Abstract
Objectives
Digital infrared thermography is a noninvasive tool used for assessing diseases, including the diabetic foot. This study aims to analyze thermal patterns of the foot sole in patients with type 2 diabetes mellitus using thermography and explore correlations with clinical variables. Additionally, a machine learning approach was developed for classification.
Methods
A total of 23 diabetic patients and 27 age- and sex-matched controls were included. Thermograms of the plantar foot surface were acquired and segmented into regions of interest. Mean foot temperature and temperature change index were calculated from predefined regions of interest. Pearson’s correlation analysis was conducted for temperature measures, glycated hemoglobin, and body mass index. A two-layered cross-validation model using principal component analysis and support vector machines were employed for classification.
Results
Significant positive correlations were found between mean foot temperature and glycated hemoglobin (ρ = 0.44, p = 0.0015), as well as between mean foot temperature and body mass index (ρ = 0.35, p = 0.013). Temperature change index did not show significant correlations with clinical variables. The machine learning model achieved high overall accuracy (90%) and specificity (100%) with a moderate sensitivity (78.3%) for classifying diabetic and control groups based on thermal data.
Conclusions
Thermography combined with machine learning shows potential for assessing diabetic foot complications. Correlations between mean foot temperature and clinical variables suggest foot temperature changes as potential indicators. The machine learning model demonstrates promising accuracy for classification, suitable for screening purposes. Further research is needed to understand underlying mechanisms and establish clinical utility in diagnosing and managing diabetic foot complications.
Keywords: Digital infrared thermography, Diabetes mellitus, Diabetic foot, Machine learning, Principal component analysis, Support vector machine
Introduction
Digital infrared thermography is a noninvasive and fast imaging tool that provides useful information for the diagnosis of several diseases [1]. In particular, it has been applied in the study of the diabetic foot, a growing healthcare burden, and its assessment is challenging [2]. Diabetic foot is a complication of diabetes mellitus that can lead to severe consequences such as foot ulcers and amputations [3, 4]. Foot ulcerations affect approximately 2–4% of patients with type 2 diabetes mellitus (T2DM) [5]. Risk factors for foot lesions include peripheral and autonomic neuropathy, vascular disease, previous foot ulceration, and other microvascular complications such as retinopathy and end-stage renal disease [5]. Infrared thermography can provide a quantitative estimation of temperature variations in plantar angiosomes, which is important in analyzing the diabetic foot since it could bring knowledge regarding ulceration [6]. In addition, several studies have suggested that infrared thermography is a reliable method for determining foot skin temperature and investigating the relationship between foot skin temperature and blood flow in type 2 diabetes mellitus patients [2, 7]. Diagnosis of foot infection in patients with diabetes seems valid and reliable using photographic imaging in combination with infrared thermography [8, 9].
Additionally, infrared thermography has been used to detect inflammation in the acute diabetic foot and the foot in remission [10]. Furthermore, infrared thermography has been used to evaluate the treatment effect of percutaneous transluminal angioplasty in patients with peripheral arterial disease [11]. Therefore, digital infrared thermography in diagnosing diabetic foot complications has been suggested to be a complementary tool in diagnosing peripheral nerve disorders [12]. In a recent study that used a sock-based remote temperature monitoring device, temperature data was evaluated for patients who presented with a diabetic foot injury [13]. This study highlights the importance of monitoring foot sole temperature in diabetic patients to identify potential injuries or complications. Another reason to study temperature patterns in the foot sole of diabetic patients is to determine the distribution of load on the foot sole [14]. This distribution can be affected by factors such as the type of sole of the foot and the effect of standing posture [14]. Monitoring adherence to offloading treatment using temperature-based sensors can be a feasible and relevant method with a wide range of possible research and patient care applications [15].
Taken together, digital infrared thermography is a useful and important tool in diagnosing diabetic foot, providing noninvasive and quantitative information that can help in the analysis of temperature variations, the detection of foot infections, and the evaluation of treatment effects, providing valuable insights for healthcare providers to prevent complications and improve patient outcomes.
Despite the promising applications of digital infrared thermography in diagnosing and managing diabetic foot complications, several gaps in the current literature necessitate further investigation. Many existing studies focus primarily on the correlation between foot temperature and diabetic neuropathy or vascular disorders, but they often do not consider the integration of advanced machine learning techniques to enhance diagnostic accuracy [16]. Additionally, while some research has explored the relationship between foot skin temperature and blood flow in diabetic patients, there is a lack of comprehensive analyses that combine thermographic data with clinical variables such as glycated hemoglobin and body mass index [7].
Moreover, previous studies often do not employ robust classification models to distinguish between diabetic and non-diabetic individuals based on thermal patterns. While some research has utilized machine learning algorithms, the performance metrics of these models are not always clearly reported or optimized, leading to potential data leaking, overfitting, and unreliable results [17].
