Abstract
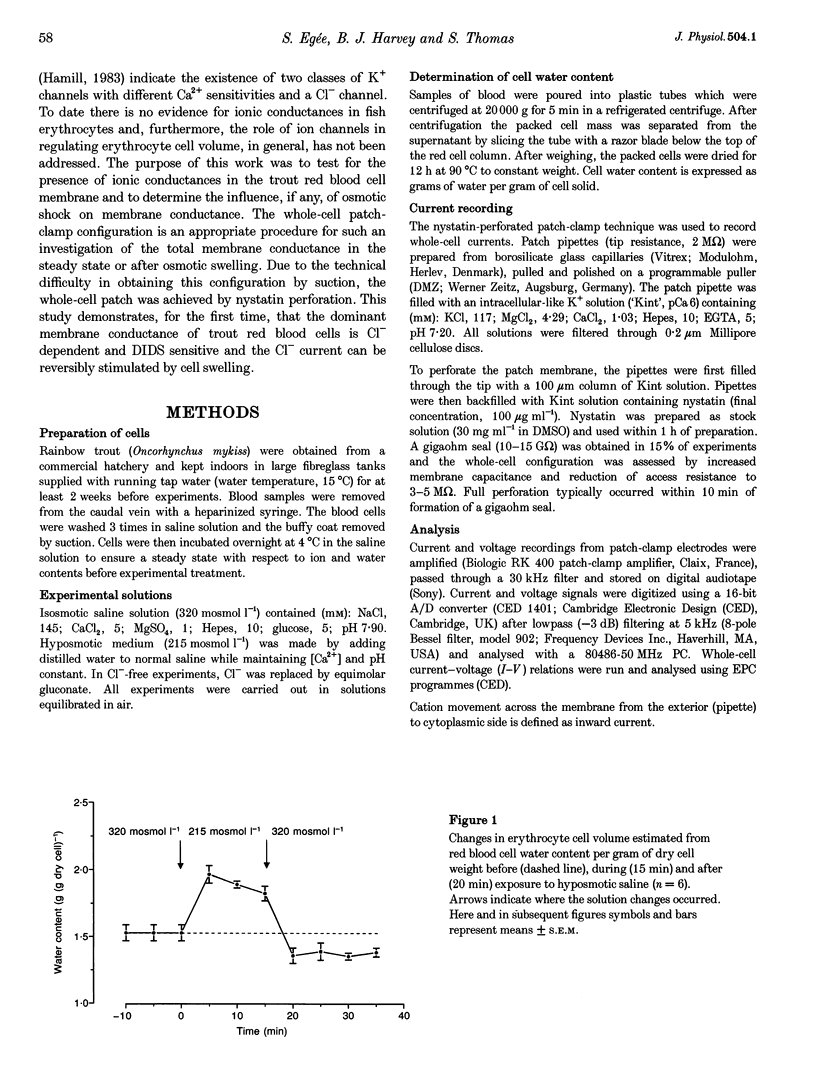
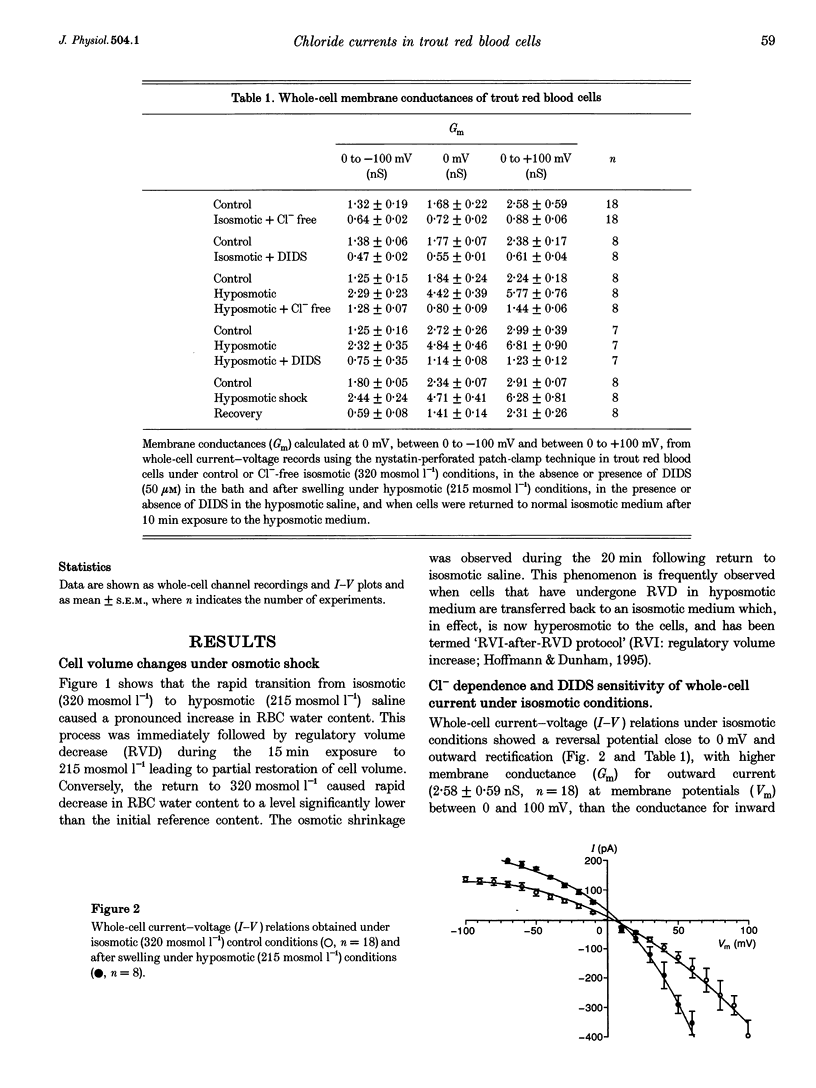
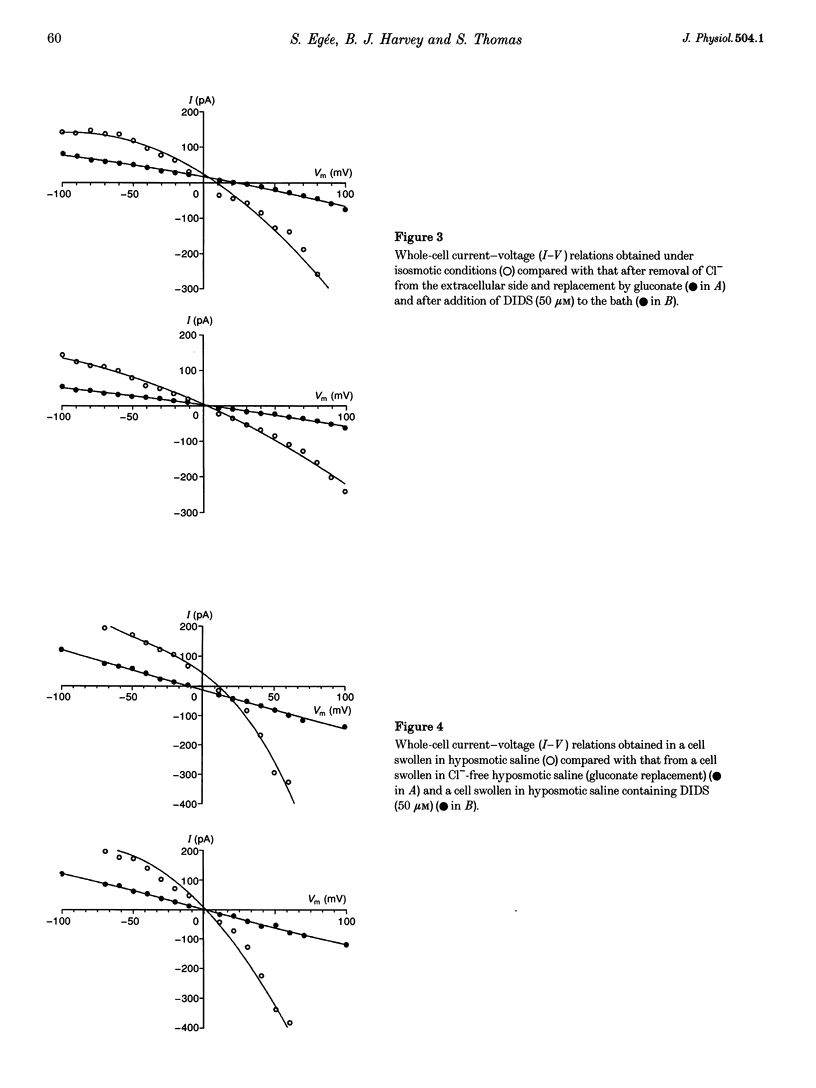
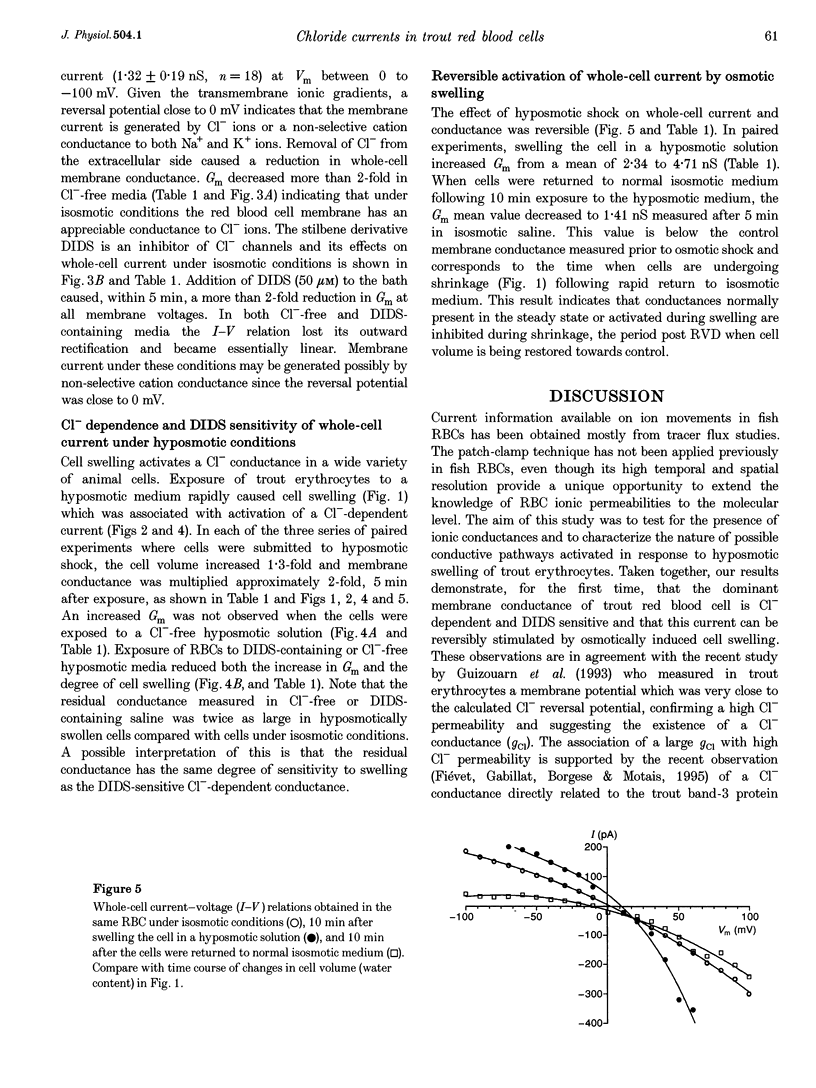
1. The nystatin-perforated whole-cell recording mode of the patch-clamp technique was used to investigate the membrane conductance of trout (Oncorhynchus mykiss) red blood cells in the steady state, 5 min after exposure to hyposmotic medium and 10 min after return to normal isosmotic medium. 2. Whole-cell I-V relations showed outward rectification when red blood cells were bathed in isosmotic (320 mosmol l-1) saline solution and the patch pipette was filled with 117 mM KCl. The membrane conductance was 2.58 +/- 0.59 nS (number of experiments, n = 18) between 0 and 100 mV and 1.32 +/- 0.19 nS (n = 18) between 0 and -100 mV. Removal of Cl- from the extracellular side or incubation with the Cl- channel blocker DIDS caused a reduction in whole-cell membrane conductance by more than 50%, indicating that the membrane current was generated by Cl- ions. The remaining conductance was voltage independent and probably due to non-selective cation conductance. 3. The membrane conductance increased approximately 2-fold after cell swelling induced by exposure to hyposmotic saline solution (215 mosmol l-1). This effect was abolished in Cl(-)-free hyposmotic medium or in the presence of DIDS. 4. The return to isosmotic solution produced a fall in membrane conductance to, or below, control values. 5. We conclude that trout red blood cells possess a significant Cl- conductance in the steady state which is reversibly activated during cell swelling and contributes to volume recovery.