Abstract
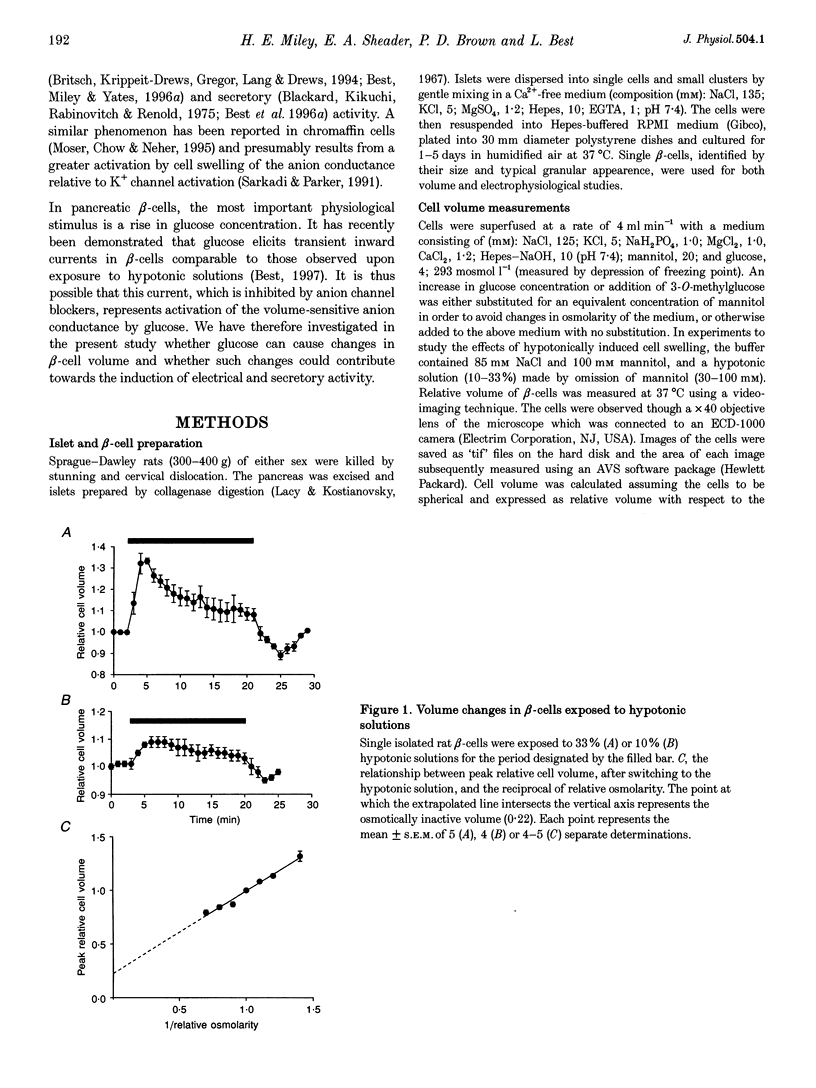
1. Changes in relative cell volume in response to hypotonic solutions and glucose were studied in single isolated rat pancreatic beta-cells using a video-imaging technique. beta-cell electrical activity was recorded under similar conditions using the perforated patch technique. 2. Exposure of beta-cells to hypotonic solutions (10 and 33% hypotonicity) caused an immediate increase in cell volume to relative values of 1.09 and 1.33, respectively. This was followed by a gradual regulatory volume decrease. 3. Raising the concentration of glucose from 4 to 20 mM or 12 mM (with substitution of mannitol) increased beta-cell volume by 12 and 10%, respectively. This effect of glucose persisted when CO2+ was added to inhibit insulin release. Glucose-induced volume increases were sustained for the duration of exposure to elevated hexose concentration. The addition of 16 mM 3-O-methylglucose, which is transported into the beta-cell but not metabolized, produced only a transient 5% increase in beta-cell volume. 4. Exposure of beta-cells to a 15% hypotonic solution resulted in a transient depolarization and electrical activity. Raising the glucose concentration to 20 or 12 mM caused a sustained depolarization and generation of electrical activity. However, the addition of 16 mM 3-O-methylglucose had no effect on beta-cell membrane potential. The glucose-induced increase in volume and induction of electrical activity, when measured in single beta-cells simultaneously, showed comparable kinetics. 5. The secretion of insulin from intact pancreatic islets was stimulated by exposure to hypotonic solutions (10-33% hypotonicity). A 15% hypotonic solution stimulated insulin release to a peak value comparable to that elicited by raising the glucose concentration from 4 to 20 mM. Whereas hypotonic solutions caused a transient stimulation of insulin release, the effect of glucose was sustained. 6. It is suggested that glucose increases the volume in rat pancreatic beta-cells by a mechanism dependent upon metabolism of the sugar. The extent of cell swelling evoked by raised glucose concentrations is sufficient to depolarize the cells and induce electrical and secretory activity and may involve activation of a volume-sensitive anion conductance.
