analysis. After checking the proportional hazards assumption, hazard ratios (HR) and 95% CIs were estimated using multivariate-adjusted Cox proportional hazards regression model. To minimize a treatment selection bias in the SEER cohort, propensity score matching (PSM) was performed using a 1:1 matching protocol with a nearest neighbor-matching algorithm and a caliper of 0.01. All statistical analysis was performed using SPSS statistical software version 22.0 (SPSS Inc., IBM Corporation, Chicago, IL). A two-tailed P value < 0.05 was considered statistically significant for all analyses.
Results
Patient characteristics in ypT1 rectal cancer after nCRT stratified by LN status
We identified 1303 patients with ypT1 rectal cancer who underwent nCRT followed by extended resection, 1212 patients from the SEER database (SEER cohort) and 91 patients from the Zhejiang Cancer Hospital (ZJCH cohort). Demographic and clinicopathological characteristics of the participants stratified by LN status are summarized in Table 1. Regional LN metastasis was observed in 257 patients (20.7%) in the SEER cohort and 15 patients (16.4%) in the ZJCH cohort, respectively. In the SEER cohort, a close correlation was found between age and LN metastasis (P < 0.001). Compared with patients ≥ 70 years, the rate of LN metastasis was twofold higher in patients younger than 50 years (12.8% vs 26.1%). The proportions of nodal metastasis gradually increased along the years of diagnosis, from 15.4 (2004–2008) to 28.5% (2014–2018) (P < 0.001). LN harvest and perineural invasion were astonishingly connected with LN metastasis (both P < 0.001). There was no association between preoperative CEA level and LN metastasis. The agreement of the clinical diagnosis with pathology were 43.4% and 66.7% for LN metastasis in the SEER and ZJCH cohorts, respectively. Patients with pathologically positive nodes in both cohorts were more likely to receive ACT, especially in the ZJCH cohort (93.3% vs 36.8% in the SEER cohort) (Table 1).
Table 1.
Clinicopathologic characteristics of patients with ypT1 rectal cancer after neoadjuvant chemoradiotherapy stratified by LN status
| Variable | SEER cohort (n = 1212) | ZJCH cohort (n = 91) | ||||
|---|---|---|---|---|---|---|
| LN positive (%) | LN negative (%) | P† | LN positive (%) | LN negative (%) | P† | |
| Total patients | 251 (20.7) | 961 (79.3) | 15 (16.4) | 76 (83.6) | ||
| Age at diagnose (years) | < 0.001 | 0.672 ‡ | ||||
| < 50 | 58 (23.1) | 164 (17.1) | 4 (26.7) | 13 (13.1) | ||
| 50–69 | 150 (59.8) | 505 (52.5) | 9 (60.0) | 49 (64.5) | ||
| ≥ 70 | 43 (17.1) | 292 (30.4) | 2 (13.3) | 14 (18.4) | ||
| Gender | 0.043 | 0.260 | ||||
| Male | 147 (58.6) | 629 (65.5) | 8 (53.3) | 52 (68.4) | ||
| Female | 104 (41.4) | 332 (34.5) | 7 (46.7) | 24 (31.6) | ||
| Race | 0.019 ‡ | NA | ||||
| White | 202 (80.5) | 798 (83.0) | ||||
| Black | 21 (8.4) | 79 (8.2) | ||||
| Asian/PI/AI | 25 (10.0) | 84 (8.7) | ||||
| Unknown | 3 (1.2) | 0 (0.0) | ||||
| Year of diagnosis | < 0.001 | NA | ||||
| 2004–2008 | 88 (35.1) | 484 (50.4) | ||||
| 2009–2013 | 100 (39.8) | 319 (33.2) | ||||
| 2014–2018 | 63 (25.1) | 158 (16.4) | ||||
| Grade | 0.873 | 0.139 ‡ | ||||
| Well/moderately differentiated | 183 (72.9) | 712 (74.1) | 9 (60.0) | 55 (72.4) | ||