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baroin A., Garcia-Romeu F., Lamarre T., Motais R. A transient sodium-hydrogen exchange system induced by catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1984 Nov;356:21–31. doi: 10.1113/jphysiol.1984.sp015450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgese F., Garcia-Romeu F., Motais R. Control of cell volume and ion transport by beta-adrenergic catecholamines in erythrocytes of rainbow trout, Salmo gairdneri. J Physiol. 1987 Jan;382:123–144. doi: 10.1113/jphysiol.1987.sp016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne P. K., Cossins A. R. Sodium and potassium transport in trout (Salmo gairdneri) erythrocytes. J Physiol. 1984 Feb;347:361–375. doi: 10.1113/jphysiol.1984.sp015070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M. Cell volume regulation by Amphiuma red blood cells. The role of Ca+2 as a modulator of alkali metal/H+ exchange. J Gen Physiol. 1983 Dec;82(6):761–784. doi: 10.1085/jgp.82.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen O. Mediation of cell volume regulation by Ca2+ influx through stretch-activated channels. Nature. 1987 Nov 5;330(6143):66–68. doi: 10.1038/330066a0. [DOI] [PubMed] [Google Scholar]
- Cossins A. R., Weaver Y. R., Lykkeboe G., Nielsen O. B. Role of protein phosphorylation in control of K flux pathways of trout red blood cells. Am J Physiol. 1994 Dec;267(6 Pt 1):C1641–C1650. doi: 10.1152/ajpcell.1994.267.6.C1641. [DOI] [PubMed] [Google Scholar]
- Delpire E., Lauf P. K. Kinetics of DIDS inhibition of swelling-activated K-Cl cotransport in low K sheep erythrocytes. J Membr Biol. 1992 Feb;126(1):89–96. doi: 10.1007/BF00233463. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981 Sep;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic D., Sackin H. A calcium-permeable stretch-activated cation channel in renal proximal tubule. Am J Physiol. 1991 Jan;260(1 Pt 2):F119–F129. doi: 10.1152/ajprenal.1991.260.1.F119. [DOI] [PubMed] [Google Scholar]
