Abstract
Due to the limitations of organ transplantation and the urgent need for treatment of chronic diseases, the benefit of stem cells for treatment has been studied and evaluated as an effective approach worldwide. One of the leading countries in this field is Iran. In this respect, several research and treatment institutes, including endocrinology and metabolism research institute are active in the use of stem cells in Iran. Herein, the aim is to review strategies, challenges, and opportunities for stem cell research and treatment in endocrinology and metabolism research institute.
Keywords: Cell Therapy, CTRM, Endocrinology and Metabolism, EMRI, Research Institutes, Regenerative Medicine, Stem Cell Research, Stem Cell Transplantation
Introduction
For many years, organ transplantation has been an effective treatment for end-stage organ disease, especially those related to the liver and kidney. In this context, a suitable donor deficiency for organ donation is a major problem that cannot be ignored [1–3]. On the other hand, chronic metabolic disorders as part of global health care concerns require advanced therapeutic strategies [4, 5]. Therein, stem cell transplantation (SCT) can be considered as a proper treatment decision [6, 7]. Different types of stem cells are established in each stage of an organism’s development. Considering their unique regenerative potency, they can provide infinite sources for repairing and regenerating damaged tissues and organs in the human body [8, 9]. Due to the benefits and the importance of cell transplantation, many projects and researches with the aim of developing the science of exploiting cells to help treat various diseases and disorders, have been carried out around the world [10]. Hereupon, Iran is one of the pioneers in this field. Specifically, Endocrinology and Metabolism Research Institute (EMRI) with various research groups including medical ethics, traditional medicine, innovation, modeling and simulation in medical sciences, bionanotechnology, immunogenetics, and Islamic fasting is one of the important research institutions of Iran that have a brilliant scientific and research background in cell transplantation (https://emri.tums.ac.ir/En) (Fig. 1). Herein, the purpose of writing a current review is to provide insight into the strategies, challenges, and opportunities for stem cell research and therapy in EMRI.
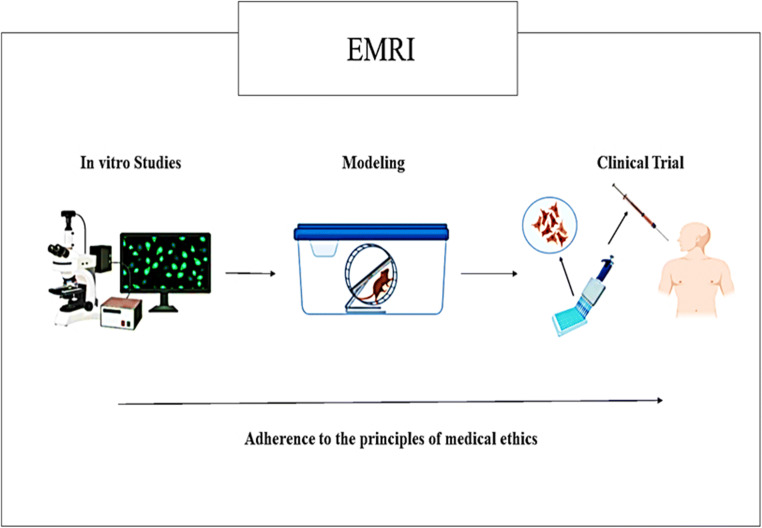
Fig. 1.