Full text
PDF
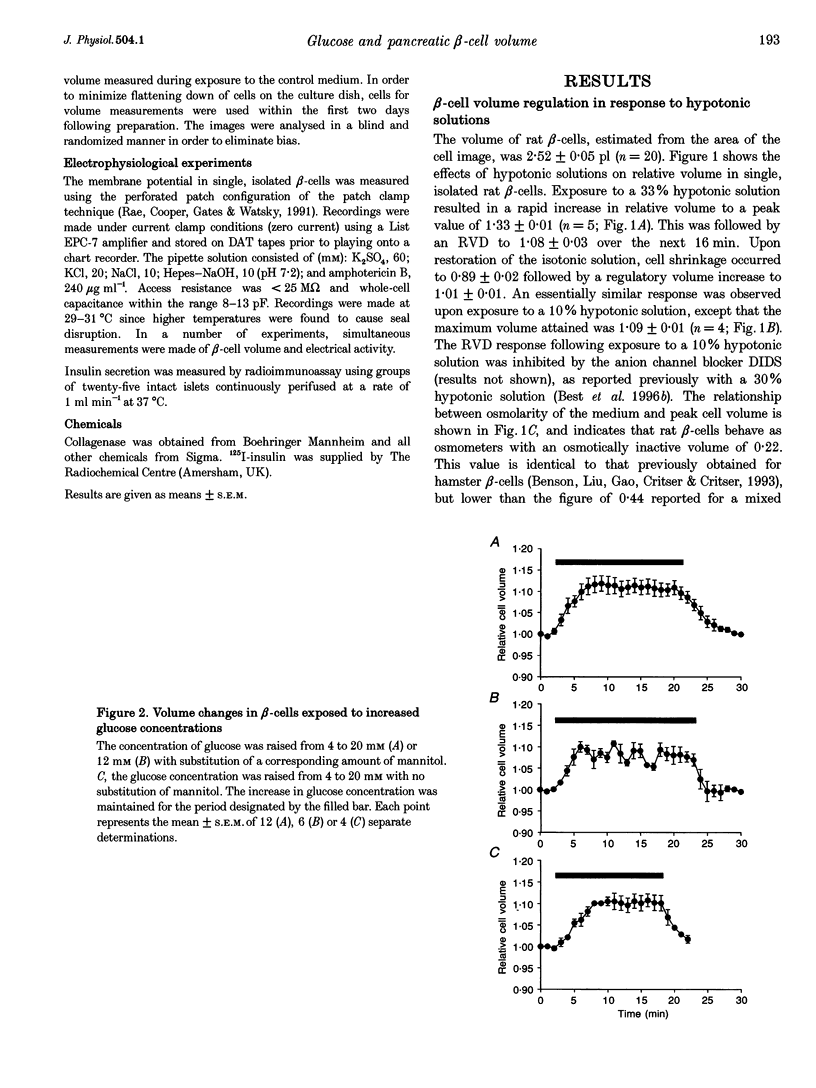
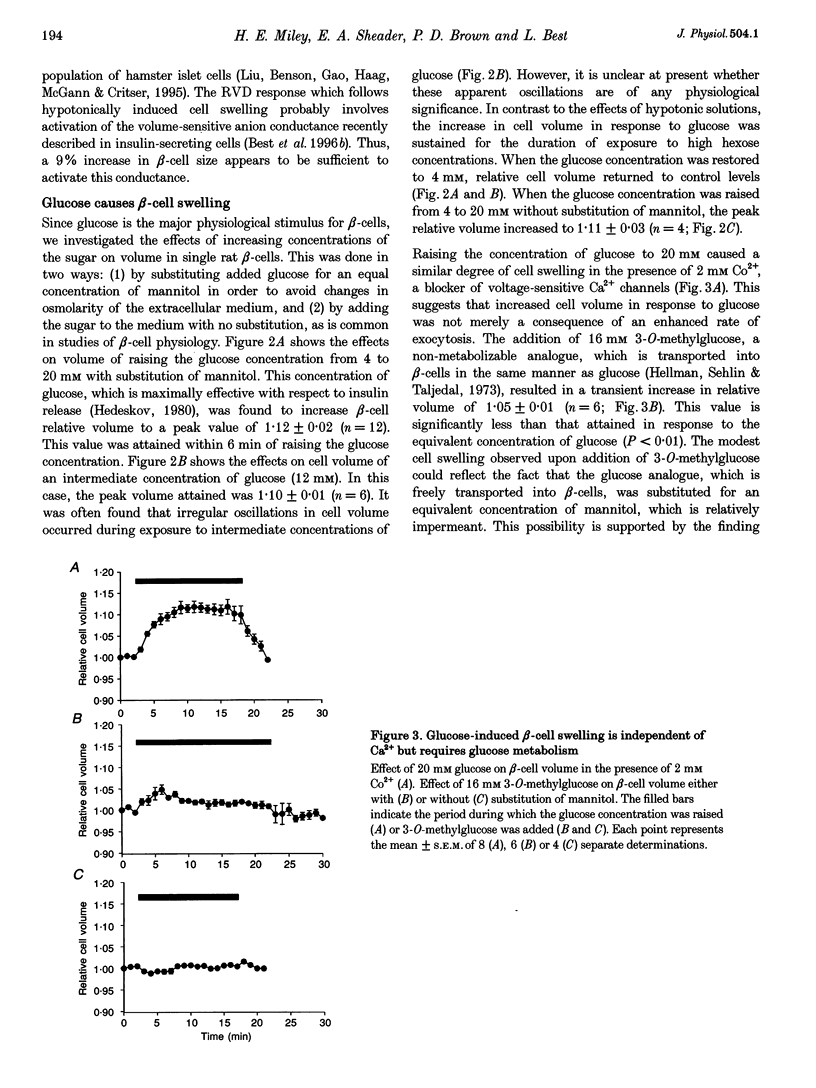
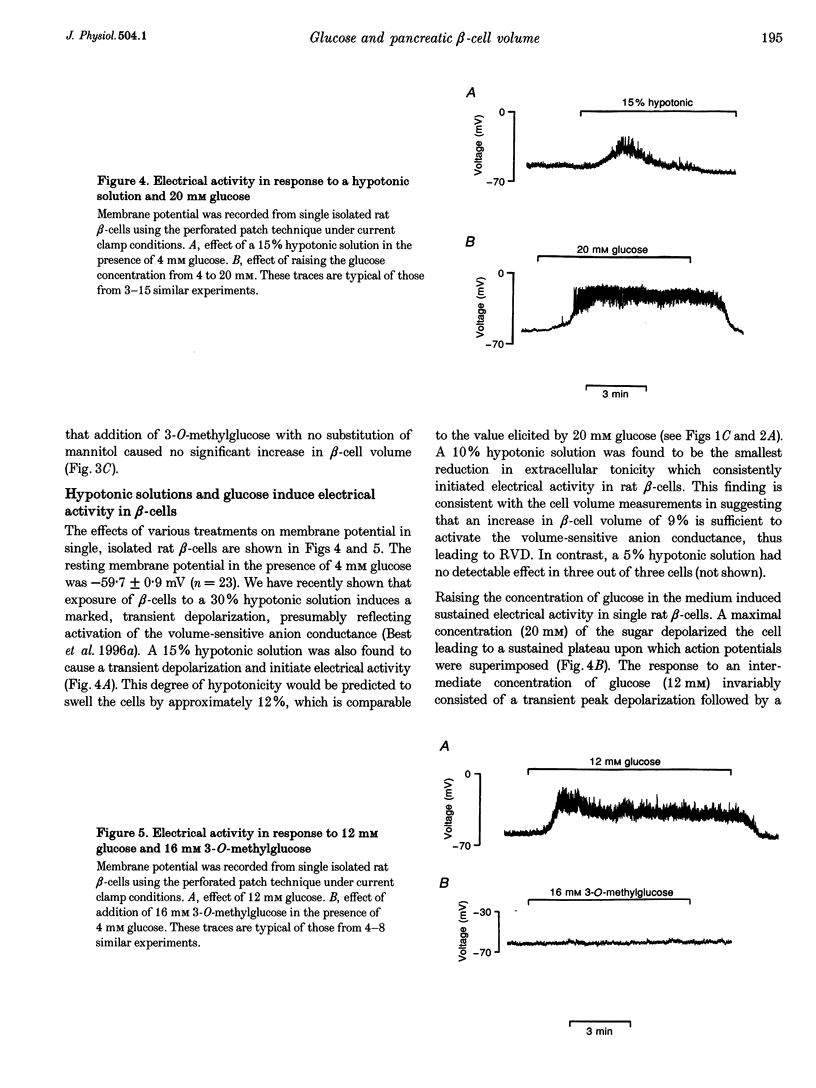


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benson C. T., Liu C., Gao D. Y., Critser E. S., Critser J. K. Determination of the osmotic characteristics of hamster pancreatic islets and isolated pancreatic islet cells. Cell Transplant. 1993 Nov-Dec;2(6):461–465. doi: 10.1177/096368979300200604. [DOI] [PubMed] [Google Scholar]
- Best L. Glucose and alpha-ketoisocaproate induce transient inward currents in rat pancreatic beta cells. Diabetologia. 1997 Jan;40(1):1–6. doi: 10.1007/s001250050635. [DOI] [PubMed] [Google Scholar]
- Best L., Miley H. E., Yates A. P. Activation of an anion conductance and beta-cell depolarization during hypotonically induced insulin release. Exp Physiol. 1996 Nov;81(6):927–933. doi: 10.1113/expphysiol.1996.sp003993. [DOI] [PubMed] [Google Scholar]
- Best L., Sheader E. A., Brown P. D. A volume-activated anion conductance in insulin-secreting cells. Pflugers Arch. 1996 Jan;431(3):363–370. doi: 10.1007/BF02207273. [DOI] [PubMed] [Google Scholar]
