Abstract
The alpha C protein, a protective surface protein of group B streptococci (GBS), is present in most non-type III GBS strains. Conjugate vaccines composed of the alpha C protein and type III capsular polysaccharide (CPS) might be protective against most GBS infections. In this study, the type III CPS was covalently coupled to full-length, nine-repeat alpha C protein (resulting in III-α9r conjugate vaccine) or to two-repeat alpha C protein (resulting in III-α2r conjugate vaccine) by reductive amination. Initial experiments with the III-α9r vaccine showed that it was poorly immunogenic in mice with respect to both vaccine antigens and was suboptimally efficacious in providing protection in mice against challenge with GBS. Therefore, modified vaccination protocols were used with the III-α2r vaccine. Female mice were immunized three times with 0.5, 5, or 20 μg of the III-α2r vaccine with an aluminum hydroxide adjuvant and bred. Ninety-five percent of neonatal mice born to dams immunized with the III-α2r vaccine survived challenge with GBS expressing type III CPS, and 60% survived challenge with GBS expressing wild-type (nine-repeat) alpha C protein; 18 and 17%, respectively, of mice in the negative control groups survived (P, <0.0001). These protection levels did not differ significantly from those obtained with the type III CPS-tetanus toxoid conjugate vaccine and the unconjugated two-repeat alpha C protein, which protected 98 and 58% of neonates from infection with GBS expressing type III CPS or the alpha C protein, respectively. Thus, the two-repeat alpha C protein in the vaccine was immunogenic and simultaneously enhanced the immunogenicity of type III CPS. III-α vaccines may be alternatives to GBS polysaccharide-tetanus toxoid vaccines, eliciting additional antibodies protective against GBS infection.
Group B streptococci (GBS) are the leading cause of meningitis, pneumonia, and sepsis in neonates (1). The type-specific capsular polysaccharides (CPS) expressed on the surface of GBS are considered protective antigens (23). Thus far, nine CPS serotypes are known: Ia, Ib, II, III, IV, V, VI, VII, and VIII. Human vaccine trials with CPS of types Ia, II, and III showed low and variable levels of CPS-specific antibodies following immunization of volunteers and pregnant women (2, 3). Coupling of type III CPS to a carrier protein, such as tetanus toxoid (TT), enhanced the immunogenicity of type III CPS and resulted in antibody levels that were less variable. Vaccination of healthy nonpregnant women with a type III CPS-TT (III-TT) conjugate vaccine elicited a 50-fold increase in type III CPS-specific antibody levels, while vaccination with an unconjugated type III CPS vaccine elicited a 7-fold increase (18). Even at lower doses, 90% of recipients reacted to the III-TT vaccine with a fourfold increase in type III CPS-specific antibody concentrations, as opposed to only 50% of recipients of the unconjugated type III CPS vaccine.
Protection by CPS is highly specific, and cross-protection by the different serotypes does not occur. To be fully effective, a multivalent vaccine would require the inclusion of several clinically important serotypes. The most prevalent GBS serotypes causing infections in the United States are type Ia (approximately 35 to 50% of all GBS infections) and type III (approximately 30% of all GBS infections) (17, 23a). Recently, type V has emerged as the most common serotype in nonpregnant adults (5).
TT is a component of several other vaccines, and most adults have high levels of preexisting antibody to it (16). Since TT is not relevant to GBS infection, it might be advantageous to incorporate a GBS protein into the vaccine for the purpose of enhancing protection and expanding the number of strains covered by the vaccine. Replacement of TT with a protective cell surface protein of GBS might eliminate the need for many types of CPS in a multivalent GBS vaccine and avoid the overuse of TT, which may lead to toxicity or suppression of the anti-CPS antibody response (7, 9, 16).
Among the surface proteins of GBS that confer immunity, the first to be identified were two molecules designated the alpha and beta C proteins (4, 21, 35). In addition, the R protein (10, 22), Rib protein (33), and alpha-like proteins purified from type III GBS (19) and from type V GBS (20) were identified. Among these GBS surface proteins, alpha C protein is the most frequently found in clinical isolates (9a, 17). The alpha C protein is present in approximately 50% of all clinical isolates and in approximately 70% of non-type III GBS isolates. Therefore, the alpha C protein is a potentially valuable replacement for TT in conjugate vaccines with type III CPS. A type III CPS-alpha C protein conjugate vaccine could, by itself, be effective in providing protection against most GBS infections.
The cloned alpha C protein gene (bca) contains nine identical tandem repeats flanked by N and C termini (29). However, the size of the protein, which corresponds to the number of repeats, varies in clinical GBS isolates (25, 28). Using a neonatal mouse model, we have shown that the number of repeats is important for vaccine efficacy (11–13). Alpha C proteins containing 1 or 2 repeats are more protective when used for passive (11) or active (12) immunization than are alpha C proteins with 9 or 16 repeats. Alpha C proteins with fewer repeats seemed to be more immunogenic than alpha C proteins with more repeats (12). In addition, spontaneous mutants with fewer repeats could escape from nine-repeat protein-elicited antibodies (26) but not from one-repeat protein-elicited antibodies (13).
