Abstract
Background
To investigate patients with disorders of consciousness (DoC) for residual awareness, guidelines recommend quantifying glucose brain metabolism using positron emission tomography. However, this is not feasible in the intensive care unit (ICU). Cerebral blood flow (CBF) assessed by arterial spin labeling magnetic resonance imaging (ASL-MRI) could serve as a proxy for brain metabolism and reflect consciousness levels in acute DoC. We hypothesized that ASL-MRI would show compromised CBF in coma and unresponsive wakefulness states (UWS) but relatively preserved CBF in minimally conscious states (MCS) or better.
Methods
We consecutively enrolled ICU patients with acute DoC and categorized them as being clinically unresponsive (i.e., coma or UWS [≤ UWS]) or low responsive (i.e., MCS or better [≥ MCS]). ASL-MRI was then acquired on 1.5 T or 3 T. Healthy controls were investigated with both 1.5 T and 3 T ASL-MRI.
Results
We obtained 84 ASL-MRI scans from 59 participants, comprising 36 scans from 35 patients (11 women [31.4%]; median age 56 years, range 18–82 years; 24 ≤ UWS patients, 12 ≥ MCS patients; 32 nontraumatic brain injuries) and 48 scans from 24 healthy controls (12 women [50%]; median age 50 years, range 21–77 years). In linear mixed-effects models of whole-brain cortical CBF, patients had 16.2 mL/100 g/min lower CBF than healthy controls (p = 0.0041). However, ASL-MRI was unable to discriminate between ≤ UWS and ≥ MCS patients (whole-brain cortical CBF: p = 0.33; best hemisphere cortical CBF: p = 0.41). Numerical differences of regional CBF in the thalamus, amygdala, and brainstem in the two patient groups were statistically nonsignificant.
Conclusions
CBF measurement in ICU patients using ASL-MRI is feasible but cannot distinguish between the lower and the upper ends of the acute DoC spectrum. We suggest that pilot testing of diagnostic interventions at the extremes of this spectrum is a time-efficient approach in the continued quest to develop DoC neuroimaging markers in the ICU.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12028-024-02031-0.
Keywords: Brain injury, Cerebral blood flow, Coma, Disorders of consciousness, Functional neuroimaging
Introduction
Coma affects 2 in 1,000 people each year [1], many of whom are admitted to the intensive care unit (ICU). A patient’s consciousness level has major implications for prognosis, treatment, resource allocation, and end-of-life decisions, but physicians are underestimating consciousness levels in up to 40% of unresponsive patients with brain injury [2, 3]. This is particularly worrisome in the ICU because underestimation of awareness levels may put patients at risk of suboptimal treatment decisions [4, 5]. Indeed, seven of ten deaths in the ICU occur because a good clinical outcome is deemed unlikely and life-sustaining therapy is withdrawn [4], so accurate estimation of preserved consciousness is crucial to avoid erroneous medical decisions, including premature withdrawal of life-sustaining therapy. Conversely, overestimation of awareness may lead to futile treatment, putting a strain on limited health care resources and causing caregiver distress. Therefore, precise estimation of consciousness levels after acute brain injury would lead to better prediction of clinical outcomes, optimization of neurorehabilitation potential, and a decrease in caregiver burden and health costs.
The European Academy of Neurology recommends 18-fluorodeoxyglucose positron emission tomography (FDG-PET) for the workup of unresponsive patients with chronic brain injury [3] because brain glucose metabolism is an excellent proxy for consciousness levels in these patients [6]. FDG-PET is known to be superior to functional magnetic resonance imaging (MRI) in detecting residual consciousness and prognosticating clinical outcomes in patients with chronic disorders of consciousness (DoC) [3, 6]. In patients with acute brain injury, however, FDG-PET is not feasible owing to logistical challenges in the ICU: There is rarely a clinical indication for an FDG-PET scan in ICU patients, and after administration of the radioactive tracer, there is a very limited time to perform the scan. Moreover, the scan duration of 30–60 min extends patient time outside the ICU, which increases the risk of complications. Hence, other neuroimaging measures than FDG-PET to assess consciousness levels in the ICU must be sought.
Arterial spin labeling magnetic resonance imaging (ASL-MRI) is a noninvasive method to quantify cerebral blood flow (CBF) using magnetically labeled arterial blood water as an endogenous tracer [7]. CBF is tightly coupled with glucose metabolism and neuronal activity, supporting ASL as a reliable measure of brain function [8]. ASL-MRI is validated against other established CBF measures [9–13], and ASL-MRI CBF measurements overlap considerably with FDG-PET measures in patients with dementia and epilepsy [14–18]. Notably, ASL-MRI can be performed in ICU patients in continuation of a clinically indicated structural MRI scan; the scan time is short (approximately 10 min), it is relatively inexpensive, and patients are not exposed to radioactivity [7, 16, 19].
In this proof-of-principle study, we hypothesized that CBF assessed by ASL-MRI can distinguish between clinically unresponsive and clinically low responsive ICU patients (and thus might be able to identify residual consciousness). Hence, we enrolled, on one hand, patients with DoC with known residual consciousness levels, in whom ASL-MRI should show relatively preserved CBF, and on the other hand, patients with clinically definite loss of consciousness, in whom ASL-MRI should reveal severely compromised CBF. In addition, we acquired ASL-MRI at both 1.5 T and 3 T in a sample of healthy volunteers to assess the reliability of CBF measurements across different magnetic field strengths, as some patients are contraindicated for 3 T scans. We reasoned that, if positive, this proof-of-principle study would lay the foundation for a larger prospective trial to investigate the usefulness of ASL-MRI to predict consciousness levels in ICU patients with acute DoC.
Methods
Patient Cohort
We enrolled a consecutive sample of mechanically ventilated patients with DoC admitted to the ICU of a tertiary referral center (Rigshospitalet, Copenhagen University Hospital) who fulfilled the following inclusion criteria: (1) minimum age > 18 years, (2) clinically nonresponding with an acute DoC after traumatic or nontraumatic brain injury, (3) < 31 days since injury, (4) clinical stability allowing transportation to an MRI scanner outside the ICU, and (5) written informed consent obtained from legal guardian. Exclusion criteria were (1) acute life-threatening conditions, (2) high-dose sedation, (3) clinically overt seizures or nonconvulsive seizures on (continuous) electroencephalogram (EEG), (4) a history of high-grade carotid artery stenosis (90%), (5) body temperature < 35 °C, and (6) contraindications to MRI.