Endocrinology and metabolism research institute. EMRI with various research groups is one of the leading research institutions of Iran that have a great scientific and research background in cell transplantation. In this respect, the required facilities for in vitro studies to characterize the cell sources, modeling diseases to evaluate the effects of cell-based therapies, and finally cell transplantation in accordance with the guidelines and standards of medical ethics are provided in EMRI
Historical background of stem cell researches
In 1868, Ernst Haeckel was the first person who coined the “Stem Cell” term to the ancestor unicellular organism in science. Subsequently, Valentin Hacker ascribed this term to the gonadal cells [11, 12]. Thereupon, Alexander Maximo propounded the idea of blood stem cells in 1909 [13]. Later, in the 1960s McCulloch and till showed the presence of stem cells in bone marrow which are the progenitors of various blood cells through their experiments on mice. Embryonic stem cells were stated by university of San Francisco and Cambridge in 1981. Martin Evans and Matthew Kaufman from Cambridge university isolated the stem cell from the mouse embryos which was called “embryonic stem cells” by Gail Martin from the San Francisco university [14]. Later in 1998, first human embryonic stem cells derived from inner cell mass of human embryos by James Thomson. Simultaneously, John Gearhart isolated the embryonic germ cells from human fetal gonadal tissues. A successful transplant of insulin making cells from cadavers was reported in 1999. These accomplishments encouraged the related institutions and organizations to pay more money and attention to develop this field and optimize these techniques as novel future treatments. Accordingly, the induced pluripotent stem cells (iPSCs) were one the achievements in 2006 by Shinya Yamanaka. In this regard, Iran became the tenth pioneer country in the human embryonic stem cell technologies in 2003. Subsequently, in 2005 the relevant guidelines were issued by Iranian Ministry of Health and Medical Ethics and the History of Medicine Research Center at Tehran University [15].
History of transplantation in Iran
The history of transplantation in Iran goes back to 1935. Subsequently, about 30 years ago (1991) Qavamzadeh launched the first bone marrow transplant department at Shariati Hospital, Tehran. Since then, a lot of researches and projects in the field of SCT have been conducted at other research centers such as EMRI Institute (Fig. 2) [16, 17].
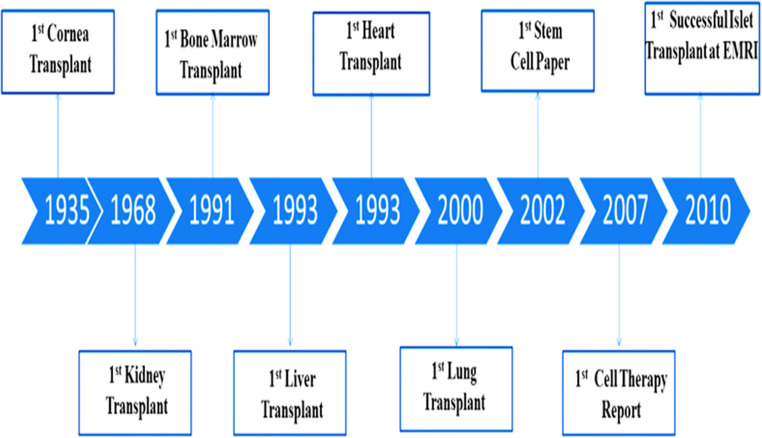
Fig. 2.
History of transplantation in Iran. Due to the limitations of organ transplantation, the application of stem cells as an effective therapeutic target has been studied and the first article in this field was published in 2002. Subsequently, the first report of cell therapy in Iran was made in 2007[18–25]
The first successful clinical islet isolation and transplantation in EMRI
After the establishment of a good manufacturing practices (GMP) facility in 2005, investigations around islet isolation were conducted in the EMRI. Ultimately, the first pancreatic islet isolation and transplantation was successfully performed in 2010 [25]. Indeed, islet transplantation has developed as one of the most promising approaches to achieve insulin independence and ameliorate glycometabolic control in diabetic subjects [26, 27]. However, there are some limitations for islet transplantation including a human pancreas shortage, immunosuppression requirement, and the inefficacy of the islet isolation procedure [28].
Fetal and mesenchymal stem cell transplantation in EMRI
A number of clinical trials have been carried out using liver-derived fetal stem-cells for treatment of diabetes at the EMRI [29]. The first experience demonstrated no significant decrease in the dose of insulin needed for treatment of the patients, a finding that can be attributed to the heterogeneity of the subjects who were consisted of 30 patients with type 1 and 26 with type 2 diabetes [30]. It should be mentioned, however, that in a comprehensive safety study conducted on 44 out of 56 patients three years later, we observed no detectable safety concerns [31]. The only reported possible side effect was a case of meningioma which could not attributed to the procedure in the subsequent in-depth evaluations [32].