| Poorly differentiated/undifferentiated | 22 (8.8) | 75 (7.8) | 3 (20.0) | 3 (3.9) | ||
| Unknown | 46 (18.3) | 174 (18.1) | 3 (20.0) | 18 (23.7) | ||
| Histologic type | 0.1780 ‡ | 0.083 ‡ | ||||
| Adenocarcinoma | 237 (94.4) | 932 (97.0) | 12 (80.0) | 72 (94.7) | ||
| Mucinous adenocarcinoma | 11 (4.4) | 22 (2.3) | 3 (20.0) | 4 (5.3) | ||
| Signet ring cell carcinoma | 3 (1.2) | 7 (0.7) | - | - | ||
| CEA level | 0.321 | 0.990 ‡ | ||||
| Normal | 106 (42.2) | 356 (37.0) | 14 (93.3) | 71 (93.4) | ||
| Elevated | 46 (18.3) | 194 (20.2) | 1 (6.7) | 5 (6.6) | ||
| Borderline/unknown | 99 (39.4) | 411 (42.8) | - | - | ||
| No. of LN examined | < 0.001‡ | 0.744 | ||||
| LN < 12 | 104 (41.4) | 605 (63) | 8 (53.3) | 42 (55.3) | ||
| LN ≥ 12 | 144 (57.4) | 342 (35.6) | 7 (46.7) | 34 (44.7) | ||
| Unknown | 3 (1.2) | 14 (1.5) | - | - | ||
| Clinical LN metastasis | < 0.001 | < 0.001 | ||||
| Negative | 43 (17.1) | 465 (48.4) | 5 (33.3) | 61 (80.3) | ||
| Positive | 109 (43.4) | 40 (4.2) | 10 (66.7) | 15 (19.7) | ||
| Unknown | 99 (39.4) | 456 (47.5) | - | - | ||
| Perineural invasion | < 0.001 | NA | ||||
| No | 107 (42.6) | 296 (30.8) | ||||
| Yes | 6 (2.4) | 5 (0.5) | ||||
| Unknown | 138 (55.0) | 660 (68.7) | ||||
| Adjuvant chemotherapy | < 0.001 | 0.035 ‡ | ||||
| No | 132 (52.6) | 608 (63.3) | 1 (6.7) | 23 (30.2) | ||
| Yes | 77 (30.7) | 132 (13.7) | 14 (93.3) | 53 (69.7) | ||
| Unknown | 42 (16.7) | 221 (23.0) | - | - | ||
LN lymph node, PI Pacific Islander, AI American Indian, NA not available, CEA carcinoembryonic antigen
†Pearson chi-square test
‡Fisher’s exact test
Preoperative predictors and prognostic value of LN metastasis in ypT1 rectal cancer
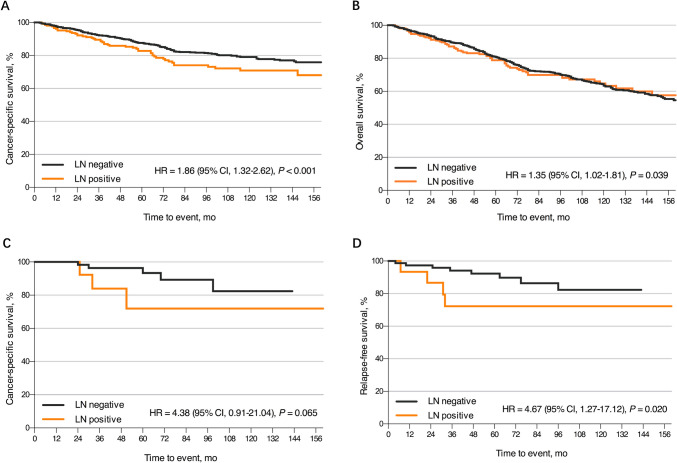
In the SEER cohort, a higher risk of pathologically confirmed LN involvement was associated with age at diagnosis, numbers of retrieved LNs, clinical LN metastasis, and perineural invasion status instead of gender, race/ethnicity, or the year of diagnosis (Table 2). The odds of LN metastasis in patients older than 70 years were 0.43 times lower than the odds in patients younger than 50 (odds ratio (OR) 0.43, 95% CI 0.26–0.73, P < 0.001). Patients with 12 or more LNs retrieved (OR 1.95, 95% CI 1.38–2.75, P < 0.001) and perineural invasion (OR 4.58, 95% CI 1.00–20.89, P = 0.050) were more likely to have regional LN involvement. Consistent with finding from the SEER cohort, clinical LN metastasis remain significant preoperative predictors of pathological LN metastasis in the ZJCH cohort (OR 8.80, 95% CI 2.37–32.61, P = 0.001). One hundred and ninety-three deaths were caused by ypT1 rectal cancer in the SEER cohort, and the 10-year CSS and OS rates were 77.5% and 63.5%, respectively. LN metastasis increased the risk of cancer-specific mortality (HR 1.86, 95% CI 1.32–2.62, P < 0.001, Fig. 1A) and all-cause mortality (HR 1.35, 95% CI 1.02–1.81, P = 0.039, Fig. 1B). During follow-up, 12 of the 91 patients (13.2%) experienced local recurrence or distant metastasis in the ZJCH cohort. Among 78 patients, all 8 deaths were related to tumor progression and the cumulative 10-year CSS rate was 80.2%. LN metastasis appeared to be associated with poor CSS (HR 4.38, 95% CI 0.91–21.04, P = 0.065, Fig. 1C) and RFS (HR 4.67, 95% CI 1.27–17.12, P = 0.020, Fig. 1D) in patients with ypT1 rectal cancer following nCRT.