We aimed for normoventilation and the lowest possible levels of sedation. If patients could not be fully weaned off sedation, dosages were reduced to the lowest possible levels as previously described [20, 21]. Levels of sedation were graded as (1) none or minimal, indicating absence of intravenous fentanyl, remifentanil, propofol, midazolam, sodium thiopental, or sevoflurane; (2) low to moderate, indicating fentanyl < 500 µg/h or < 200 µg/h if combined with propofol, remifentanil < 1000 µg/h or < 250 µg/h if combined with propofol, propofol < 100 mg/h, midazolam < 10 mg/h, or sevoflurane < 3%; or (3) high or very high, indicating propofol ≥ 100 mg/h, fentanyl ≥ 500 µg/h or ≥ 200 µg/h if combined with propofol, remifentanil ≥ 1,000 µg/h or ≥ 250 µg/h if combined with propofol, midazolam ≥ 10 mg/h, sevoflurane ≥ 3%, or any dosage of sodium thiopental [22].
Consciousness levels were determined by a clinical examination just prior to the scan on the same day by trained medical staff (EWG, MHO, and MA) under the supervision of a board-certified neurologist with > 15 years of experience in neurocritical care (DK). The neurological examination included the following: (1) assessment of cranial nerves and sensorimotor status, (2) Glasgow Coma Scale (GCS), (3) Full Outline of Unresponsiveness (FOUR) score [23], (4) Simplified Evaluation of Consciousness Disorders (SECONDs) [24], (5) visual pursuit/fixation with a mirror, (6) ability to follow simple motor commands (including with the family, when possible, to stimulate arousal), (7) reaction to central and peripheral noxious stimuli (in the absence of command-following), and (8) assessment of verbal and nonverbal communication, as described earlier [25] and in accordance with the European Academy of Neurology Guideline on Coma and other Disorders of Consciousness [3]. Patients were subclassified into the following categories: coma, unresponsive wakefulness state (UWS), minimally conscious state with/without language processing (MCS−/MCS+), or emerged from MCS. For the group analysis, patients were dichotomized into the following groups: those with residual consciousness (MCS or better [≥ MCS]) and those without residual consciousness (coma or UWS [≤ UWS]). In addition, we accessed patients’ electronic health records to identify relevant health information, including medical history, diagnoses, treatments, and laboratory and imaging test results.
Healthy Controls
Healthy volunteers were recruited through a local database of potential research participants with an interest in participating in brain research. We included 24 healthy controls, two men and two women from each age decade ranging from 20 to 80 years without any of the following exclusion criteria: (1) major neurological or psychiatric disease, including (but not limited to) prior cognitive impairment; (2) antipsychotic medication; (3) systolic blood pressure > 200 mm Hg and diastolic blood pressure > 115 mm Hg; (4) average weekly alcohol consumption of > 14 and > 21 units for women and men, respectively; (5) use of euphoric drugs, including cannabis, within the preceding 3 months; (6) pregnant or breastfeeding; (7) previous participation in trials with radioactivity (with accumulated radiation doses of > 10 mSv) within the last year or significant work-related exposure to radioactivity; and (8) contraindications to MRI. Participants were screened for cognitive impairment using the Mini Mental Status Examination, and neurological deficits were ruled out by a standard clinical neurological examination. Blood pressure and routine blood tests were obtained. Healthy participants received 140 Danish kroner (US $20) per hour as economic compensation. All participants gave written informed consent.
ASL-MRI
ASL-MRI was performed on 1.5 T (Signa Artist) or 3 T (Signa Premier) MRI scanners (GE Healthcare) with 21- or 48-channel head coils, respectively. Patients were not sedated or, if necessary, receiving sevoflurane or propofol at the lowest possible dosages to limit movement artifacts; they were mechanically normoventilated and monitored for sedation, ventilator rate, blood pressure, pulse, and blood oxygenation by experienced neuroanesthesiologists. Hemoglobin levels (which are known to influence ASL-MRI results [26–28]) were retrieved from same-day blood samples in accordance with clinical routine laboratory procedures.
ASL-MRI images were acquired twice using the GE product 3D pcASL sequence with a post-labeling delay (PLD) of 1.525 or 2.525 s. Parameters at 3 T were as follows: labeling duration = 1.45 s, 512 sampling points on eight spirals, field of view = 24 cm, reconstructed matrix = 128, repetition time (TR) = 4.810 s (PLD = 1.525 s) or 5.810 s (PLD = 2.525 s), echo time 52.8 ms, bandwidth 62.5 Hz/pixel, slice thickness = 4 mm, number of slices = 36, acquisition time = 4:32 min (PLD = 1.525 s) or 5:21 min (PLD = 2.525 s), and number of excitations = 3. Parameters at 1.5 T were unchanged, except TR = 4.816 s (PLD = 1.5 s) or 5.816 s (PLD = 2.5 s), echo time 10.7 ms, and acquisition time 4:19/5:08 min. Quantitative CBF images were calculated automatically on the MRI scanners by vendor-provided software. A high-resolution three-dimensional T1-weighted structural image was acquired using a sagittal, magnetization-prepared rapid gradient echo sequence (repetition time/echo time/inversion time = 7.7/3.2/450 ms at 3 T and 7.3/3.0/450 ms at 1.5 T, flip angle = 12°, in-plane acquired matrix 256 × 256, number of slices = 220, slice thickness = 0.9 mm, [field of view (FOV); 230 mm]. Gray and white matter segmentation was performed with the FreeSurfer image analysis suite version 7.1.0–1 (http://surfer.nmr.mgh.harvard.edu/). Briefly, high-resolution T1-weighted scans were analyzed in FreeSurfer with tissue type segmentation as well as parcellations of the cortical areas of the brain according to the Desikan-Killiany atlas. Registration of the ASL CBF maps was conducted in FreeSurfer using the boundary-based registration algorithm. The cortical segmentation and parcellations were then resliced into the native ASL space from which median CBF values were extracted. Because ICU patients often have intracranial devices or are connected to medical equipment that is only compatible with 1.5 T MRI, we acquired ASL-MRI in patients at either 1.5 T or 3 T. To determine the effect of MRI field strength on the measured CBF, we acquired both 1.5 T and 3 T ASL-MRI data (performed at the same day) in all healthy controls.
Data Analyses
The proposed sample size was an empirical estimate based on clinical feasibility and the number of study participants in similar proof-of-principle research testing the clinical extremes on the spectrum of consciousness levels to assess the value of prognostication methods in brain injury [29, 30] as well as the number of volunteers in our database. As stated previously, whole-brain gray matter CBF, as well as regional CBF levels in the amygdala, thalamus, and brainstem, was derived based on the cortical gray matter segmentation from FreeSurfer software resliced to the ASL-MRI. Furthermore, because cortical metabolism of the most preserved hemisphere correlates better with residual consciousness in chronic DoC than the whole-brain average [31], we also did a subanalysis based on CBF measurements in the best hemisphere of each patient.