In the next fetal stem cell experience with tighter inclusion criteria that included solely patients with type 1 diabetes (72 patients), significant therapeutic response presented through c-peptide changes and insulin-free periods (from 4 to 24 months) was demonstrated in a considerable proportion of patients [33]. Interestingly, later analysis of the recorded data demonstrated remarkable changes in different lymphocyte populations which can be definitely attributable to the procedure [34].
Another clinical trial carried out at the EMRI aimed to assess the safety and efficacy of transplantation of autologous BM-MSC in patients with T1DM (diagnosed through measurement of Anti-GAD antibody). That study, which is elaborated in detail elsewhere,[31] was conducted in two phases. In the phase one, 23 T1DM patients received autologous BM-MSC. Three months later, in the phase two of the trial, 21 patients who still needed exogenous insulin for hyperglycemia received a second dose of autologous BM-MSC. Our results demonstrated that daily insulin requirement was significantly decreased in 39 percent of the subjects, and two patients did not need to inject insulin any longer as a result of the intervention [31].
The first Iranian tissue bank
Iranian Tissue Bank (I.T.B) began operating in 1994 by providing heart valves homograft. The I.T.B complies with the guidelines and standards of the European association of tissue banks (EATB), American association of tissue banks (AATB), and food and drug administration (FDA). Additionally, tissue production processes in this complex are performed in accordance with GMP principles at the cleanroom facilities of Imam Khomeini Hospital in Tehran [35, 36].
Evidences for Iran Stem cell research and therapies
In Iran researches and technologies related to the application of stem cells for disease, have made significant progress by profiting from religious blessings, the efforts of public and private research organizations such as EMRI, and political supports. However, human stem cell investigations are still correlated with some ethical and regulatory concerns. Therefore, many studies also have been carried out to address these concerns [23, 37].
Experimental studies
Given the importance of pre-clinical investigations (as experimental studies) before clinical trials, several pieces of research on the benefits of applying stem cells and their products in the treatment of various diseases including metabolic and neurological diseases in EMRI have been conducted. In this context, in addition to in vitro studies, different animal models have been used [8, 38–45].
The EMRI scientific status in the TUMS and Iran in the field of stem cells and regenerative medicine
EMRI in 2010 has begun cell based therapies with pancreatic islet transplantation [25]. Later, in 2011 fetal liver derived stem cell transplantation in diabetes was its next achievement in mesenchymal stem cell transplantation [46]. Finally, in 2015 cell therapy and regenerative medicine (CTRM) research center was established.
Establishment of the cell therapy and regenerative medicine research center in EMRI
According to importance of cell therapy as a promising method for treatment of incurable diseases [47, 48] CTRM was established to create a suitable platform to perform research and development (R&D) in the field of stem cell therapy.
Cell therapy and regenerative medicine research center; Strategies, initiatives, opportunities
CTRM can develop new products, improve the quality of services and products including GMP facilities [25, 49], and adhere to current national and international standards as well as Good laboratory practices (GLP) and good clinical practice (GCP) principles [47, 48, 50]. Cellular therapies can provide a personalized approach to achieve the best therapeutic results and outcomes [48]. The key strategic plans in this center are including;
Introducing and institutionalizing cell therapy sciences in national and international universities and institutes [50].
Design and implementation of multifaceted and international and fundamental applied research projects in the areas of diabetes, obesity, Alzheimer’s, Parkinson’s, and etc. [41, 48, 49, 51, 52].
Training of dedicated and specialized human resources with the benefit of experienced professors in the fields of cell therapy and regenerative medicine [47].
Empowering a committed and proficient human resources with knowledge of modern laboratory equipment [47].
Providing optimal and appropriate infrastructure of the cell manufacturing facilities and modern, standard laboratory equipment to communicate with the industry [47].
Applying grant from non-university support agencies to fund extensive multidisciplinary studies and produce standard evidence in this regard [47].
Research to standardize existing laboratory protocols and present new laboratory methods [49].
Upgrading and enhancing the knowledge of the specialists of the center and establishing a scientific network on national and international levels [47].
Establishment of general quality management systems (ISO 9001) as well as quality management systems specific to diagnostic and medical laboratories (ISO 15,189-GLP) [25, 47, 49].