Table 2.
Multivariable logistic regression analysis of potential factors associated with LN metastasis in patients with ypT1 rectal cancer after neoadjuvant chemoradiotherapy
| Variable | SEER cohort (n = 1212) | ZJCH cohort (n = 91) | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |||
| Age at diagnose (years) | ||||||
| < 50 | 1 (reference) | 1 (reference) | ||||
| 50–69 | 0.89 (0.58–1.34) | 0.565 | 0.66 (0.14–3.13) | 0.602 | ||
| ≥ 70 | 0.43 (0.26–0.72) | 0.001 | 0.54 (0.07–4.28) | 0.557 | ||
| Gender | ||||||
| Male | 1 (reference) | 1 (reference) | ||||
| Female | 1.20 (0.85–1.68) | 0.299 | 1.55 (0.42–5.64) | 0.509 | ||
| Race | NA | |||||
| White | 1 (reference) | |||||
| Black | 0.90 (0.49–1.64) | 0.731 | ||||
| Asian/PI/AI | 1.25 (0.73–2.16) | 0.414 | ||||
| Year of diagnosis | NA | |||||
| 2004–2008 | 1 (reference) | |||||
| 2009–2013 | 1.55 (0.90–2.66) | 0.117 | ||||
| 2014–2018 | 1.48 (0.81–2.69) | 0.201 | ||||
| CEA level | ||||||
| Normal | 1 (reference) | 1 (reference) | ||||
| Elevated | 0.77 (0.48–1.22) | 0.263 | 0.65 (0.05–7.74) | 0.731 | ||
| No. of LN examined | ||||||
| LN < 12 | 1 (reference) | 1 (reference) | ||||
| LN ≥ 12 | 1.95 (1.38–2.75) | < 0.001 | 1.22 (0.33–4.52) | 0.770 | ||
| Clinical LN metastasis | ||||||
| Negative | 1 (reference) | 1 (reference) | ||||
| Positive | 28.10 (17.11–46.17) | < 0.001 | 8.80 (2.37–32.61) | 0.001 | ||
| Perineural invasion | NA | |||||
| No | 1 (reference) | |||||
| Yes | 4.58 (1.00–20.89) | 0.050 | ||||
LN lymph node, OR odds ratio, CI confidence interval, PI Pacific Islander, AI American Indian, NA not available, CEA carcinoembryonic antigen
Fig. 1.