- Best L., Trebilcock R., Tomlinson S. Lactate transport in insulin-secreting beta-cells: contrast between rat islets and HIT-T15 insulinoma cells. Mol Cell Endocrinol. 1992 Jul;86(1-2):49–56. doi: 10.1016/0303-7207(92)90174-5. [DOI] [PubMed] [Google Scholar]
- Best L., Yates A. P., Meats J. E., Tomlinson S. Effects of lactate on pancreatic islets. Lactate efflux as a possible determinant of islet-cell depolarization by glucose. Biochem J. 1989 Apr 15;259(2):507–511. doi: 10.1042/bj2590507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackard W. G., Kikuchi M., Rabinovitch A., Renold A. E. An effect of hyposmolarity on insulin release in vitro. Am J Physiol. 1975 Mar;228(3):706–713. doi: 10.1152/ajplegacy.1975.228.3.706. [DOI] [PubMed] [Google Scholar]
- Britsch S., Krippeit-Drews P., Gregor M., Lang F., Drews G. Effects of osmotic changes in extracellular solution on electrical activity of mouse pancreatic B-cells. Biochem Biophys Res Commun. 1994 Oct 28;204(2):641–645. doi: 10.1006/bbrc.1994.2507. [DOI] [PubMed] [Google Scholar]
- Falke L. C., Gillis K. D., Pressel D. M., Misler S. 'Perforated patch recording' allows long-term monitoring of metabolite-induced electrical activity and voltage-dependent Ca2+ currents in pancreatic islet B cells. FEBS Lett. 1989 Jul 17;251(1-2):167–172. doi: 10.1016/0014-5793(89)81448-6. [DOI] [PubMed] [Google Scholar]