Effective collaboration with cell therapy specialists at accredited national and international centers [25].
Contribute to the dissemination, development and enhancement of the quality and quantity of scientific products such as evidences, books and papers [50].
Commercialization of diagnostic kits according to the highest international standards [25].
Developing guidelines related to the field of cell therapy and regenerative medicine and its useful and effective role in health system status [47].
Accelerate the commercialization of research ideas and the production of standard and optimal kits [53].
The importance of establishing policy, ethics, and guidelines
The Milgram experiment on obedience to authority figures and the Tuskegee study of untreated syphilis in the negro male are two of the obnoxious experiments in contemporary history [54]. Since the 1960’s the ethical issues were more propounded and in this regard, some committees and related organizations became responsible to codify the special related rules and laws. There are a lot of national and international guidelines and policies however each country must be considered its own traditional, religious, and cultural background in dealing with controversial ethical issues [55].
National guideline for cell therapy manufacturing in EMRI
Stem cells offer hope for novel treatments although their application in researches and clinics causes controversy. Ethical concerns as one of the important subjects should be taken into account in every research. Proper guidelines are needed to ensure appropriate conduct of research. A committee has prepared a preliminary draft of guideline which was reformed by a group of experts in two-day meeting. In 2014, the final draft of guideline was proffered to the Ministry of Health and Medical education for approval. The main topics of the guideline includes: (1) structure of cell processing centers and cell banks, (2) ethical and legal regal regulations, (3) coding and traceability, (4) cell and tissue recovery and transport, (5) processing and production of cell products, (6) storage and transport, (7) cell transplantation and post transplantation surveillance, (8) quality assurance and quality management system, (9) documentation, and (10) environmental Health and safety. This guideline helps to improve the efficiency and quality of cellular products in clinical researches and therapeutics [56].
Iranian national guideline for trans boundaries transfer of biomedical samples
In recent years, biomedical sciences have made significant progress and also international cooperation is expanded [48, 50]. In this regard, the transfer of biological materials between different countries is essential. Therefore, it is necessary to set a framework to clarify ethical and legal issues and provide specific criteria for researchers, physicians, and supervisory authorities [47]. In Islamic Republic of Iran the national guideline for trans boundaries transfer of biomedical samples was established to facilitate the process of transferring [57]. National Biomedical Research Ethics Committee is an authority which has the responsibility of setting guidelines for biomedical researches in Islamic Republic of Iran [58]. Iranian national guideline for trans boundaries transfer of biomedical samples is one of these guidelines which covers the transfer of all biomedical samples including cellular samples, feta, genetic samples, gametes, embryos, bacterial and viral samples, DNA, RNA, various tissues and organs, blood or their derivatives. In this guideline labelling, coding, traceability, packaging, storage, transport, hygienic, safety methods, waste disposal, and agreement for trans boundaries transfer of biological samples have been discussed [47, 53]. Moreover, transportation of biomedical specimens of human or other living organisms require a safe, timely, legal and ethical system based on principles, scientific and updated standards. Therefore, this guideline is provided based on reliable resources and experts’ guidance [47, 58].
National and international collaborations
As mentioned, the EMRI has made an important contribution to the production of medical sciences specifically in the field of stem cell and medical technologies by using the necessary facilities, infrastructure, and the benefit of experienced personnel and researchers. In this respect, the taken measures have led to the first cell therapy trials in the country. Among them was the establishment of the first pancreatic islet isolation laboratory by the EMRI in collaboration with the University of British Columbia (Canada) in 2007 and the first successful pancreatic islet transplantation (2011) in the country. Moreover, for the first time carried out a cell therapy project with embryonic stem cells in diabetes and a number of incurable diseases in collaboration with international scientists. These experiences lead to the formation of standard workspaces (including cell products production room, cell-molecular laboratory and cell therapy clinic), donation network and provision of legally aborted embryos, production of various cell lines with clinical applicability, attracting and training clinicians and basic sciences in this field, setting up an embryonic cell bank and creating a registry system for patients undergoing cell therapy (https://emri.tums.ac.ir/ctrm/item).