Kaplan–Meier survival curves for patients with ypT1 rectal cancer after nCRT stratified by LN status. A CSS in the SEER cohort. B OS in the SEER cohort. C CSS in the ZJCH cohort. D RFS in the ZJCH cohort. nCRT, neoadjuvant chemoradiotherapy; LN, lymph node; CSS, cancer-specific survival; OS, overall survival; RFS, relapse-free survival
Extended resection versus local excision for ypT1 rectal cancer
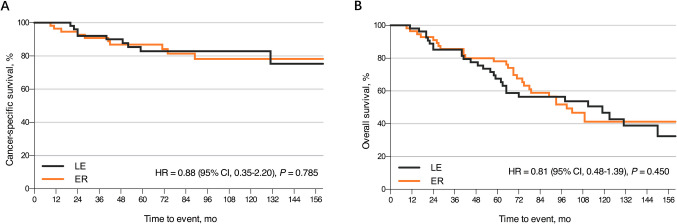
In the SEER cohort, we identified another 62 patients with ypT1 rectal cancer underwent nCRT followed by local excision from 2004 to 2018. After PSM, a total of 120 patients (60 patients in each group) were analyzed and the baseline characteristics were well-balanced between the local excision and extended resection groups (Supplemental Table 2). For multivariable survival analysis, additional LN dissection was not significantly associated with favorable CSS (HR 0.81, 95% CI 0.35–2.20, P = 0.785, Fig. 2A) or OS (HR 0.81, 95% CI 0.48–1.39, P = 0.450, Fig. 2B).
Fig. 2.
Kaplan–Meier survival curves for patients with ypT1 rectal cancer after nCRT according to the surgical approaches. A CSS comparison between ER and LE. B OS comparison between ER and LE. nCRT, neoadjuvant chemoradiotherapy; CSS, cancer-specific survival; ER, extended resection; LE, local excision; OS, overall survival
The role of ACT in patients with ypT1 rectal cancer after nCRT
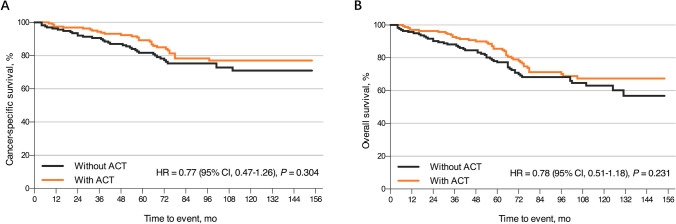
After nCRT with subsequent regional lymphadenectomy, 209 patients (17.2%) in the SEER cohort and 67 patients (73.6%) in the ZJCH cohort received ACT (Table 1). However, for multivariable Cox regression analysis in the SEER cohort, ACT did not provide apparent survival benefit in patients with ypT1 rectal cancer (CSS: HR 0.99, 95% CI 0.55–1.50, P = 0.746; OS: HR 0.95, 95% CI 0.68–1.34, P = 0.784, Supplemental Table 1). PSM in ypT1 rectal cancer with and without ACT resulted in the selection of 187 matched pairs of patients. Supplemental Table 3 summarizes the baseline characteristics of the entire unmatched and propensity score-matched cohorts. Regrettably, as shown in Fig. 3, ACT was not associated with additional survival benefit after performing PSM in terms of CSS (HR 0.77, 95% CI 0.47–1.26, P = 0.304) and OS (HR 0.78, 95% CI 0.51–1.18, P = 0.231). Subgroup analysis evaluated gender, CEA level, and number of LN examined without revealing any good evidence of improved survival in any subgroup. Of note, no related survival benefit was observed for either node-positive or node-negative patients (Supplemental Figs. 1 and 2). However, for patients younger than 50 years, ACT was shown to be significant associated with improved CSS (HR 0.25, 95% CI 0.07–0.89, P = 0.033, Supplemental Fig. 1) and OS (HR 0.30, 95% CI 0.10–0.84, P = 0.022, Supplemental Fig. 2).
Fig. 3.
Kaplan–Meier survival curves for ypT1 rectal cancer patients with or without ACT. A CSS comparison between the ACT and observation groups. B OS comparison between the ACT and observation groups. ACT, adjuvant chemotherapy; CSS, cancer-specific survival; OS, overall survival
Discussion
Currently, nCRT is the standard therapy for patients with LARC to downstage disease and reduce locoregional recurrence. Pathologic downstaging to ypT1 stage after nCRT is relatively uncommon, which means a favorable response to preoperative therapy. Given its relative rarity and the lack of prospective randomized trials, the optimal sequential treatment strategies of ypT1 patients have not been established. Thus, we designed this first and largest population-based study with long-term follow-up to shed light on the therapeutic value of local excision and ACT in patients with ypT1 rectal cancer after nCRT.