In this study, two-repeat alpha C protein was conjugated to GBS type III CPS (resulting in III-α2r vaccine) by reductive amination. We tested whether this protein could act as a carrier protein for type III CPS and simultaneously whether it could protect from infection with GBS expressing nine-repeat alpha C protein. Antisera elicited to the III-α2r vaccine were analyzed for antibodies to type III CPS and two-repeat alpha C protein by an enzyme-linked immunosorbent assay (ELISA). Protective efficacy was studied by passive and active immunization in a neonatal mouse model with GBS strains M781, which expresses type III CPS but lacks C proteins (III/C−), and A909, which expresses type Ia CPS and the alpha and loeta C proteins (Ia/Cα+β+), for challenge. In a separate study, type III CPS was coupled to nine-repeat alpha C protein (resulting in III-α9r vaccine) and tested for its immunogenicity and protective capacity in neonatal mice. Results obtained in each vaccine study with III-α9r or III-α2r vaccine were individually compared to results obtained with the III-TT vaccine, which was included in both studies.
MATERIALS AND METHODS
Bacterial strains.
Wild-type GBS strain A909 (Ia/Cα+β+), which expresses nine repeats, and GBS strain M781 (III/C−) were used in the protection studies. Escherichia coli HMS174(DE3) and BL21(DE3), which contain plasmids with different numbers of repeats, were used for the purification of two- and nine-repeat alpha C proteins. GBS strain M781 was used as a source of type III polysaccharide.
Purification of proteins and polysaccharide.
Recombinant two- and nine-repeat alpha C proteins were expressed and purified as described previously (11). TT (Institut Armand Frappier, Montreal, Quebec, Canada) was purified to its monomeric form as detailed elsewhere (34). Type III polysaccharide was purified from GBS strain M781 by a previously described method (34).
Conjugation by reductive amination.
TT was coupled to type III CPS by reductive amination as detailed in a previous study (34). In the current study, reductive amination was also used, with minor modifications, to couple the alpha C proteins (with two or nine repeats) to type III CPS. In brief, type III CPS was oxidized with NaIO4. The proportion of sialic acid residues oxidized was determined by gas chromatography-mass spectrometry of trimethylsilyl derivatives. Equal amounts of oxidized type III CPS and alpha C protein were conjugated in 0.2 M Na2HPO4 (pH 7.2) by use of sodium cyanoborohydrate, and the mixture was incubated at 37°C for up to 4 days. The conjugation process was monitored by chromatography of the conjugation sample on a Superose-6 column (Pharmacia, Piscataway, N.J.) with phosphate-buffered saline, and the A280 was monitored at the beginning of conjugation and 24, 48, and 66 h later. Subsequently, the conjugate was separated from free alpha C protein by gel filtration chromatography (S300 column; Pharmacia) with phosphate-buffered saline. The void-volume fractions monitored by A280 were pooled, reduced with sodium borohydride, dialyzed against water, and lyophilized. The III-TT conjugate vaccine was produced in a previous study by use of type III CPS with 25% of the sialic acid residues oxidized (34); the conjugate vaccine contained 61% (wt/wt) CPS and 39% (wt/wt) protein.
Analysis of the conjugate vaccines.
III-α2r and III-α9r vaccines were analyzed for their protein (24) and carbohydrate (8) contents; purified alpha C protein and type III CPS were used as the standards. The quality of the conjugate was tested by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) followed by silver staining (30) or by Western blotting with rabbit antisera to type III CPS and monoclonal antibody 4G8 to the alpha C protein.
Production of rabbit antisera.
Two New Zealand White female rabbits (6 to 8 weeks old) were subcutaneously immunized on days 0, 21, and 42 with 50 μg of the III-α2r vaccine in the presence of aluminum hydroxide or with 100 μg of the III-α9r vaccine in the presence of complete Freund’s adjuvant on day 0; booster doses were given on days 21 and 42 with incomplete Freund’s adjuvant. Serum was collected before each immunization and again 2 weeks after the last immunization.
Immunogenicity of the conjugate vaccines.
Antibodies elicited to the two- and nine-repeat alpha C proteins by the unconjugated proteins or by the III-α2r or III-α9r vaccines in rabbits or mice were measured in an ELISA developed for the alpha C proteins as described previously (11). Antibodies elicited to type III CPS by the III-TT, III-α2r, and III-α9r vaccines in rabbits or mice were measured by an ELISA developed for this polysaccharide (14).
Passive immunization.