Stem cell therapy challenges in Iran
There are some challenges in performing stem cell therapies in Iran as a developing country, which are as follows [59–61]:
The lack of belief in cell therapy and regenerative medicine as a therapeutic approach because of the low level of information in this area.
Lack of skilled human resources which training of qualified students and researchers has greatly helped to solve this challenge.
Inadequacy of stem cells providing and transplanting facilities.
In recent years, there have been gaps in scientific and ethical guidelines in the field of stem cell therapy, which many of them have been addressed today.
Challenges in keeping pace with the rapid changes in technologies.
Considering the mentioned challenges, providing the conditions to increase public awareness about the importance of stem cell therapeutic application as well as increasing government support in this area can help advance the use of stem cell-based science.
Conclusion and future perspectives
At the EMRI as a pioneering institute in stem cell science, education, research, and clinical activities related to the application of stem cells in different diseases, especially endocrine diseases in some subsets such as clinical endocrinology and metabolism, cellular and molecular, and population sciences are performed. These activities are aiming to preserve the national, regional and international situation of Iran in research and providing clinical services. Additionally, there are ongoing studies in different parts of EMRI such as CTRM to achieve the better stem cells using as well as the development of medical ethics observation in pre-clinical and clinical researches. Further, based on the overall motivation in Iran, it seems that the focus of future stem cells researches will be more on multidisciplinary studies, especially in the treatment of cancer and the cardiovascular system.
Abbreviations
- SCT
Stem Cell Transplantation
- EMRI
Endocrinology and Metabolism Research Institute
- iPSCs
induced Pluripotent Stem Cells
- GMP
Good Manufacturing Practices
- I.T.B
Iranian Tissue Bank
- EATB
European association of tissue banks
- AATB
American association of tissue banks
- FDA
Food and Drug Administration
- EMRC
Endocrinology and Metabolism Research Centre
- TUMS
Tehran University of medical sciences
- CTRM
Cell Therapy and Regenerative Medicine
- R&D
Research and Development
- GLP
Good Laboratory Practices
- GCP
Good Clinical Practice.
Declarations
Conflict of interest
There is no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grinyó JM. Why is organ transplantation clinically important? Cold Spring Harb Perspect Med. 2013;3(6):a014985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saidi R, Kenari SH. Challenges of organ shortage for transplantation: solutions and opportunities. Int J Organ Transplant Med. 2014;5(3):87. [PMC free article] [PubMed] [Google Scholar]
- 3.Girlanda R. Deceased organ donation for transplantation: challenges and opportunities. World J Rransplant. 2016;6(3):451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niessen LW, Squire SB. Universal health coverage and chronic conditions. Lancet Glob Health. 2019;7(10):e1290–e1292. [DOI] [PubMed] [Google Scholar]
- 5.Peek SF, Divers TJ. Chapter 14 - Metabolic diseases. In: Divers TJ, Peek SF, editors. Rebhun’s diseases of dairy cattle. 2nd ed. Saint Louis: W.B. Saunders; 2008. p. 590–605. [Google Scholar]
- 6.Gasparetto C. Stem cell transplantation for multiple myeloma. Cancer Control. 2004;11(2):119–29. [DOI] [PubMed] [Google Scholar]
- 7.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–26. [DOI] [PubMed] [Google Scholar]
- 8.Larijani B, NASLI EE, Amini P, Nikbin B, Alimoghaddam K, Amiri S, et al. Stem cell therapy in treatment of different diseases. Acta Med Iran. 2012;50(2):79-96 [PubMed]
- 9.Goodarzi P, Falahzadeh K, Aghayan H, Payab M, Larijani B, Alavi-Moghadam S, et al. Therapeutic abortion and ectopic pregnancy: alternative sources for fetal stem cell research and therapy in Iran as an Islamic country. Cell Tissue Bank. 2019;20(1):11–24. [DOI] [PubMed] [Google Scholar]
- 10.Rajabzadeh N, Fathi E, Farahzadi R. Stem cell-based regenerative medicine. Stem Cell Investig. 2019;6:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Pham P. Stem Cell Processing. Berlin: Springer; 2016.