Despite patients with ypT1 rectal cancer are considered to have a good therapeutic response to nCRT, the risk of regional LN involvement cannot be ignored and approximately 12.0–20.0% of these patients presented with nodal metastasis after LN sampling [17–19]. In our study, the pathologic node-positive rates were 20.7% in the SEER cohort and 16.4% in the ZJCH cohort, respectively. Further, multivariate Cox regression analysis demonstrated that LN involvement was an independent negative prognostic factor in ypT1 rectal cancer, associated with poor CSS (HR 1.86, 95% CI 1.32–2.62, P < 0.001) and OS (HR 1.35, 95% CI 1.02–1.81, P = 0.039), in tandem with previous findings from a meta-analysis [18]. The optimal surgical approach for patients with ypT1 rectal cancer is still controversial and the role of local excision among these individuals who respond well to nCRT has not been clearly elucidated. Previously, several small retrospective studies demonstrated that local excision seems to offer a better evacuation and continence function after surgery and comparable oncological outcomes for ypT0-1 rectal cancer after nCRT [20, 21]. However, Hallam et al. [22] reported the rates of local recurrence and median disease-free survival for patients with ypT1 or higher tumors were 21.9% and 68.0%. They emphasized that local excision after nRCT could be regarded as a curative treatment only when pathological complete response was confirmed. In our study, radical surgery was still the mainstream treatment for ypT1 rectal cancer according to the NCCN guideline. However, consistent with previous findings [20, 21], additional regional LN dissection was not found to improve oncologic outcomes after a longer period of follow-up (CSS: HR 0.88, 95% CI 0.35–2.20, P = 0.785; OS: HR 0.81, 95% CI 0.48–1.39, P = 0.450). Compared to radical surgery, local excision seems to be a promising minimally invasive approach with a lower incidence of postoperative complications and better anal sphincter function [20, 21]. However, given the non-negligible propensity of LN metastasis, the recommendation of an organ-sparing approach by local excision for ypT1 rectal cancer may still require some caution. A strict patient selection criteria and close follow-up is needed to minimize the risk of recurrence and metastasis, which posed by a non-radical surgery. The clinicopathological predictors of residual nodal disease in ypT1 rectal cancer such as age, the distance of the tumor margin to the anal verge, tumor grade, residual tumor diameter, and clinical LN status, should to be taken into account when formulating a surgical decision-making [20, 23, 24].
In fact, the adjuvant treatment of LARC still refer to the regimens of ACT for colon cancer, which are supported by several landmark clinical trials. The clinical significance of ACT following nCRT and surgery in patients with LARC remains controversial and the clinical guidelines offer discordant recommendations of adjuvant treatment for patients with a good therapeutic response to nRCT [15]. In our study, we found that ACT did not appear to provide survival benefit in ypT1 rectal cancer, regardless of whether LN status, grade, number of LN examined, and CEA level varied. For node-negative patients, most of the previous studies were performed with a small sample size and indicated that ACT seems not to be required for patients with yp stage I rectal cancer downstaged by nCRT, which was consistent with our study [25, 26]. More high-level evidence is required to validate this finding, and a RCT of ACT for patients with pathologic complete response or yp stage I is currently ongoing [27]. Also, it is worth noting that no survival benefit of ACT was found for node-positive patients in our population-based propensity score analysis, which seems to be different from the expectation. Indeed, there is insufficient evidence to support the routine use of ACT for yp stage III rectal cancer. Up to now, it is disappointing that all of randomized phase III trials (CHRONICLE, I-CNR-RT, PROCTOR-SCRIPT) aimed at demonstrating the effects of fluorouracil-based ACT following nRCT and surgery in LARC have failed [12–14]. A meta-analysis of RCTs similarly reported that additional ACT was not associated with a survival benefit in yp stage III rectal cancer [28]. To our knowledge, this is the first study to perform a subgroup analysis by age in the SEER cohort. A key finding in our study was that patients younger than 50 years seemed to benefit from ACT, whereas those older than 50 years did not. The possible explanation of this difference in efficacy is the heterogeneity of potential for tolerating chemotherapy-related toxicities. For elder patients, the survival benefit from ACT might be counteracted by chemotherapy-induced adverse events. Recently, for patients with LARC, NCCN guidelines have shifted the attention from adjuvant treatment to total neoadjuvant treatment (TNT) [4]. ACT does not significantly improve long-term survival but increased risk of chemotherapy-related toxicities. The RAPIDO and STELLAR trials support that TNT was associated with a significant improvement in pathologic complete response rate, disease-free survival, and OS as opposed to ACT [29, 30]. Therefore, the TNT strategy can be considered as an alternative to standard treatment in LARC and may facilitate “watch-and-wait” strategy aimed at organ preservation.