With data from healthy controls, we report the replicability of CBF estimates across field strengths (1.5 T vs. 3 T) and PLDs (1.525 and 2.525 s) using Person’s ρ correlation coefficients and population intraclass correlation coefficients (ICC) based on a random effects model. Statistical analysis of CBF of patients and healthy controls employed linear mixed-effects modeling with study participant as a random effect and hemoglobin level as a fixed effect. Group was also modeled as a fixed effect, either with two levels (healthy controls vs. patients) or three levels (healthy control, ≤ UWS, and ≥ MCS, using either healthy control or ≥ MCS as reference). Results were considered significant at p < 0.05. Owing to the exploratory nature of the investigation, we did not correct for multiple testing. All analyses were done using MATLAB (version 2021a; The MathWorks Inc., Natick, MA).
Ethics and Data Handling
Written informed consent was obtained from all participants or their next-of-kin. All procedures followed the Declaration of Helsinki. The study received ethical permission from the regional ethical committee (VEK Region Hovedstaden; H-21015473). All personal data were handled and stored according to the General Data Protection Regulations and Act, after approval of the local data protection authorities (P-2021-288).
Results
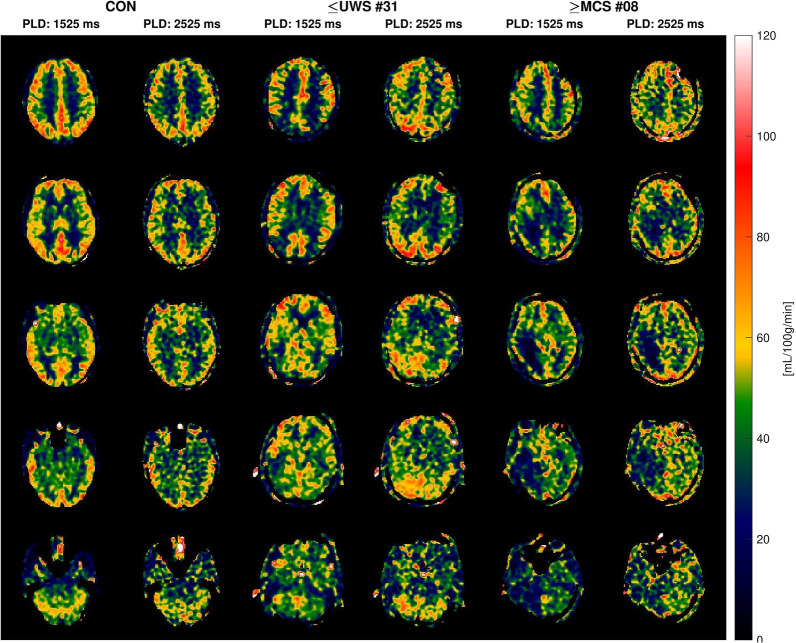
Between December 2021 and April 2023, we obtained 84 ASL-MRI scans from 59 participants: 35 ICU patients (32 nontraumatic brain injuries; 11 women; median age 56 years, range 18–82 years) who were investigated with a total of 36 ASL-MRI scans (at scan time: 24 times ≤ UWS, 12 times ≥ MCS, i.e., one patient was scanned twice) and 48 scans from 24 healthy volunteers (12 women [50%]; median age 50 years, range 21–77 years; i.e., healthy volunteers were investigated twice, at 1.5 T and 3 T). The primary cause of ICU admission was ischemic stroke in 13 (37.1%) patients, anoxic-ischemic encephalopathy in 4 (11.4%) patients, traumatic brain injury in 3 (8.6%) patients, intracerebral hemorrhage in 3 (8.6%) patients, neuroinfections in 3 (8.6%) patients, subarachnoid hemorrhage in 2 (5.7%) patients, and other etiologies in 7 (20.0%) patients. On average, ≤ UWS patients were scanned slightly earlier after their brain injury than ≥ MCS patients, but the difference was not statistically significant (7.9 ± 6.1 days vs. 10.5 ± 6.9 days, p = 0.27). Table 1 provides baseline characteristics of the patient cohort. Figure 1 provides representative ASL-MRI scans of a patient in a coma, an MCS + patient, and a healthy volunteer.
Table 1.
Baseline characteristics of the patient cohort with disorders of consciousness
| Scan ID | Sex | Age | Brain injury | Consciousness levela | Timing of ASL-MRI scan, days after injury | Sedationb | Hemoglobin (mmol/liter) | 3-month outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 56 | Brainstem infarction (vertebrobasilar dolichoectasia) | Coma | 3 | None or minimal | 9.0 | Dead |
| 2 | M | 25 | Global anoxia, epileptic seizure, hydrocephalus | UWS | 9 | None or minimal | 6.0 | Dead |
| 3 | F | 78 | Multiple embolic strokes, hypertensive encephalopathy, sepsis | Coma | 2 | None or minimal | 7.1 | Dead |
| 4 | M | 40 | Posterior fossa tumor (ependymoma) | eMCS | 3 | Low to moderate | 5.7 | Alive |
| 5 | F | 22 | Cardiac arrest (strangulation) | UWS | 19 | Low to moderate | 6.2 | Alive |
| 6 | M | 75 | Thalamic hemorrhage (hypertensive) | Coma | 18 | Low to moderate | 6.1 | Alive |
| 7 | M | 32 | Hyponatremia (polydipsia) | MCS+ | 6 | Low to moderate | 9.1 | Alive |
| 8 | F | 55 | Posterior fossa epidermoid cyst with postsurgical hemorrhage | MCS− | 25 | None or minimal | 5.7 | Alive |
| 9 | M | 75 | Brainstem infarction (basilar artery thrombosis) | Coma | 1 | High or very high | 8.7 | Dead |
| 10 | M | 71 | Subarachnoid hemorrhage with cardiac arrest | Coma | 7 | Low to moderate | 7.6 | Dead |
| 11 | M | 72 | Multifactorial encephalopathy (NPH, strokes, epilepsy) | MCS+ | 6 | Low to moderate | 7.7 | Alive |
| 12c | See ID 11 | 72 | See ID 11 | MCS+ | 14 | Low to moderate | 7.4 | See ID 11 |