- 12.Ramalho-Santos M, Willenbring H. On the origin of the term “stem cell”. Cell Stem Cell. 2007;1(1):35–8. [DOI] [PubMed] [Google Scholar]
- 13.Lagarkova M. Such various stem cells. Biochemistry. 2019;84(3):187–9. [DOI] [PubMed] [Google Scholar]
- 14.Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol. 2014;30:647–75. [DOI] [PubMed] [Google Scholar]
- 15.Aramesh K, Dabbagh S. An Islamic view to stem cell research and cloning: Iran’s experience. Am J Bioeth. 2007;7(2):62–3. [DOI] [PubMed] [Google Scholar]
- 16.Ghavamzadeh A, Alimoghaddam K, Ghaffari F, Derakhshandeh R, Jalali A, Jahani M. Twenty years of experience on stem cell transplantation in Iran. Iran Red Crescent Med J. 2013;15(2):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghavamzadeh A, Alimoghaddam K, Jahani M, Mousavi SA, Iravani M, Bahar B, et al. Stem cell transplantation; Iranian experience. Arch Iran Med. 2009;12(1):69–72. [PubMed] [Google Scholar]
- 18.Broumand B. Transplantation activities in Iran. Exp Clin Transplant. 2005;3(1):333–7. [PubMed] [Google Scholar]
- 19.Ghavamzadeh A, Alimogaddam K, Jahani M, Mousavi S, Iravani M, Bahar B, et al. Hematopoietic stem cell transplantation in Iran: 1991 to 2008. Hematology/Oncol Stem Cell Ther. 2008;1(4):231–8. [DOI] [PubMed] [Google Scholar]
- 20.Malek HS, Salahi H, Lahsai M, Bahador A, Bagheri LK, Fatahi M, et al. The first report of liver transplantation in Iran. Arch Iran Med. 5(4):213-215
- 21.Mandegar MH, Bagheri J, Chitsaz S, Jebelli M, Javidi D, Sarzaeem MR, et al. Heart transplantation in iran; a comprehensive single-center review of 15-year performance. Arch Iran Med. 2009;12(2):111–5. [PubMed] [Google Scholar]
- 22.Abbasi-Dezfouli A, Pojhan S, Behgam-Shadmehr M, Najafizadeh K, Ghorbani F, Lorgard-Dezfuli-Nejad M, et al. The cost of lung transplantation in Iran. Ann Transplant. 2009;14(2):30–3. [PubMed] [Google Scholar]
- 23.Shaiegan M. Stem cell therapy and research status in Iran: at A glance. Iran J Blood Cancer. 2010;2(3):99–100. [Google Scholar]
- 24.Massarrat S. Stem cell therapy in Iran. Arch Iran Med. 2007;10(4):435–8. [PubMed] [Google Scholar]
- 25.Larijani B, Arjmand B, Amoli MM, Ao Z, Jafarian A, Mahdavi-Mazdah M, et al. Establishing a cGMP pancreatic islet processing facility: the first experience in Iran. Cell Tissue Bank. 2012;13(4):569–75. [DOI] [PubMed] [Google Scholar]
- 26.McCall M, Shapiro AJ. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2(7):a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rheinheimer J, Bauer AC, Silveiro SP, Estivalet AA, Bouças AP, Rosa AR, et al. Human pancreatic islet transplantation: an update and description of the establishment of a pancreatic islet isolation laboratory. Arch Endocrinol Metab. 2015;59(2):161–70. [DOI] [PubMed] [Google Scholar]
- 28.Rother KI, Harlan DM. Challenges facing islet transplantation for the treatment of type 1 diabetes mellitus. J Clin Investig. 2004;114(7):877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tootee A, Esfahani EN, Larijani B. Application of fetal stem cells in diabetes: Iran’s experience. Iran J Public Health. 2015;44(Supple 2):1–5. 26060770 [Google Scholar]
- 30.Ghodsi M, Heshmat R, Amoli M, Keshtkar A-A, Arjmand B, Aghayan H, et al. The effect of fetal liver-derived cell suspension allotransplantation on patients with diabetes: first year of follow-up. Acta Med Iran. 2012;50(8):541–6. [PubMed] [Google Scholar]