- Hedeskov C. J. Mechanism of glucose-induced insulin secretion. Physiol Rev. 1980 Apr;60(2):442–509. doi: 10.1152/physrev.1980.60.2.442. [DOI] [PubMed] [Google Scholar]
- Hellman B., Sehlin J., Täljedal I. B. Transport of 3-O-methyl-D-glucose into mammalian pancreatic -cells. Pflugers Arch. 1973;340(1):51–58. doi: 10.1007/BF00592196. [DOI] [PubMed] [Google Scholar]
- Hoffmann E. K., Simonsen L. O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol Rev. 1989 Apr;69(2):315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- Häussinger D., Lang F. The mutual interaction between cell volume and cell function: a new principle of metabolic regulation. Biochem Cell Biol. 1991 Jan;69(1):1–4. doi: 10.1139/o91-001. [DOI] [PubMed] [Google Scholar]
- Kinard T. A., Satin L. S. An ATP-sensitive Cl- channel current that is activated by cell swelling, cAMP, and glyburide in insulin-secreting cells. Diabetes. 1995 Dec;44(12):1461–1466. doi: 10.2337/diab.44.12.1461. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Sener A., Levy J., Herchuelz A. The stimulus-secretion coupling of glucose-induced insulin release. XXII. Qualitative and quantitative aspects of glycolysis in isolated islets. Acta Diabetol Lat. 1976 Sep-Dec;13(5-6):202–215. doi: 10.1007/BF02581118. [DOI] [PubMed] [Google Scholar]