| 13 | F | 56 | Aorta dissection, multiple cerebral emboli | MCS+ | 6 | Low to moderate | 6.2 | Alive |
| 14 | M | 82 | Brainstem infarction (basilar artery thrombosis) | Coma | 2 | Low to moderate | 6.8 | Dead |
| 15 | M | 67 | Aorta dissection, multiple cerebral emboli | Coma | 4 | Low to moderate | 6.6 | Dead |
| 16 | F | 44 | Pontine hemorrhage (hypertensive) | Coma | 5 | High or very high | 7.3 | Alive |
| 17 | F | 59 | Hepatic/uremic encephalopathy, rhabdomyolysis, seizures | Coma | 10 | Low to moderate | 5.1 | Alive |
| 18 | F | 52 | Traumatic brain injury, epilepsy | UWS | 6 | High or very high | 6.1 | Alive |
| 19 | M | 55 | Posterior circulation stroke (basilar artery thrombosis) | UWS | 5 | Low to moderate | 5.7 | Dead |
| 20 | M | 51 | Pontine hemorrhage (hypertensive) | MCS− | 11 | Low to moderate | 7.3 | Alive |
| 21 | M | 23 | Cardiac arrest (strangulation) | UWS | 15 | Low to moderate | 6.8 | Alive |
| 22 | M | 40 | Cerebellar infarct with fossa posterior decompression | MCS+ | 13 | None or minimal | 5.7 | Alive |
| 23 | M | 78 | Posterior circulation stroke, status epilepticus | Coma | 3 | Low to moderate | 12.4 | Alive |
| 24 | F | 76 | Pneumococcal meningitis | Coma | 10 | Low to moderate | 5.0 | Dead |
| 25 | M | 50 | Acute leukoencephalopathy, COVID-19 | Coma | 5 | None or minimal | 9.0 | Dead |
| 26 | M | 73 | Multiple embolic strokes | MCS− | 3 | Low to moderate | 9.3 | Dead |
| 27 | M | 58 | Posterior circulation stroke (basilar artery thrombosis) | Coma | 1 | None or minimal | 9.3 | Dead |
| 28 | M | 67 | Third ventricle tumor | UWS | 21 | Low to moderate | 4.8 | Dead |
| 29 | M | 30 | Traumatic brain injury | Coma | 15 | High or very high | 5.4 | Alive |
| 30 | M | 48 | Subarachnoid hemorrhage | Coma | 19 | High or very high | 5.7 | Alive |
| 31 | F | 18 | Traumatic brain injury | Coma | 7 | Low to moderate | 6.3 | Alive |
| 32 | F | 48 | Astrocytoma, postsurgical abscess | MCS− | 24 | Low to moderate | 4.9 | Dead |
| 33 | M | 77 | Posterior cerebellar stroke | MCS− | 5 | Low to moderate | 8.6 | Alive |
| 34 | M | 52 | Left middle cerebral artery infarction | MCS− | 10 | Low to moderate | 5.7 | Dead |
| 35 | F | 61 | Myocardial infarction, hypotension | UWS | 10 | Low to moderate | 9.8 | Alive |
| 36 | M | 75 | Herpes simplex encephalitis | UWS | 9 | Low to moderate | 5.9 | Alive |
ASL-MRI arterial spin labeling magnetic resonance imaging, eMCS emerged from minimally conscious state, F female, M male, MCS+ minimally conscious state with command-following, MCS− minimally conscious state without command-following, NPH normal pressure hydrocephalus, UWS unresponsive wakefulness state
aFor group analysis, patients were dichotomized into coma and UWS (≤ UWS) and MCS or better (≥ MCS)
bSee Methods for details
cThis patient was investigated twice with 9 days in-between the two scans
Fig. 1.
Arterial spin labeling magnetic resonance imaging examples of a healthy volunteer (CON), a patient in a coma (≤ UWS), and a minimally conscious state patient with command-following abilities (≥ MCS). Examples are study participants with median whole-brain gray matter cerebral blood flow in their groups. Images were acquired at 1.5 T field strength with post-labeling delays (PLDs) of 1.525 and 2.525 s; 10 equidistant slices are shown for each study participant
CBF at Different Field Strengths and PLDs in Healthy Controls
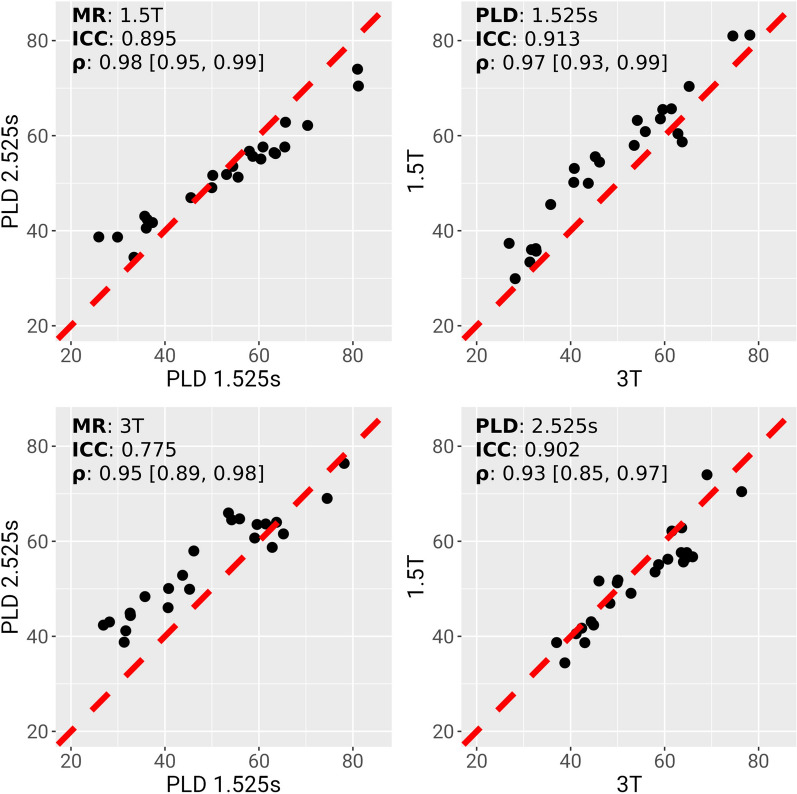
In healthy controls, CBF ASL-MRI showed a strong correlation across field strengths of 1.5 T and 3 T, irrespective of PLD (PLD 1.525 s: correlation coefficient = 0.97 [95% confidence interval (CI) 0.93–0.99], ICC = 0.91; PLD 2.525 s: correlation coefficient = 0.93 [95% CI 0.85–0.97], ICC = 0.90; Fig. 2). Similarly, CBF was strongly correlated across PLDs (1.5 T: correlation coefficient = 0.98 [95% CI 0.95–0.99], ICC = 0.90; 3 T: correlation coefficient = 0.95 [95% CI 0.89–0.98], ICC = 0.78; Fig. 2). Visually, the image quality of the PLD 1.525 s ASL images was better, and those images were chosen for subsequent statistical analyses.