- Fincham D. A., Wolowyk M. W., Young J. D. Volume-sensitive taurine transport in fish erythrocytes. J Membr Biol. 1987;96(1):45–56. doi: 10.1007/BF01869333. [DOI] [PubMed] [Google Scholar]
- Fiévet B., Gabillat N., Borgese F., Motais R. Expression of band 3 anion exchanger induces chloride current and taurine transport: structure-function analysis. EMBO J. 1995 Nov 1;14(21):5158–5169. doi: 10.1002/j.1460-2075.1995.tb00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Romeu F., Cossins A. R., Motais R. Cell volume regulation by trout erythrocytes: characteristics of the transport systems activated by hypotonic swelling. J Physiol. 1991;440:547–567. doi: 10.1113/jphysiol.1991.sp018724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L., Brill S. R. Volume-activated taurine efflux from skate erythrocytes: possible band 3 involvement. Am J Physiol. 1991 May;260(5 Pt 2):R1014–R1020. doi: 10.1152/ajpregu.1991.260.5.R1014. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983 Mar;396(4):325–334. doi: 10.1007/BF01063938. [DOI] [PubMed] [Google Scholar]
- Guizouarn H., Harvey B. J., Borgese F., Gabillat N., Garcia-Romeu F., Motais R. Volume-activated Cl(-)-independent and Cl(-)-dependent K+ pathways in trout red blood cells. J Physiol. 1993 Mar;462:609–626. doi: 10.1113/jphysiol.1993.sp019572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann E. K., Dunham P. B. Membrane mechanisms and intracellular signalling in cell volume regulation. Int Rev Cytol. 1995;161:173–262. doi: 10.1016/s0074-7696(08)62498-5. [DOI] [PubMed] [Google Scholar]