- 31.Nasli-Esfahani E, Ghodsi M, Amini P, Keshtkar AA, Amiri S, Mojahed-Yazdi N, et al. Evaluation of fetal cell transplantation safety in treatment of diabetes: a three-year follow-up. J Diabetes Metab Disord. 2015;14(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasli-Esfahani E, Ghadami M, Amini P, Amiri S, Ghodsi M, Rambod C, et al. Transitional meningioma after fetal liver-derived cell suspension allotransplant: a case report. Exp Clin Transplant. 2017;15(2):231–4. [DOI] [PubMed] [Google Scholar]
- 33.Tootee A, Esfahani EN, Ghodsi M, Farideh R, Amini M, Larijani B, et al. Application of allotransplantation of fetal liver-derived stem-cells for treatment of type 1 Diabetes: a single-arm, phase 3 clinical trial. Iran J Public Health. 2015;44(2):36–41. 26060774 [Google Scholar]
- 34.Tootee A, Esfahani EN, Ghodsi M, Razi F, Adibi H, Heshmat R, et al. Flowcytometric assessment of lymphocyte subsets in type-1 diabetic patients following allotransplantation of liver-derived fetal stem-cells. Iran J Public Health. 2015;44(2):48. [Google Scholar]
- 35.Rouchi AH, Tavakoli SA, Mahdavi-Mazdeh M. Iranian homograft tissue processing. Glob Cardiol Sci Pract. 2016;2016(1):e201607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dashti-Khavidaki S, Dabardani F, Mahdavi-Mazdeh M, Ravanasa E, Hosseini SK. Efficacy of decontamination protocol by antimicrobial treatment in Iranian Tissue Bank (ITB). Cell Tissue Bank. 2015;16(3):381–8. [DOI] [PubMed] [Google Scholar]
- 37.Miremadi T, Salekdeh GH, Aghdami N, Gharanfoli M, Vasei M, Kouhkan A, et al. Stem cell research and therapy in the Islamic republic of Iran: pioneering in the Islamic world. Stem Cells Dev. 2012;22(1):51–7. [DOI] [PubMed] [Google Scholar]
- 38.Larijani B, Zahedi F, editors. Islamic perspective on human cloning and stem cell research. Transplantation Proceedings; 2004: Elsevier. [DOI] [PubMed]
- 39.Arjmand B, Goodarzi P, Aghayan H, Payab M, Rahim F, Alavi-Moghadam S, et al. Co-transplantation of human fetal mesenchymal and hematopoietic stem cells in type 1 diabetic mice model. Front Endocrinol. 2019;10:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goodarzi P, Larijani B, Alavi-Moghadam S, Tayanloo-Beik A, Mohamadi-Jahani F, Ranjbaran N, et al. Mesenchymal stem cells-derived exosomes for wound regeneration. Cell biology and translational medicine, vol 4: Berlin: Springer; 2018. p. 119 – 31. [DOI] [PubMed]
- 41.Goodarzi P, Aghayan HR, Larijani B, Soleimani M, Dehpour A-R, Sahebjam M, et al. Stem cell-based approach for the treatment of Parkinson’s disease. Med J Islam Repub Iran. 2015;29:168. [PMC free article] [PubMed] [Google Scholar]
- 42.Goodarzi P, Aghayan HR, Soleimani M, Norouzi-Javidan A, Mohamadi-Jahani F, Jahangiri S, et al. Stem cell therapy for treatment of epilepsy. Acta Medica Iranica. 2014:651–5. [PubMed]
- 43.Payab M, Goodarzi P, Heravani NF, Hadavandkhani M, Zarei Z, Falahzadeh K, et al. Stem cell and obesity: Current state and future perspective. Cell biology and translational medicine, vol 2: Berlin: Springer; 2018. p. 1–22. [DOI] [PubMed]
- 44.Gilany K, Payab M, Goodarzi P, Tayanloo-Beik A, Alavi-Moghadam S, Mousavi M, et al. Lipidomics of adipogenic differentiation of mesenchymal stem cells. Genomics: Springer; 2019. p. 123–40. [Google Scholar]