Fig. 2.
Replicability of cerebral blood flow (CBF) estimates across post-labeling delays (PLDs) and magnetic resonance (MR) field strengths. Four plots show the correspondence between global gray-matter-weighted CBF estimates acquired on one of two MR scanners (3 T or 1.5 T) and at one of two PLDs (1.525 or 2.525 s). Top left, two PLDs acquired on 1.5 T scanner; top right, two MR strengths at PLD 1.525 s; bottom left, two PLDs acquired on 3 T scanner; bottom right, two MR strengths at PLD 2.525 s. x- and y-axes are in units of CBF. Black dots represent individual participants. Red hatched lines show the identity line. ρ denotes Pearson’s correlation coefficient and associated 95% confidence interval. ICC intraclass correlation coefficient
CBF in ICU Patients with DoC Compared with Healthy Controls
Group comparisons between patients and healthy controls are presented here for data from 1.5 T scans and PLD 2.525 s. In the linear mixed-effects model comparing healthy controls and patients, ASL-MRI whole-brain cortical CBF values showed a significant group difference (p = 0.0041; Fig. 3, Table 2). Patients had on average 16.2 mL/100 g/min lower CBF compared with healthy controls. Hemoglobin was significantly negatively correlated with CBF in the linear mixed-effects model (p = 0.0001; Table 2). A post hoc linear regression analysis within groups showed that hemoglobin levels were significantly negatively correlated with CBF for healthy controls (p = 0.007, R2 = 0.28) and ≤ UWS patients (p = 0.02, R2 = 0.29), whereas a nonsignificant negative correlation was observed for ≥ MCS patients (p = 0.25, R2 = 0.19; Fig. 3). Group comparisons with patient data from both 1.5 T and 3 T did not reveal statistically different results (data not shown).
Fig. 3.
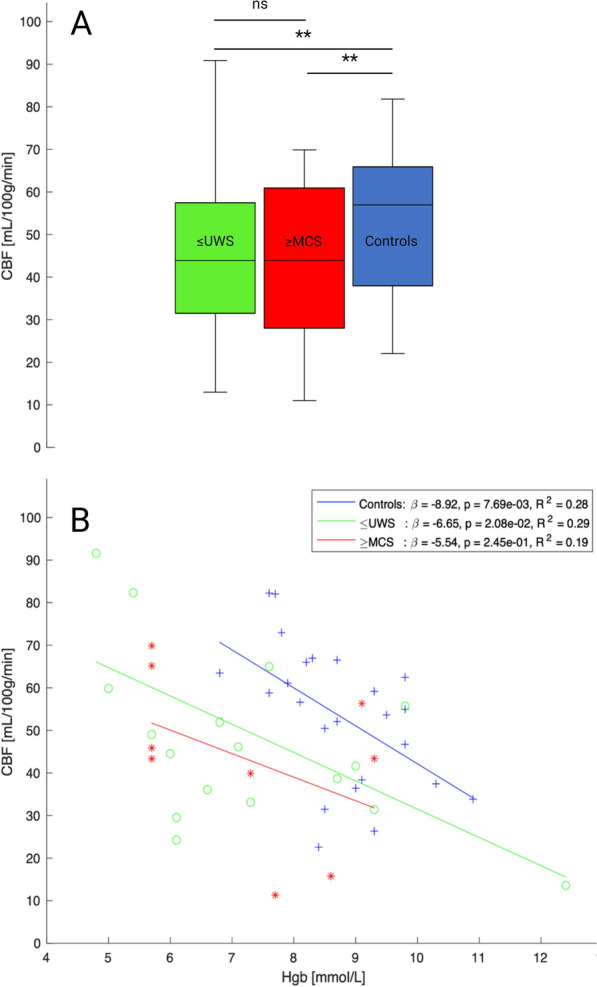
a Box and whisker plot showing cerebral blood flow (CBF) levels measured at 1.5 T arterial spin labeling magnetic resonance imaging in coma or unresponsive wakefulness state (≤ UWS) and minimally conscious state or better (≥ MCS) patients and healthy controls. ns, not significant. ** p < 0.005. b CBF of the three groups as a function of hemoglobin levels. Although statistically significant only in controls (p = 0.001), all groups showed decreasing CBF with increasing hemoglobin levels
Table 2.
Multiple regression results from linear mixed-effects models
| Reference group | Region | CBF difference, mL/100 g/min, (95% CI) | Effect of Hgb, mL/100 g/min/(mmol/L) | |
|---|---|---|---|---|
| ≤ UWS plus ≥ MCSa | Controls | Cortex (mean) | − 16.2 (− 26.9 to − 5.4), p = 0.0041 | − 6.92, p = 0.0001 |
| ≤ UWSb | Controls | Cortex (mean) | − 13.9 (− 25.6 to − 2.3), p = 0.02 | − 6.91, p = 0.0001 |
| ≥ MCSb | Controls | Cortex (mean) | − 20.6 (− 34.7 to − 6.5), p = 0.005 | |
| ≤ UWSc | ≥ MCS | Cortex (mean) | 6.7 (− 7.1 to 20.5), p = 0.33 | |
| ≤ UWSc | ≥ MCS | Cortex (median) | 8.3 (− 6.6 to 23.1), p = 0.27 | − 6.72, p = 0.0002 |
| ≤ UWSc | ≥ MCS | Thalamus | 6.0 (− 7.1 to 19.1), p = 0.36 | − 6.02, p = 0.0002 |
| ≤ UWSc | ≥ MCS | Amygdala | 3.9 (− 7.1 to 14.9), p = 0.48 | − 4.34, p = 0.001 |
| ≤ UWSc | ≥ MCS | Brainstem | 3.5 (− 8.0 to 15.0), p = 0.54 | − 4.19, p = 0.0025 |
Bold p values are statistically significant (see Methods)
CBF, cerebral blood flow, CI, confidence interval, Hgb, hemoglobin, MCS, minimally conscious state with or without command-following, UWS, unresponsive wakefulness state
aCombined patient groups (i.e., unresponsive [≤ UWS] and low responsive [≥ MCS] ICU patients) versus healthy controls
bSeparate patient groups of ≤ UWS and ≥ MCS patients versus healthy controls
c ≤ UWS patients versus ≥ MCS patients
Whole-Brain, Hemispheric, and Regional CBF in ICU Patients with DoC Stratified According to Consciousness Levels
In the linear mixed-effects model, compared with controls, the whole-brain CBF was 13.9 mL/100 g/min lower in ≤ UWS patients (p = 0.02) and 20.6 mL/100 g/min lower in ≥ MCS patients (p = 0.005; Table 2). However, there was no statistically significant difference in ASL-MRI scans between consciousness levels (i.e., ≤ UWS vs. ≥ MCS) in patients (p = 0.33; Table 2). In a subanalysis of hemispheric CBF, absolute differences between the best and worst brain hemispheres in patients were overall small (Supplemental Fig. S1), and best hemispheric CBF did not differ between patient groups (p = 0.41; Supplemental Fig. S2). Numerical differences between ≤ UWS and ≥ MCS patients regarding regional CBF in the thalamus, amygdala, and brainstem were statistically nonsignificant. For all inspected brain areas, hemoglobin levels were negatively correlated with CBF (Table 2).