Although this study had a large sample size of consecutive patients and long follow-up, there are several limitations that warrant consideration because of its retrospective nature and intrinsic limitations in the SEER database. Firstly, pathological parameters such as tumor budding, depth of submucosal invasion, lymphovascular, and vascular invasions, have been identified as possible risk factors for LN metastasis. Unfortunately, these pathological characteristics have not been publicly released by the SEER cancer registries so far. Secondly, our analyses of ypT1 rectal cancer with local excision may have been underpowered due to insufficient sample size. Moreover, we were unable to evaluate the therapeutic value of local excision combined with ACT, which appears to show a similar efficacy compared with radical surgery in high-risk pT1 rectal cancer [31]. Thirdly, the SEER database does not provide detailed information regarding baseline comorbidities, postoperative complications, specific ACT regimens, or toxicity. Therefore, we are unable to determine if these factors might impact our results. Finally, The SEER dataset lacks data on recurrences, meaning that we could not evaluate the impact of different therapeutic strategies on local recurrence, distant metastasis, or RFS.
Conclusions
In conclusion, our population-based study yielded an unexpectedly high rate of LN metastasis in patients with ypT1 rectal cancer after nCRT. However, regional LN dissection does not seem to confer additional survival benefit. Local excision following nCRT may provide comparable long-term oncologic outcomes to radical surgery. Moreover, the application of additional ACT did not effectively improve the prognosis in patients with ypT1 rectal cancer, except for those younger than 50 years of age. Further multicenter randomized clinical trials are warranted to evaluate the true role of local excision and ACT in the management of ypT1 rectal cancer after nCRT.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Forest plots of ACT effects on cancer-specific survival within subgroups. Abbreviations: ACT, Adjuvant Chemotherapy (JPG 1063 KB)
Supplementary file2 Forest plots of ACT effects on overall survival within subgroups. Abbreviations: ACT, Adjuvant Chemotherapy (JPG 1053 KB)
Acknowledgements
The authors harbor sincere gratitude to all the staff members of the SEER program for their efforts in establishing this national database.
Authors contributions
Conceptualization: Yibo Cai, Yuping Zhu, Zhuo Liu; Methodology: Yibo Cai, Lai Jiang, Haixing Ju, Zhuo Liu; Formal analysis: Yibo Cai, Lai Jiang, Zhuo Li; Data curation: Yibo Cai, Lai Jiang; Investigation: Haixing Ju, Yuping Zhu, Zhuo Liu; Writing—original draft preparation: Yibo Cai, Zhuo Liu; Writing—review and editing: Haixing Ju,Yuping Zhu, Zhuo Liu; Supervision: Haixing Ju,Yuping Zhu, Zhuo Liu.
Funding
This study is partially supported by the Basic Public Welfare Research Project of Zhejiang Province (grant TGY23H160044), the Medical Science and Technology Project of Zhejiang Province (grant 2023KY567), and the Traditional Chinese Medicine Science and Technology of Zhejiang Province (grant 2024ZL026).