- Moser T., Chow R. H., Neher E. Swelling-induced catecholamine secretion recorded from single chromaffin cells. Pflugers Arch. 1995 Dec;431(2):196–203. doi: 10.1007/BF00410191. [DOI] [PubMed] [Google Scholar]
- Sarkadi B., Parker J. C. Activation of ion transport pathways by changes in cell volume. Biochim Biophys Acta. 1991 Dec 12;1071(4):407–427. doi: 10.1016/0304-4157(91)90005-h. [DOI] [PubMed] [Google Scholar]
- Sehlin J. Interrelationship between chloride fluxes in pancreatic islets and insulin release. Am J Physiol. 1978 Nov;235(5):E501–E508. doi: 10.1152/ajpendo.1978.235.5.E501. [DOI] [PubMed] [Google Scholar]
- Semino M. C., Gagliardino A. M., Bianchi C., Rebolledo O. R., Gagliardino J. J. Early changes in the rat pancreatic B cell size induced by glucose. Acta Anat (Basel) 1990;138(4):293–296. doi: 10.1159/000146958. [DOI] [PubMed] [Google Scholar]
- Smith P. A., Ashcroft F. M., Rorsman P. Simultaneous recordings of glucose dependent electrical activity and ATP-regulated K(+)-currents in isolated mouse pancreatic beta-cells. FEBS Lett. 1990 Feb 12;261(1):187–190. doi: 10.1016/0014-5793(90)80667-8. [DOI] [PubMed] [Google Scholar]