Discussion
We investigated whether CBF measured by ASL-MRI could provide information about residual awareness in ICU patients with acute DoC. However, although ASL-MRI was feasible in this logistically challenging patient population, CBF values could not discriminate between ≤ UWS and ≥ MCS patients.
CBF is Decreased in Acute DoC
CBF in ICU patients with DoC was overall reduced compared with that in healthy controls, with 16 mL per 100 g brain tissue per minute on average. However, despite reduced global CBF at the group level, we found a striking interindividual difference in CBF both in ≤ UWS and ≥ MCS patients. The range of CBF values in patients with DoC (from around 10 to 90 mL per 100 g per minute) exceeded that of healthy controls on both sides of the spectrum (around 20–80 mL per 100 g per minute), consistent with a 15–20% CBF variation between young healthy study participants [32]. This might reflect a combination of disturbed cerebral autoregulation in acute brain injury [33] and iatrogenic interventions with inotropic drugs to increase the cerebral perfusion pressure [34]. Other possible factors include different levels of partial pressure of carbon dioxide (PaCO2), body temperature, and sedation levels (even though the latter were kept to a minimum). However, this CBF variability may reveal an opportunity to study trajectories of CBF over time (as opposed to isolated CBF measurements) as markers for declining or improving consciousness in acute brain injury. Given that hemoglobin levels are correlated with CBF [35] and influence ASL-MRI results [26–28], such trajectory studies would need to account for daily hemoglobin values.
Although CBF is well studied in neurological conditions such as epilepsy and dementia, few studies have investigated it in patients with DoC [36–39]. Using whole-brain computed tomography brain perfusion, Xiong et al. [36] examined the relationship between consciousness level on one side and CBF, cerebral blood volume, and time to peak on the other side in 29 patients with chronic UWS and 47 with chronic MCS. UWS patients had significantly decreased CBF in bilateral frontal, temporal, and occipital lobes, as well as in the thalamus and the brainstem. GCS and FOUR scores were positively correlated with CBF, cerebral blood volume, and time to peak in almost all regions of interest. However, this study differed from ours by use of another neuroimaging modality (i.e., computed tomography brain perfusion), the setting (i.e., prolonged DoC in chronic brain injury as opposed to ICU patients with acute brain injury), and the lack of a control group.
ASL-MRI Cannot Discriminate Between Consciousness Levels in Acute DoC
After adjustment for hemoglobin levels, CBF was decreased in patients compared with healthy controls; however, global CBF was not significantly different between patient groups. Regional CBF levels in the cortex, thalamus, amygdala, or brainstem were also not statistically different in ≤ UWS patients compared with ≥ MCS patients, and neither was best hemispheric CBF.
Less than a handful of studies, including ours, have investigated CBF in DoC using ASL-MRI [37–39]. Except for possibly one [38], none of these studies showed meaningful differences between patients with and without residual consciousness. In a retrospective pilot study of 12 ICU patients with aneurysmal subarachnoid hemorrhage, Nelson et al. [37] analyzed CBF within gray matter nodes of the default mode network (DMN), which include bilateral medial prefrontal cortices, thalami, and posterior cingulate cortices, yet not all these patients had a DoC. There were no correlations between CBF and admission GCS scores for any DMN nodes, perhaps because of the small sample size, ASL data acquisition at variable times after the brain injury, and the possibility that CBF analysis in DMN nodes may not reflect the functional integrity of the entire neural network. In an even smaller study of four study participants who met MCS criteria [39], CBF was decreased in gray matter compared with that in normal controls (n = 10), especially in the medial prefrontal and midfrontal regions. Although CBF patterns showed considerable variability (from 7.7 to 33.1 mL/100 g/min), in the one study participant who was studied longitudinally, global CBF values increased over time and correlated with clinical improvement [39]. In yet another study (n = 23 patients), Wu et al. [38] found that regional CBF in MCS patients was increased in the putamen, anterior cingulate gyrus, and medial frontal cortex compared with that in UWS patients. A difference between MCS and UWS in a left-lateralized pattern was observed, but the authors did not probe predictive utility. Unlike our study, these were patients with DoC in the postacute to chronic setting with a median time from brain injury onset of 3 months (range 1–47 months). It might therefore be that contrary to the acute phase, ASL-MRI correlates better with consciousness levels in the chronic stage when reactive cerebral inflammatory processes and dysregulation of cerebral autoregulation have resolved. Ideally, this hypothesis should be tested in longitudinal follow-up studies with repeated ASL-MRI measurements in the acute and chronic stages of brain injury.