The mouse protection study was adapted from that of Rodewald et al. (31). In our study, groups of four CD-1 outbred pregnant mice were given 0.5 ml of undiluted rabbit antiserum by intraperitoneal injection 3 to 4 days before delivery. Within 48 h of birth, pups were challenged intraperitoneally with 5 × 104 CFU of GBS strain A909 or with 1 × 106 CFU of GBS strain M781 (103 times the 50% lethal dose, as determined in pilot studies). Survival was assessed 48 h after challenge, and survival data for groups whose dams had been immunized with different antisera were compared by use of Fisher’s exact test.
Active immunization.
A neonatal mouse model of GBS infection was used to compare the protective efficacies of the III-α2r and III-TT vaccines or to compare those of the III-α9r and III-TT vaccines (31). Groups of four CD-1 outbred mice (6 to 8 weeks old) were immunized intraperitoneally with 0.5, 5, or 20 μg of the III-α2r vaccine or with 2 μg of the III-TT vaccine in the presence of aluminum hydroxide adjuvant on day 0 and boosted on days 21 and 42. For immunization with the III-α9r vaccine, mice were given 5, 10, or 20 μg in the presence of aluminum hydroxide adjuvant and boosted once (on day 21) instead of twice. Mice were bred 1 week after the last immunization, and pups (born 4 weeks after the last booster) were challenged intraperitoneally with 5 × 104 CFU of GBS strain A909 or with 1 × 106 CFU of GBS strain M781 (103 times the 50% lethal dose, as determined in a pilot study) within 48 h of birth. Survival was assessed 48 h after challenge, and survival data for groups whose dams had been immunized with different vaccines were compared by use of Fisher’s exact test.
RESULTS
III-α9r conjugate vaccine. (i) Analysis of the conjugate vaccine.
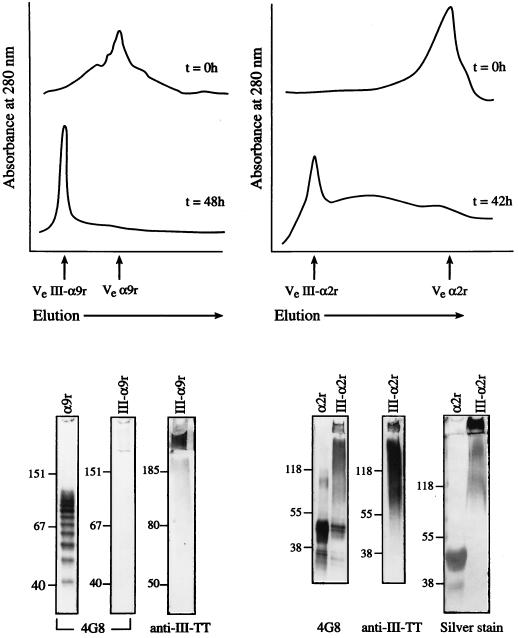
For the III-α9r vaccine, 40% of the sialic acid residues in the polysaccharides were oxidized to create aldehyde groups for covalent linkage with the ɛ-amino groups of lysine residues in the alpha C protein. An A280 profile of Superose-6 fractions as well as an SDS-PAGE analysis of S300 fractions (Fig. 1) revealed conjugation with a high degree of cross-linking between the nine-repeat alpha C protein (109 kDa) and type III CPS (150 kDa). The purified III-α9r conjugate vaccine was composed of 51% (wt/wt) CPS and 49% (wt/wt) protein.
FIG. 1.
Analysis of the conjugate vaccines. (Top) The elution volumes of uncoupled nine-repeat alpha C protein (Ve α9r) and of the type III polysaccharide–nine-repeat alpha C protein conjugate vaccine (Ve III-α9r) were 11.4 and 6.8 ml, respectively. The shift to the left of the protein profile 48 h after conjugation indicates the formation of high-molecular-weight polymers. The elution volumes of uncoupled two-repeat alpha C protein (Ve α2r) and of the type III polysaccharide–two-repeat alpha C protein conjugate vaccine (Ve III-α2r) were 16.6 and 8.2 ml, respectively. The shift to the left of the protein profile 42 h after conjugation indicates the formation of high-molecular-weight polymers. (Bottom) SDS-PAGE was followed by silver staining or by Western blotting (with monoclonal antibody 4G8 to detect the alpha C protein or rabbit antiserum to detect type III CPS); the results obtained with III-α9r conjugation are shown at the lower left, while those obtained with III-α2r conjugation are shown at the lower right. Molecular masses in kilodaltons are shown to the left of each panel. For both vaccines, the high-molecular-mass conjugate is reactive with antibodies to both the protein and the polysaccharide.
(ii) Active immunization.
No dose-response effect on antibody titers to either antigen (type III CPS or nine-repeat alpha C protein) was observed for mice (Tables 1 and 2). Moreover, the immunogenicity of the vaccine, as assessed by antibody titers elicited to each of the components, was moderate to low.