Data availability
The data obtained from SEER database in this study is publicly available from: https://seer.cancer.gov/data-software/documentation/seerstat/nov2020/.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 71(3):209–249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Keller DS, Berho M, Perez RO et al (2020) The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol 17(7):414–429. 10.1038/s41575-020-0275-y [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Torre LA, Soerjomataram I et al (2019) Global patterns and trends in colorectal cancer incidence in young adults. Gut 68(12):2179–2185. 10.1136/gutjnl-2019-319511 [DOI] [PubMed] [Google Scholar]
- 4.Benson AB, Venook AP, Al-Hawary MM et al (2022) Rectal cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20(10):1139–1167. 10.6004/jnccn.2022.0051 [DOI] [PubMed] [Google Scholar]
- 5.Rahma OE, Yothers G, Hong TS et al (2021) Use of total neoadjuvant therapy for locally advanced rectal cancer: initial results from the pembrolizumab arm of a phase 2 randomized clinical trial. JAMA Oncol 7(8):1225–1230. 10.1001/jamaoncol.2021.1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodel C, Graeven U, Fietkau R et al (2015) Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 16(8):979–989. 10.1016/S1470-2045(15)00159-X [DOI] [PubMed] [Google Scholar]
- 7.Borelli B, Germani MM, Carullo M et al (2023) Total neoadjuvant treatment and organ preservation strategies in the management of localized rectal cancer: a narrative review and evidence-based algorithm. Crit Rev Oncol Hematol 186:103985. 10.1016/j.critrevonc.2023.103985 [DOI] [PubMed] [Google Scholar]
- 8.Halverson AL, Morris AM, Cleary RK et al (2019) For patients with early rectal cancer, does local excision have an impact on recurrence, survival, and quality of life relative to radical resection? Ann Surg Oncol 26(8):2497–2506. 10.1245/s10434-019-07328-5 [DOI] [PubMed] [Google Scholar]
- 9.Rizzo G, Pafundi DP, Sionne F et al (2022) Transanal endoscopic microsurgery versus total mesorectal excision in ypT0–1 rectal cancer after preoperative radiochemotherapy: postoperative morbidity, functional results, and long-term oncologic outcome. Dis Colon Rectum 65(11):1306–1315. 10.1097/DCR.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 10.Rizzo G, Zaccone G, Magnocavallo M et al (2017) Transanal endoscopic microsurgery after neoadjuvant radiochemotherapy for locally advanced extraperitoneal rectal cancer. Eur J Surg Oncol 43(8):1488–1493. 10.1016/j.ejso.2017.05.011 [DOI] [PubMed] [Google Scholar]
- 11.Perez RO, Habr-Gama A, Lynn PB et al (2013) Transanal endoscopic microsurgery for residual rectal cancer (ypT0–2) following neoadjuvant chemoradiation therapy: another word of caution. Dis Colon Rectum 56(1):6–13. 10.1097/DCR.0b013e318273f56f [DOI] [PubMed] [Google Scholar]
- 12.Glynne-Jones R, Counsell N, Quirke P et al (2014) Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 25(7):1356–1362. 10.1093/annonc/mdu147 [DOI] [PubMed] [Google Scholar]
- 13.Sainato A, Cernusco Luna Nunzia V, Valentini V et al (2014) No benefit of adjuvant fluorouracil leucovorin chemotherapy after neoadjuvant chemoradiotherapy in locally advanced cancer of the rectum (LARC): Long term results of a randomized trial (I-CNR-RT). Radiother Oncol 113(2):223–229. 10.1016/j.radonc.2014.10.006 [DOI] [PubMed] [Google Scholar]
- 14.Breugom AJ, van Gijn W, Muller EW et al (2015) Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 26(4):696–701. 10.1093/annonc/mdu560 [DOI] [PubMed] [Google Scholar]
- 15.Carvalho C, Glynne-Jones R (2017) Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol 18(6):e354–e363. 10.1016/S1470-2045(17)30346-7 [DOI] [PubMed] [Google Scholar]
- 16.Howlader N NA, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2018, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2018/, based on November 2020 SEER data submission, posted to the SEER web site, April 2021