Strengths and Limitations
Our work has limitations. First, patients were scanned at either 1.5 T or 3 T MRI depending on clinical availability and contraindications. This reflects real-world challenges inherent to clinical neuroimaging of ICU patients. Nonetheless, our data from healthy volunteers (who were scanned twice, on 1.5 T and 3 T) indicate that ASL-MRI was highly replicable across magnetic field strengths. Second, our patient cohort was somewhat skewed toward nontraumatic brain injuries, possibly reflecting the greater clinical need for high-resolution imaging in these etiologies, but we believe it is unlikely that a more balanced patient cohort would have yielded substantially different results. Third, except for hemoglobin levels, we did not correct our statistical models for other physiological markers, such as PaCO2, body temperature, and sedation levels, which might be worthwhile to investigate in replication studies. Moreover, we did not correct for focal brain lesions, but visual inspection of ASL CBF maps and brain tissue segmentation revealed no evidence that they were negatively affected by focal lesions. Also, because we used the median value of the entire cortex, we do not assume that focal lesions or infarcts had a large influence on the results. In fact, we did a subanalysis by calculating CBF for the best hemisphere only and found no difference between unresponsive and low responsive patients. In future studies, one might consider adjusting for volumetric measurements when analyzing smaller regions such as the brainstem or subcortical gray matter to decrease partial volume effects from adjacent low-signal regions. Finally, we might have identified a discriminative signal of ASL-MRI had we included a larger patient cohort. However, if we have missed such a signal, it cannot be clinically useful given that such an effect size would be small. We therefore conclude that the number of patients was sufficient for our overarching goal, which was to decide whether a large multicenter trial to test the diagnostic accuracy of a single ASL-MRI study early after brain injury is warranted (the answer is no).
We think the present work also has strengths: It involves a larger patient cohort than previous studies, and, to our knowledge, it is the first prospective ASL-MRI study in patients with acute DoC in the ICU with an equally large control group. Furthermore, we showed that ASL-MRI is feasible in acute DoC and that CBF measurements are reliable across different field strengths and PLDs, possibly supporting broader clinical implementation for serial CBF measurements. Finally, as stated previously, we fulfilled our primary objective: Before embarking on resource-intensive diagnostic phase 2b trials in the ICU [20], we suggest that feasibility studies like ours that test diagnostic accuracy of interventions at the extremes of the DoC spectrum are valuable for go/no-go decisions, depending on the apparent potential of the diagnostic procedure in question. We have previously used a similar approach based on relatively small DoC ICU cohorts investigated with measurements of otoacoustic emissions [29], mental arithmetic [30], neurovascular coupling [40], and brimonidine eye drops [41] to probe for clinical outcome predictions and residual consciousness in this challenging patient population.
Conclusions
ASL-MRI is feasible in the ICU and (at least in healthy controls) reliable across different magnetic field strengths and PLDs. CBF measurements can distinguish well between ICU patients with brain injury and healthy controls but lack discriminatory value to identify the absence or presence of residual consciousness in acute DoC. Intraindividual CBF variability and CBF trajectories over time may yield greater insights than cross-sectional ASL-MRI measurements, which may be an approach worth further investigation. Testing novel diagnostic interventions at the extremes of the DoC spectrum might help to tell promising interventions apart from less promising ones. This design appears to be a resource-sensitive and time-efficient approach to continue the quest for refined neuroimaging technologies to detect residual consciousness after acute brain injury in the ICU.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
DK was involved in conceptualization and design. UL, PMF, UMC, AEH, and DK were involved in the data analysis. DK and AEH were involved in writing the article. All authors were involved in data acquisition, important intellectual contribution and approved the manuscript.
Source of Support
Open access funding provided by National Hospital. Open access funding was provided by Royal Danish Library. This study was funded by Offerfonden, Region Hovedstadens Forskningsfond, Lundbeck Foundation, and Rigshospitalets Forskningspuljer. Funding was given to support PhD students and research scholarship students. The authors are solely responsible for the material, its content, and results. The views expressed in the material are the authors’ own and do not necessarily reflect those of the funders. The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Data and Code Availability
Anonymous raw data and code will be shared upon reasonable request. Structural MRI and ASL-MRI scans cannot be anonymized and will not be shared, but radiological reports of both structural MRI and ASL-MRI scans are available on request.
Conflict of interest
The authors report no competing interests.
Ethical approval/informed consent
This study was approved by the Ethics Committee of the Capital Region of Denmark (H-21015473).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elisabeth Waldemar Grønlund and Ulrich Lindberg are shared first authors.
Adam Espe Hansen and Daniel Kondziella are shared last authors.
References
- 1.Kondziella D, Amiri M, Othman MH, et al. Incidence and prevalence of coma in the UK and the USA. Brain Commun. 2022;4:fcac188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schnakers C, Vanhaudenhuyse A, Giacino J, et al. Diagnostic accuracy of the vegetative and minimally conscious state: clinical consensus versus standardized neurobehavioral assessment. BMC Neurol. 2009;9:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondziella D, Bender A, Diserens K, et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur J Neurol. 2020;27:741–56. [DOI] [PubMed] [Google Scholar]
- 4.Turgeon AF, Lauzier F, Simard J-F, et al. Mortality associated with withdrawal of life-sustaining therapy for patients with severe traumatic brain injury: a Canadian multicentre cohort study. CMAJ. 2011;183:1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerges PRA, Moore L, Léger C, et al. Intensity of care and withdrawal of life-sustaining therapies in severe traumatic brain injury patients: a post-hoc analysis of a multicentre retrospective cohort study. Can J Anaesth. 2018;65:996–1003. [DOI] [PubMed] [Google Scholar]
- 6.Stender J, Gosseries O, Bruno MA, et al. Diagnostic precision of PET imaging and functional MRI in disorders of consciousness: a clinical validation study. Lancet. 2014;384:514–22. [DOI] [PubMed] [Google Scholar]
- 7.Chen JJ, Jann K, Wang DJJ. Characterizing Resting-state brain function using arterial spin labeling. Brain Connect. 2015;5:527–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoge RD, Pike GB. Oxidative metabolism and the detection of neuronal activation via imaging. J Chem Neuroanat. 2001;22:43–52. [DOI] [PubMed] [Google Scholar]