- Jensen F. Regulatory volume decrease in carp red blood cells: mechanisms and oxygenation-dependency of volume-activated potassium and amino acid transport. J Exp Biol. 1995;198(Pt 1):155–165. doi: 10.1242/jeb.198.1.155. [DOI] [PubMed] [Google Scholar]
- Kaji D. Volume-sensitive K transport in human erythrocytes. J Gen Physiol. 1986 Dec;88(6):719–738. doi: 10.1085/jgp.88.6.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk K., Ellory J. C., Young J. D. Transport of organic substrates via a volume-activated channel. J Biol Chem. 1992 Nov 25;267(33):23475–23478. [PubMed] [Google Scholar]
- Kregenow F. M. The response of duck erythrocytes to nonhemolytic hypotonic media. Evidence for a volume-controlling mechanism. J Gen Physiol. 1971 Oct;58(4):372–395. doi: 10.1085/jgp.58.4.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauf P. K., Bauer J., Adragna N. C., Fujise H., Zade-Oppen A. M., Ryu K. H., Delpire E. Erythrocyte K-Cl cotransport: properties and regulation. Am J Physiol. 1992 Nov;263(5 Pt 1):C917–C932. doi: 10.1152/ajpcell.1992.263.5.C917. [DOI] [PubMed] [Google Scholar]
- Motais R., Guizouarn H., Garcia-Romeu F. Red cell volume regulation: the pivotal role of ionic strength in controlling swelling-dependent transport systems. Biochim Biophys Acta. 1991 Oct 10;1075(2):169–180. doi: 10.1016/0304-4165(91)90248-f. [DOI] [PubMed] [Google Scholar]
- Nielsen O. B., Lykkeboe G., Cossins A. R. Oxygenation-activated K fluxes in trout red blood cells. Am J Physiol. 1992 Nov;263(5 Pt 1):C1057–C1064. doi: 10.1152/ajpcell.1992.263.5.C1057. [DOI] [PubMed] [Google Scholar]