TABLE 1.
Immunogenicity and protective efficacy of III-α9r conjugate vaccine in micea
| Immunizing antigen | Dose of immunization (μg/mouse)b | No. of pups alive/total no. of pups (% survival)c | Type III CPS-specific IgG titerd |
|---|---|---|---|
| III-α9r | 5 | 0/24 (0)* | 2,000 |
| III-α9r | 10 | 11/38 (29)** | 5,000 |
| III-α9r | 20 | 9/33 (27)*** | 2,000 |
| III-TT (positive control) | 2 | 16/16 (100) | 10,000 |
| Saline (negative control) | 0 | 0/32 (0) | 200 |
Protection of neonatal mice against type III GBS challenge was tested.
Mice were immunized at days 0 and 21; blood was drawn at day 60.
Pups were challenged with GBS strain M781. Compared to saline, P values were as follows: *, 1.000; **, 0.0006; and ***, 0.0021. Compared to III-TT, P values were as follows: *, **, and ***, <0.0001.
Blood was drawn at day 60; sera from three mice were pooled.
TABLE 2.
Immunogenicity and protective efficacy of the III-α9r conjugate vaccine in micea
| Immunizing antigen | Dose of immunization (μg/mouse)b | No. of pups alive/total no. of pups (% survival)c | α9r-specific IgG titerd |
|---|---|---|---|
| III-α9r | 5 | 16/26 (62)* | 400 |
| III-α9r | 10 | 15/35 (37)** | 200 |
| III-α9r | 20 | 2/32 (6)*** | 600 |
| α9r (positive control) | 10 | 18/31 (58) | 1,200 |
| Saline (negative control) | 0 | 2/29 (2) | 200 |
Protection of neonatal mice against α-positive GBS challenge was tested.
Mice were immunized at days 0 and 21.
Pups were challenged with GBS strain A909. Compared to saline, P values were as follows: *, <0.0001; **, 0.0014; and ***, 1.000. Compared to α9r, P values were as follows: *, 1.000; **, 0.3240; and ***, <0.0001.
Blood was drawn at day 60; sera from three mice were pooled.
Although the III-α9r vaccine elicited moderate antibody titers to type III CPS, neonates were only weakly protected from infection with GBS strain M781 (29%) (Table 1). Surprisingly, neonates were protected from infection with GBS strain A909 (62%), despite the low antibody titers elicited to the nine-repeat alpha C protein component of the III-α9r vaccine (Table 2). The positive controls (III-TT vaccine and unconjugated nine-repeat alpha C protein) protected 100 and 58% of neonates from GBS infection, respectively. The negative control (saline) did not protect neonates from GBS infection.
(iii) Passive immunization.
Antibody titers to type III CPS and nine-repeat alpha C protein were 51,200 and 6,400, respectively, in antisera from rabbits immunized with the III-α9r vaccine. All neonates born to dams immunized with these antisera were protected from infection with GBS strain M781, while 58% were protected from infection with GBS strain A909 (Table 3). The positive controls (antisera elicited to III-TT vaccine and unconjugated nine-repeat alpha C protein) protected 98 and 45% of the neonates, respectively. The negative control (preimmunization sera) did not protect neonates from GBS infection. In pups born to control mice that received saline rather than serum, survival rates were 9% (M781 challenge) and 22% (A909 challenge).
TABLE 3.
Protection of neonatal mice from GBS infection by passive immunization with III-α9r conjugate vaccine
| Antiserum | Challenge strain | No. alive/total no. tested (% survival)a |
|---|---|---|
| III-α9r | A909 | 21/36 (58)* |
| III-α9r | M781 | 36/36 (100)** |
| Nine-repeat alpha C protein | A909 | 18/40 (45)*** |
| III-TT (positive control) | M781 | 43/44 (98)**** |
| Preimmunization (negative control) | A909 | 9/45 (20) |
| Preimmunization (negative control) | M781 | 3/45 (7) |
Significant differences in protection levels for different antisera were identified by Fisher’s exact test. Compared to preimmunization serum after challenge with strain A909, P values were as follows: *, 0.0001; ***, 0.0193. Compared to preimmunization serum after challenge with strain M781, P values were as follows: ** and ****, <0.0001.
III-α2r conjugate vaccine. (i) Analysis of the conjugate vaccine.
We were unable to achieve conjugation of one-repeat alpha C protein (39 kDa) with type III CPS (data not shown). For the III-α2r vaccine, 40% of the sialic acid residues in the type III CPS were oxidized, and conjugation with a high degree of cross-linking was obtained with two-repeat alpha C protein (47 kDa). The III-α2r vaccine was composed of 61% (wt/wt) CPS and 39% (wt/wt) protein. An A280 profile of Superose-6 fractions and an SDS-PAGE analysis of the purified (S300 column) III-α2r conjugate vaccine are shown in Fig. 1.