- 9.Ewing JR, Cao Y, Knight RA, Fenstermacher JD. Arterial spin labeling: validity testing and comparison studies. J Magn Reson Imag: Off J Int Soc Magn Reson Med. 2005;740:737–40. [DOI] [PubMed] [Google Scholar]
- 10.Heijtel DFR, Mutsaerts HJMM, Bakker E, et al. NeuroImage Accuracy and precision of pseudo-continuous arterial spin labeling perfusion during baseline and hypercapnia: a head-to-head comparison with 15 O H 2 O positron emission tomography. Neuroimage. 2014;92:182–92. [DOI] [PubMed] [Google Scholar]
- 11.Koziak AM, Winter J, Lee T, et al. Validation study of a pulsed arterial spin labeling technique by comparison to perfusion computed tomography. Magn Reson Imag. 2008;26:543–53. [DOI] [PubMed] [Google Scholar]
- 12.Warmuth C, Nagel S, Hegemann O, et al. Accuracy of blood flow values determined by arterial spin labeling: a validation study in isolated porcine kidneys. J Magn Reson Imag: Off J Int Soc Magn Reson Med. 2007;358:353–8. [DOI] [PubMed] [Google Scholar]
- 13.Ye FQ, Berman KF, Ellmore T, et al. H 215 O PET validation of steady-state arterial spin tagging cerebral blood flow measurements in humans. Magn Reson Med: Off J Int Soc Magn Reson Med. 2000;456:450–6. [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Wolk DA, Reddin JS, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musiek ES, Chen Y, Korczykowski M, et al. Direct comparison of fluorodeoxyglucose positron emission tomography and arterial spin labeling magnetic resonance imaging in Alzheimer’s disease. Alzheimer’s Dement. 2012;8:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolui S, Li Z, Nasrallah IM, et al. Arterial spin labeling versus 18F-FDG-PET to identify mild cognitive impairment. NeuroImage Clin. 2020;25:102146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sierra-Marcos A, Carreño M, Setoain X, et al. Accuracy of arterial spin labeling magnetic resonance imaging (MRI) perfusion in detecting the epileptogenic zone in patients with drug-resistant neocortical epilepsy: comparison with electrophysiological data, structural MRI, SISCOM and FDG-PET. Eur J Neurol. 2016;23:160–7. [DOI] [PubMed] [Google Scholar]
- 18.Wang YH, An Y, Fan XT, et al. Comparison between simultaneously acquired arterial spin labeling and 18F-FDG PET in mesial temporal lobe epilepsy assisted by a PET/MR system and SEEG. NeuroImage Clin. 2018;19:824–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin C, Tseng Y, Hsu H, et al. Arterial spin labeling perfusion study in the patients with subacute mild traumatic brain injury. PLoS ONE. 2016;11(2):e0149109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amiri M, Fisher PM, Raimondo F, et al. Multimodal prediction of residual consciousness in the intensive care unit: the CONNECT-ME study. Brain. 2023;146:50–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amiri M, Raimondo F, Fisher PM, et al. Multimodal prediction of 3- and 12-month outcomes in ICU Patients with acute disorders of consciousness. Neurocrit Care. 2024;40:718–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DASAIM. Sedationsstrategi: målrettet behandling af gener forbundet med kritisk sygdom. Guid: Dansk Selsk Anæstesiologi og Intensiv Med. 2020;3:1–39. [Google Scholar]
- 23.Wijdicks EFM, Bamlet WR, Maramattom BV, et al. Validation of a new coma scale: the FOUR score. Ann Neurol. 2005;58:585–93. [DOI] [PubMed] [Google Scholar]
- 24.Benni PB, Li JK, Chen B, et al. NIRS monitoring of pilots subjected to +Gz acceleration and G-induced loss of consciousness (G-LOC). Adv Exp Med Biol. 2003;530:371–9. [DOI] [PubMed] [Google Scholar]
- 25.Kondziella D, Fisher PM, Larsen VA, et al. Functional MRI for assessment of the default mode network in acute brain injury. Neurocrit Care. 2017;27:401–6. [DOI] [PubMed] [Google Scholar]
- 26.Brousse V, Pondarre C, Kossorotoff M, et al. Brain injury pathophysiology study by a multimodal approach in children with sickle cell anemia with no intra or extra cranial arteriopathy. Haematologica. 2022;107:958–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Afzali-Hashemi L, Baas KPA, Schrantee A, et al. Impairment of cerebrovascular hemodynamics in patients with severe and milder forms of sickle cell disease. Front Physiol. 2021;12:645205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang XL, Wen JQ, Zhang LJ, et al. Cerebral blood flow changes in hemodialysis and peritoneal dialysis patients: an arterial-spin labeling MR imaging. Metab Brain Dis. 2016;31:929–36. [DOI] [PubMed] [Google Scholar]
- 29.Kondziella D, Jensen AM, Hjuler T, et al. Otoacoustic emissions for outcome prediction in postanoxic brain injury. Front Neurol. 2018;9:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vassilieva A, Olsen MH, Peinkhofer C, et al. Automated pupillometry to detect command following in neurological patients: a proof-of-concept study. PeerJ. 2019;7:e6929. 10.7717/peerj.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stender J, Mortensen KN, Thibaut A, et al. The minimal energetic requirement of sustained awareness after brain injury. Curr Biol. 2016;26:1494–9. [DOI] [PubMed] [Google Scholar]
- 32.Henriksen OM, Larsson HBW, Hansen AE, et al. Estimation of intersubject variability of cerebral blood flow measurements using MRI and positron emission tomography. J Magn Reson Imaging. 2012;35:1290–9. [DOI] [PubMed] [Google Scholar]
- 33.Brasil S, Nogueira RC, Salinet ASM, et al. Contribution of intracranial pressure to human dynamic cerebral autoregulation after acute brain injury. Am J Physiol Regul Integr Comp Physiol. 2023;324:R216–26. [DOI] [PubMed] [Google Scholar]
- 34.Coppalini G, Duvigneaud E, Diosdado A, et al. Effect of inotropic agents on oxygenation and cerebral perfusion in acute brain injury. Front Neurol. 2022;13:963562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ibaraki M, Shinohara Y, Nakamura K, et al. Interindividual variations of cerebral blood flow, oxygen delivery, and metabolism in relation to hemoglobin concentration measured by positron emission tomography in humans. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2010;30:1296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiong Q, Wang Y, Wang Z, et al. Relationship between consciousness level and perfusion computed tomography in patients with prolonged disorders of consciousness. Aging (Albany NY). 2022;14:9668–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson S, Edlow BL, Wu O, et al. Default mode network perfusion in aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2016;25:237–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu B, Yang Y, Zhou S, et al. Could arterial spin labeling distinguish patients in minimally conscious state from patients in vegetative state? Front Neurol. 2018;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu AA, Voss HU, Dyke JP, et al. Arterial spin labeling and altered cerebral blood flow patterns in the minimally conscious state. Neurology. 2011;77:1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Othman MH, Bhattacharya M, Møller K, et al. Resting-state NIRS–EEG in unresponsive patients with acute brain injury: a proof-of-concept study. Neurocrit Care. 2021;34:31–44. [DOI] [PubMed] [Google Scholar]
- 41.Jakobsen EW, Nersesjan V, Albrechtsen SS, et al. Brimonidine eye drops reveal diminished sympathetic pupillary tone in comatose patients with brain injury. Acta Neurochir (Wien). 2023;165:1483–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymous raw data and code will be shared upon reasonable request. Structural MRI and ASL-MRI scans cannot be anonymized and will not be shared, but radiological reports of both structural MRI and ASL-MRI scans are available on request.