- 17.Park IJ, You YN, Skibber JM et al (2013) Comparative analysis of lymph node metastases in patients with ypT0–2 rectal cancers after neoadjuvant chemoradiotherapy. Dis Colon Rectum 56(2):135–141. 10.1097/DCR.0b013e318278ff8a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haak HE, Beets GL, Peeters K et al (2021) Prevalence of nodal involvement in rectal cancer after chemoradiotherapy. Br J Surg 108(10):1251–1258. 10.1093/bjs/znab194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Huh JW, Lee WY et al (2022) Correlation between T stage and lymph node metastasis in rectal cancer treated with preoperative chemoradiotherapy. Ther Adv Med Oncol 14:17588359221132620. 10.1177/17588359221132620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin YS, Park JH, Yoon SM et al (2018) Total mesorectal excision versus local excision after preoperative chemoradiotherapy in rectal cancer with lymph node metastasis: a propensity score-matched analysis. Int J Radiat Oncol Biol Phys 101(3):630–639. 10.1016/j.ijrobp.2018.02.032 [DOI] [PubMed] [Google Scholar]
- 21.Pan H, Gao Y, Ruan H et al (2023) Transanal local excision versus intersphincteric resection for low rectal cancer with stage ypT0–1ycN0 after neoadjuvant chemoradiotherapy: an inverse probability weighting analysis for oncological and functional outcomes. J Cancer Res Clin Oncol 149(19):17383–17394. 10.1007/s00432-023-05454-y [DOI] [PubMed] [Google Scholar]
- 22.Hallam S, Messenger DE, Thomas MG (2016) A systematic review of local excision after neoadjuvant therapy for rectal cancer: are ypT0 tumors the limit? Dis Colon Rectum 59(10):984–997. 10.1097/DCR.0000000000000613 [DOI] [PubMed] [Google Scholar]
- 23.Calmels M, Collard MK, Cazelles A et al (2020) Local excision after neoadjuvant chemoradiotherapy versus total mesorectal excision: a case-matched study in 110 selected high-risk patients with rectal cancer. Colorectal Dis 22(12):1999–2007. 10.1111/codi.15323 [DOI] [PubMed] [Google Scholar]
- 24.Bosch SL, Vermeer TA, West NP et al (2016) Clinicopathological characteristics predict lymph node metastases in ypT0–2 rectal cancer after chemoradiotherapy. Histopathology 69(5):839–848. 10.1111/his.13008 [DOI] [PubMed] [Google Scholar]
- 25.Hu X, Li YQ, Li QG et al (2019) Adjuvant chemotherapy seemed not to have survival benefit in rectal cancer patients with ypTis-2N0 after preoperative radiotherapy and surgery from a population-based propensity score analysis. Oncologist 24(6):803–811. 10.1634/theoncologist.2017-0600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao YT, Lin YL, Huang J et al (2021) Downstaged ypT0–2N0 rectal cancer after neoadjuvant chemoradiation therapy may not need adjuvant chemotherapy: a retrospective cohort study. Int J Colorectal Dis 36(3):509–516. 10.1007/s00384-020-03787-5 [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Luo D, Zhu J et al (2019) ACRNaCT trial protocol: efficacy of adjuvant chemotherapy in patients with clinical T3b/T4, N+ rectal Cancer undergoing Neoadjuvant Chemoradiotherapy: a pathology-oriented, prospective, multicenter, randomized, open-label, parallel group clinical trial. BMC Cancer 19(1):1117. 10.1186/s12885-019-6289-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamburini E, Tassinari D, Ramundo M et al (2022) Adjuvant chemotherapy after neoadjuvant chemo-radiotherapy and surgery in locally advanced rectal cancer. A systematic review of literature with a meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 172:103627. 10.1016/j.critrevonc.2022.103627 [DOI] [PubMed] [Google Scholar]
- 29.Bahadoer RR, Dijkstra EA, van Etten B et al (2021) Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 22(1):29–42. 10.1016/S1470-2045(20)30555-6 [DOI] [PubMed] [Google Scholar]
- 30.Jin J, Tang Y, Hu C et al (2022) Multicenter, randomized, phase iii trial of short-term radiotherapy plus chemotherapy versus long-term chemoradiotherapy in locally advanced rectal cancer (STELLAR). J Clin Oncol 40(15):1681–1692. 10.1200/JCO.21.01667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cutting JE, Hallam SE, Thomas MG et al (2018) A systematic review of local excision followed by adjuvant therapy in early rectal cancer: are pT1 tumours the limit? Colorectal Dis 20(10):854–863. 10.1111/codi.14340 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Forest plots of ACT effects on cancer-specific survival within subgroups. Abbreviations: ACT, Adjuvant Chemotherapy (JPG 1063 KB)
Supplementary file2 Forest plots of ACT effects on overall survival within subgroups. Abbreviations: ACT, Adjuvant Chemotherapy (JPG 1053 KB)
Data Availability Statement
The data obtained from SEER database in this study is publicly available from: https://seer.cancer.gov/data-software/documentation/seerstat/nov2020/.