(ii) Active immunization.
A clear dose-response effect of the III-α2r vaccine on antibody titers to both antigens (type III CPS and two-repeat alpha C protein) was observed for mice (Tables 4 and 5). Although the III-α2r vaccine elicited reasonably high antibody titers to the protein and CPS antigens, the positive controls (purified unconjugated two-repeat alpha C protein and III-TT vaccine) elicited higher antibody titers. Several studies with mice and humans (15, 18) have shown that unconjugated type III CPS is not adequately immunogenic; therefore, the unconjugated antigen was not tested in this study.
TABLE 4.
Immunogenicity and protective efficacy of the III-α2r conjugate vaccine in micea
| Immunizing antigen | Dose of immunization (μg/mouse)b | No. of pups alive/total no. of pups (% survival)c | Geometric mean titer of type III CPS-specific IgGd |
|---|---|---|---|
| III-α2r | 0.5 | 12/36 (33)* | 1,131 |
| III-α2r | 5 | 32/41 (78)** | 9,051 |
| III-α2r | 20 | 36/38 (95)*** | 20,278 |
| III-TT (positive control) | 2 | 45/46 (98) | 76,472 |
| Saline (negative control) | 0 | 8/45 (18) | 109 |
Protection of neonatal mice against type III GBS challenge was tested.
Mice were immunized at days 0, 21, and 42.
Pups were challenged with GBS strain M781. Compared to saline, P values were as follows: *, 0.1258; ** and ***, <0.0001. Compared to III-TT, P values were as follows: *, <0.0001; **, 0.053; and ***, 0.5872.
Blood was drawn at day 60; values are geometric means for four sera.
TABLE 5.
Immunogenicity and protective efficacy of the III-α2r conjugate vaccine in micea
| Immunizing antigen | Dose of immunization (μg/mouse)b | No. of pups alive/total no. of pups (% survival)c | Geometric mean titer of α2r-specific IgGd |
|---|---|---|---|
| III-α2r | 0.5 | 6/37 (16)* | 270 |
| III-α2r | 5 | 17/40 (43)** | 3,577 |
| III-α2r | 20 | 22/37 (60)*** | 8,170 |
| α2r (positive control) | 10 | 22/38 (58) | 14,969 |
| Saline (negative control) | 0 | 8/46 (17) | 170 |
Protection of neonatal mice against α-positive GBS challenge was tested.
Mice were immunized at days 0, 21, and 42.
Pups were challenged with GBS strain A909. Compared to saline, P values were as follows: *, 1; **, 0.0166; and ***, <0.0001. Compared to α2r, P values were as follows: *, 0.0003; **, 0.2573; and ***, 1.000.
Blood was drawn at day 60; values are geometric means for four sera.
The protective capacity of the III-α2r vaccine in neonatal mice was assessed in the same dose-response experiment as that used to evaluate its immunogenicity. A dose-response effect on protection levels was observed. At the highest dose (20 μg/mouse), 95% of neonates were protected from infection with GBS strain M781 (Table 4), while 60% were protected from infection with GBS strain A909 (Table 5). The positive controls (III-TT vaccine and unconjugated two-repeat alpha C protein) protected 98 and 58% of neonates, respectively. The negative control (saline with adjuvant) did not significantly protect neonates from GBS infection.
(iii) Passive immunization.
Antibody titers to type III CPS and two-repeat alpha C protein were 51,200 and 3,200, respectively, in antisera from rabbits immunized with the III-α2r vaccine. To confirm the results obtained with active immunization, mice were passively immunized with rabbit antisera to the III-α2r vaccine. Ninety-four percent of neonates were protected from infection with GBS strain M781, while 55% were protected from infection with GBS strain A909 (Table 6). The positive controls (antisera elicited to III-TT vaccine and unconjugated two-repeat alpha C protein) protected 98 and 55% of neonates, respectively. The negative control (preimmunization sera) did not significantly protect neonates from GBS infection. In pups born to control mice that received saline rather than serum, survival rates were 6% (M781 challenge) and 14% (A909 challenge). In each of the active and passive protection studies, little variation was observed in survival among litters within the same treatment groups.
TABLE 6.
Protection of neonatal mice from GBS infection by passive immunization with III-α2r conjugate vaccine
| Antiserum | Challenge strain | No. alive/total no. tested (% survival)a |
|---|---|---|
| III-α2r | A909 | 22/40 (55)* |
| III-α2r | M781 | 34/36 (94)** |
| Two-repeat alpha C protein (positive control) | A909 | 35/48 (73)*** |
| III-TT (positive control) | M781 | 41/42 (98)**** |
| Preimmunization (negative control) | A909 | 5/35 (19) |
| Preimmunization (negative control) | M781 | 3/47 (8) |
Significant differences in protection levels for different antisera were identified by Fisher’s exact test. Compared to preimmune antiserum after challenge with strain A909, P values were as follows: *, 0.0003; ***, <0.0001. Compared to preimmune antiserum after challenge with strain M781, P values were as follows: **, 0.0004; ****, <0.00001.