- 45.Goodarzi P, Payab M, Alavi-Moghadam S, Larijani B, Rahim F, Bana N, et al. Development and validation of Alzheimer’s disease animal model for the purpose of regenerative medicine. Cell Tissue Bank. 2019;20(2):141–51. [DOI] [PubMed] [Google Scholar]
- 46.Larijani B, Arjmand B, Ahmadbeigi N, Falahzadeh K, Soleimani M, Sayahpour FA, et al. A simple and cost-effective method for isolation and expansion of human fetal pancreas derived mesenchymal stem cells. Arch Iran Med. 2015;18(11):770–5. [PubMed] [Google Scholar]
- 47.Aghayan HR, Arjmand B, Ahmadbeigi N, Gheisari Y, Vasei M. Draft of Iranian national guideline for cell therapy manufacturing. Arch Iran Med. 2017;20(8):547–50. [PubMed] [Google Scholar]
- 48.Arjmand B, Goodarzi P, Mohamadi-Jahani F, Falahzadeh K, Larijani B. Personalized regenerative medicine. Acta Med Iran. 2017;55(3):144–9. [PubMed] [Google Scholar]
- 49.Larijani B, Aghayan HR, Goodarzi P, Arjmand B. GMP-grade human fetal liver-derived mesenchymal stem cells for clinical transplantation. Methods Mol Biol (Clifton, NJ). 2015;1283:123–36. [DOI] [PubMed] [Google Scholar]
- 50.Arjmand B, Larijani B. Personalized medicine: a new era in endocrinology. Acta Med Iran. 2017;55(3):142–3. [PubMed] [Google Scholar]
- 51.Arjmand B, Goodarzi P, Aghayan HR, Payab M, Rahim F, Alavi-Moghadam S, et al. Co-transplantation of human fetal mesenchymal and hematopoietic stem cells in type 1 diabetic mice model. Front Endocrinol (Lausanne). 2019;10:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Payab M, Goodarzi P, Foroughi Heravani N, Hadavandkhani M, Zarei Z, Falahzadeh K, et al. Stem cell and obesity: current state and future perspective. Adv Exp Med Biol. 2018;1089:1–22. [DOI] [PubMed] [Google Scholar]
- 53.Aghayan HR, Mahdavi-Mazdeh M, Goodarzi P, Arjmand B, Emami-Razavi SH. Coding and traceability in Iran. Cell Tissue Bank. 2010;11(4):397–400. [DOI] [PubMed] [Google Scholar]
- 54.Milgram S, Gudehus C. Obedience to authority. New Yor: Ziff-Davis Publishing Company; 1978.
- 55.Wheeler MA, McGrath MJ, Haslam N. Twentieth century morality: The rise and fall of moral concepts from 1900 to 2007. PloS one. 2019;14(2):e0212267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aghayan HR, Arjmand B, Ahmadbeigi N, Gheisari Y, Vasei M. Draft of Iranian national guideline for cell therapy manufacturing. Arch Iran Med. 2017;20(8):547–50. [PubMed] [Google Scholar]
- 57.Zhang X, Matsui K, Krohmal B, Zeid AA, Muthuswamy V, Koo YM, et al. Attitudes towards transfers of human tissue samples across borders: an international survey of researchers and policy makers in five countries. BMC Med Ethics. 2010;11(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Larijani B, Zahedi F, Malek Afzali H. Medical ethics in the Islamic Republic of Iran. East Mediterr Health J. 2005;11(5–6):1061–1072. [PubMed]
- 59.Sah J. Challenges of stem cell therapy in developing country. J Stem Cell Res Ther. 2016;1(3):1–3.33409004 [Google Scholar]
- 60.Ikehara S. Grand challenges in stem cell treatments. Front Cell Dev Biol. 2013;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trounson A, McDonald C. Stem cell therapies in clinical trials: progress and challenges. Cell Stem Cell. 2015;17(1):11–22. [DOI] [PubMed] [Google Scholar]