DISCUSSION
Newer vaccines against encapsulated bacteria are based on the chemical coupling of CPS to carrier proteins. Thus far, only a few carrier proteins, such as TT or diphtheria toxoid, have been used for the preparation of these vaccines. Carrier-specific epitope suppression, in which the antibody response to CPS is reduced by prior immunization with the carrier protein, has been documented in several studies (9, 16, 32). Although it has been shown that prior immunization of mice with TT does not suppress the immune response to the type III CPS component of the III-TT vaccine (13a), recent studies with humans (7) suggest that an increase in the amount of TT—for instance, in multicomponent vaccines—may reduce the efficiency of such vaccines. If a protective surface protein from the same microorganism as the polysaccharide is used as a carrier protein, the overuse of TT is avoided and protection against more serotypes may be provided.
Until now, only a few conjugate vaccines containing a protective surface protein and a polysaccharide from the same microorganism have been described. Neisseria meningitidis CPS conjugated to a meningococcal outer membrane vesicle protein from the same organism induced high bactericidal titers in monkeys (36). Another example is a CPS fimbrial protein conjugate vaccine which protected against Porphyromonas gingivalis in mice (6). We previously showed that the surface-associated beta C protein of GBS in a conjugate vaccine with type III CPS successfully protected neonatal mice from GBS expressing beta C protein (27). However, beta C protein is present in only 10% of all clinical isolates of GBS. Since alpha C protein is present in 70% of non-type III GBS strains, a combination of type III CPS and the alpha C protein in one vaccine might be protective against most GBS infections.
In the study presented here, we conjugated GBS type III CPS to a nine-repeat alpha C protein of GBS by reductive amination and tested this conjugate vaccine for its immunogenicity and protective efficacy in mice. It appeared that antibody titers elicited to type III CPS and nine-repeat alpha C protein by the III-α9r vaccine were moderate to low. In a previous study, we showed that the immunogenicity of the alpha C protein in mice was inversely related to the number of repeats (11). In other words, 9- or 16-repeat alpha C protein was less immunogenic than 1- or 2-repeat alpha C protein. This low immunogenicity of nine-repeat alpha C protein may have influenced the immunogenicity of both components in the III-α9r vaccine. Of note, there was no direct correspondence between the dose of III-α9r vaccine and its protective efficacy in active immunization (Tables 1 and 2). With other vaccines, higher doses of carrier proteins have resulted in diminished protective efficacy (7, 9, 16, 32). The antibody response to the alpha C protein moiety was so low at all doses tested that it may have been near a threshold resulting in variable protection. Because the ELISA does not measure functional activity, ELISA titers may not correlate directly with protective efficacy.
The III-α9r vaccine protected 29% of neonatal mice from GBS expressing type III CPS and 62% of neonatal mice from GBS expressing nine-repeat alpha C protein. Inhibition with rabbit antiserum raised to native type III CPS on intact GBS in an ELISA showed no significant difference between antibody binding to the III-α9r vaccine and to unconjugated type III CPS (data not shown). The implication is that epitopes in type III CPS, including conformational epitopes, were not altered by conjugation to the alpha C protein. Moreover, high antibody titers to type III CPS were elicited in rabbits by the III-α9r vaccine in the presence of Freund’s adjuvant, and 100% of pups were protected from GBS expressing type III CPS by passive immunization with this rabbit antiserum. This result indicates that the protective epitope in the type III CPS was still intact after conjugation with the alpha C protein. Although unconjugated nine-repeat alpha C protein or the III-TT vaccine protected neonatal mice well from infection with GBS expressing type III CPS or nine-repeat alpha C protein, respectively, we could not exclude the possibility that the active immunization schedule used in this study was suboptimal for the III-α9r vaccine.
Since 1- or 2-repeat alpha C protein is more immunogenic than 9- or 16-repeat alpha C protein, we initially undertook the conjugation of 1-repeat alpha C protein with type III CPS of GBS. However, this effort was not successful. Successful conjugation between two-repeat alpha C protein (47 kDa, 36 lysine residues) and type III CPS suggests that the molecular mass or the number of lysine residues in the one-repeat alpha C protein (39 kDa, 23 lysine residues) was too low for efficient conjugation. In addition, the conformation of one-repeat alpha C protein, which might differ from that of two-repeat alpha C protein, could reduce the availability of lysine residues.
Since the efficacy of the III-α9r vaccine in actively immunized mice was less than optimal, the immunization schedule used with the III-α-2r vaccine included an additional booster dose. Active immunization of mice with the III-α2r vaccine induced high antibody titers to type III CPS and two-repeat alpha C protein. The observed protection levels were not significantly different from those obtained with the III-TT vaccine (98%) and with unconjugated two-repeat alpha C protein (58%), although the immunizing dose of the III-α2r vaccine was higher. Similar protection levels were obtained by passive immunization with rabbit antiserum raised to the III-α2r vaccine plus aluminum hydroxide.
In this study, GBS two-repeat alpha C protein was an effective carrier for the type III CPS and simultaneously added protection against infection with GBS expressing the alpha C protein. We conclude that the III-α2r vaccine may be useful as an alternative to polysaccharide-TT conjugate vaccines for GBS and as a model for other conjugate vaccines.
ACKNOWLEDGMENTS
We thank Barbara Reinap for the preparation of the conjugate vaccines and Liz Gong and Julianne Pinel for technical assistance with the III-α9r conjugate vaccine.
This research was supported by NIH grant AI38424 and NIH contract AI75326.
REFERENCES
- 1.Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 980–1054. [Google Scholar]
- 2.Baker C J, Kasper D L. Group B streptococcal vaccines. Rev Infect Dis. 1985;7:458–467. doi: 10.1093/clinids/7.4.458. [DOI] [PubMed] [Google Scholar]
- 3.Baker C J, Rench M A, Edwards M S, Carpenter R J, Hays B M, Kasper D L. Immunization of pregnant women with a polysaccharide vaccine of group B Streptococcus. N Engl J Med. 1988;319:1180–1220. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 4.Bevanger L, Maeland J A. Complete and incomplete Ibc protein fraction in group B streptococci. Acta Pathol Microbiol Scand Sect B. 1979;87:51–54. doi: 10.1111/j.1699-0463.1979.tb02402.x. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg H M, Stephens D S, Modansky M, Erwin M, Elliot J, Facklam R R, Schuchat A, Baughman W, Farley M M. Invasive group B streptococcal disease: the emergence of serotype V. J Infect Dis. 1996;173:365–373. doi: 10.1093/infdis/173.2.365. [DOI] [PubMed] [Google Scholar]
- 6.Choi J I, Schifferle R E, Yoshimura F, Kim B W. Capsular polysaccharide-fimbrial protein conjugate vaccine protects against Porphyromonas gingivalis infection in SCID mice reconstituted with human peripheral blood lymphocytes. Infect Immun. 1998;66:391–393. doi: 10.1128/iai.66.1.391-393.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dagan R, Eskola J, Leclerc C, Leroy O. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect Immun. 1998;66:2093–2098. doi: 10.1128/iai.66.5.2093-2098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois M, Gilles K A, Hamilton J K, Rebers P A, Smith F. Colorimetric method for the determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1038/168167a0. [DOI] [PubMed] [Google Scholar]
- 9.Fattom A, Cho Y H, Fuller S, Naso R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Overload of homologous carrier protein at the site of injection might cause a reduced immunogenicity of capsular polysaccharide conjugate vaccines; p. 160. [Google Scholar]
- 9a.Ferrieri P, Flores A E. Surface protein expression in group B streptococcal invasive isolates. Adv Exp Med Biol. 1997;418:635–637. doi: 10.1007/978-1-4899-1825-3_148. [DOI] [PubMed] [Google Scholar]
- 10.Flores A E, Ferrieri P. Molecular species of R-protein antigens produced by clinical isolates of group B streptococci. J Clin Microbiol. 1989;27:1050–1054. doi: 10.1128/jcm.27.5.1050-1054.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gravekamp C, Horensky D S, Michel J L, Madoff L C. Variation in repeat number within the alpha C protein of group B streptococci alters antigenicity and protective epitopes. Infect Immun. 1996;64:3576–3583. doi: 10.1128/iai.64.9.3576-3583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gravekamp C, Kasper D L, Michel J L, Kling D E, Carey V, Madoff L C. Immunogenicity and protective efficacy of the alpha C protein of group B streptococci are inversely related to the number of repeats. Infect Immun. 1997;65:5216–5221. doi: 10.1128/iai.65.12.5216-5221.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gravekamp C, Rosner B, Madoff L C. Deletion of repeats in the alpha C protein enhances the pathogenicity of group B streptococci in immune mice. Infect Immun. 1998;66:4347–4354. doi: 10.1128/iai.66.9.4347-4354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Guttormsen, H. K. Personal communication.
- 14.Guttormsen H K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 15.Guttormsen H K, Wetzler L M, Finberg R W, Kasper D L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herzenberg L A, Tokuhisa T. Carrier-priming leads to hapten-specific suppression. Nature. 1980;285:664–667. doi: 10.1038/285664a0. [DOI] [PubMed] [Google Scholar]
- 17.Johnson D R, Ferrieri P. Group B streptococcal Ibc protein antigen: distribution of two determinants in wild-type strains of common serotypes. J Clin Microbiol. 1984;19:506–510. doi: 10.1128/jcm.19.4.506-510.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Investig. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kvam A I, Bevanger L, Loseth K. An apparently new strain-variable Streptococcus agalactiae protein. Adv Exp Med Biol. 1997;418:355–357. doi: 10.1007/978-1-4899-1825-3_85. [DOI] [PubMed] [Google Scholar]
- 20.Lachenauer C S, Madoff L C. A protective surface protein from type V group B streptococci shares N-terminal sequence homology with the alpha C protein. Infect Immun. 1996;64:4255–4260. doi: 10.1128/iai.64.10.4255-4260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lancefield R C. Antigens of group B streptococci relation to mouse-protective antibodies and immunity. In: Robbins J B, et al., editors. New approaches for inducing natural immunity to pyogenic organisms. Bethesda, Md: National Institutes of Health; 1975. pp. 145–151. [Google Scholar]
- 22.Lancefield R C, Perlmann G E. Preparation and properties of a protein (R antigen) occurring in streptococci of group A, type 28 and in certain streptococci of other serological groups. J Exp Med. 1952;96:83–97. doi: 10.1084/jem.96.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy N J, Nicholson-Weller A, Baker C J, Kasper D L. Potentiation of virulence by group B streptococcal polysaccharides. J Infect Dis. 1984;149:851–860. doi: 10.1093/infdis/149.6.851. [DOI] [PubMed] [Google Scholar]
- 23a.Lin F Y, Clemens J D, Azimi P H, Regan J A, Weisman L E, Philips III J B, Rhoads G G, Clark P, Brenner R A, Ferrieri P. Capsular polysaccharide types of group B streptococcal isolates from neonates with early-onset systemic infection. J Infect Dis. 1998;177:790–792. doi: 10.1086/517810. [DOI] [PubMed] [Google Scholar]
- 24.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Madoff L C, Hori S, Michel J L, Baker C J, Kasper D L. Phenotypic diversity in the alpha C protein of group B streptococcus. Infect Immun. 1991;59:2638–2644. doi: 10.1128/iai.59.8.2638-2644.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madoff L C, Michel J L, Gong E W, Kling D E, Kasper D L. Group B streptococci escape host immunity by deletion of tandem repeat elements of the alpha C protein. Proc Natl Acad Sci USA. 1996;93:4131–4136. doi: 10.1073/pnas.93.9.4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madoff L C, Paoletti L C, Tai J Y, Kasper D L. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J Clin Investig. 1994;94:286–292. doi: 10.1172/JCI117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michel J L, Beseth B D, Madoff L C, Olken S K, Kasper D L, Ausubel F M. Genotypic diversity and evidence for two distinct classes of the C protein alpha antigen of group B Streptococcus. In: Totolian A, editor. Pathogenic streptococci: present and future. St. Petersburg, Russia: Lancer Publications; 1994. pp. 331–332. [Google Scholar]
- 29.Michel J L, Madoff L C, Olson K, Kling D E, Kasper D L, Ausubel F M. Large identical tandem repeating units in the C protein alpha antigen gene, bca, of group B streptococci. Proc Natl Acad Sci USA. 1992;89:10060–10065. doi: 10.1073/pnas.89.21.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morrissey J H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 31.Rodewald A K, Onderdonk A B, Warren H B, Kasper D L. Neonatal mouse model of group B streptococcal infection. J Infect Dis. 1992;166:635–639. doi: 10.1093/infdis/166.3.635. [DOI] [PubMed] [Google Scholar]
- 32.Schutze M P, Leclerc C, Jolivet M, Audibert F, Chedid L. Carrier-induced epitopic suppression, a major issue for future synthetic vaccines. J Immunol. 1985;135:2319–2322. [PubMed] [Google Scholar]
- 33.Stalhammar-Carlemalm M, Stenberg L, Lindahl G. Protein Rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J Exp Med. 1993;177:1593–1603. doi: 10.1084/jem.177.6.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wessels M R, Paoletti L C, Kasper D L, DiFabio J L, Michon F, Holme K, Jennings H J. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Investig. 1990;86:1428–1433. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilkinson H W, Eagon R G. Type-specific antigens of group B type Ic streptococci. Infect Immun. 1971;4:596–604. doi: 10.1128/iai.4.5.596-604.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zollinger W D, Moran E E, Devi S J, Frasch C E. Bactericidal antibody responses of juvenile rhesus monkeys immunized with group B Neisseria meningitidis capsular polysaccharide-protein conjugate vaccines. Infect Immun. 1997;65:1053–1060. doi: 10.1128/iai.65.3.1053-1060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]