Therefore, our study aims to fill these gaps by analyzing the thermal patterns of the foot sole in patients with type 2 diabetes mellitus, integrating clinical variables, and employing a rigorous machine learning approach to enhance classification accuracy. By addressing these deficiencies, our research seeks to provide a more comprehensive understanding of the potential of digital infrared thermography as a screening tool for diabetic foot status and to establish its clinical utility in diagnosing and managing diabetic foot complications.
Materials and methods
Study population and data acquisition
This study included a T2DM group of adults previously diagnosed by their physicians and a control group without diabetes mellitus. The T2DM group was diagnosed using standard methods such as fasting plasma glucose and glycated hemoglobin (HbA1C) levels. Both groups consisted of 50 volunteers of both sexes, aged between 40 and 65 years (Table 1). The exclusion criteria for both groups included amputations in their feet, current or previous ulcers, foot infections and fractures, Charcot arthropathy, surgery of lower limbs, and severe peripheral vascular disease. Study participants underwent blood extraction (5 mL) to determine HbA1C levels [18].
Table 1.
Demographic information from the study population (mean ± standard deviation)
| Control Group | T2DM Group | p-value | |
|---|---|---|---|
| Participants | 27 | 23 | - |
| Sex (male/female) | (10,17) | (9,14) | 0.8791† |
| Age | 50 (± 6.7) | 53 (± 6.4) | 0.0664* |
| BMI | 26.3 (± 3.6) | 30. 8(± 6.5) | 0.0185 * |
| HbA1C | 5.7 (± 0.34 | 8.2 (± 1.7) | 5.3e-6 * |
†Chi-square p-value; *two-sample t-test p-value; numbers printed in bold are statistically significant.
The preparation for image acquisition was conducted in a room with a temperature of 20 °C. Participants were asked to remove their shoes and socks and rest in a supine position for 15 min to reach a state of thermodynamic equilibrium [19, 20]. In order to receive only the heat radiation coming from the feet, a curtain was placed over the ankles, blocking the radiation from the rest of the body. Recommendations from the Thermography Guidelines of the International Academy of Clinical Thermology were followed to obtain accurate thermograms [21, 22].
Images were acquired using a FLIR One Pro® infrared camera (Teledyne FLIR LLC, Wilsonville, OR, USA). The emissivity settings in the thermographic camera were set to 0.98, which is the emissivity of human skin [2, 21, 23, 24].
Data preprocessing
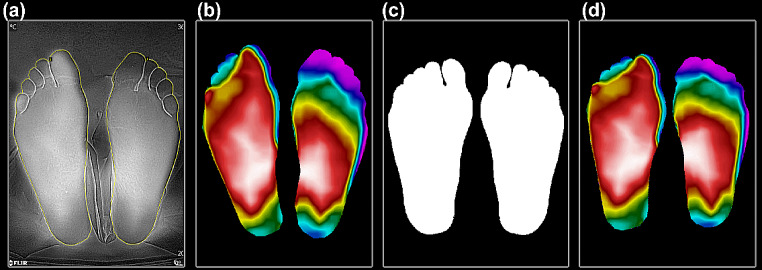
Each thermogram obtained for both groups was segmented and labeled to their respective group with the free software VGG image annotator (VIA) from the Visual Geometry Group of the Department of Engineering Science, University of Oxford [25]. Once the thermogram was manually segmented, a register of the foot shape was performed through lineal geometrical transformation by pairs, using a general template of the feet to get a similar shape for all the thermograms [26] (Fig. 1).
Fig. 1.
Manual segmentation and thermogram register. (a) The thermogram is segmented manually with VIA; (b) Previous segmentation is used for masking the original thermogram and getting the background removed; (c) Feet shape is used as a general template; (d) Registration method is applied to transform the thermogram’s shape
Median Foot Sole temperature
In order to get an initial overview, as well as an initial measurement, it is proposed to use the mean of all temperature values contained within the sole of the foot, which will be denoted as Median Foot Sole (MFS), making use of the Equation (1):
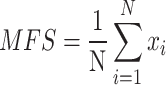 |
1 |
Where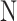 represents the total number of temperatures values in foot sole.
represents the total number of temperatures values in foot sole.
Median foot temperature
Measuring the temperature differences in the soles of the feet allows for detecting possible thermal asymmetries between both feet. Calculating the mean foot temperature (MFT) provides a quantitative measure for comparing regions of interest (ROI) and determining if there is an asymmetry between both feet of the same patient [27]. Different ROIs are used to calculate MFT [28–30]; for this study, we selected the hallux, 3rd and 5th toes, 1st,3rd and 5th metatarsal heads, medial arch, lateral arch and heel, as depicted in Fig. 4b. The MFT is calculated using the following Equation (2):
 |
2 |
Fig. 4.
Thermography results: (a) Mean temperature of both groups; (b) ROIs used in this study; (c) Angiosomes of the control group used to compute TCI and (d) Angiosomes of the T2DM group
Where 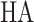 represents the hallux,
represents the hallux,  and
and 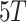 represent the present 3rd and 5th toes,
represent the present 3rd and 5th toes,  ,
, 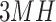 , and
, and  represents 1st, 3rd and 5th metatarsal heads,
represents 1st, 3rd and 5th metatarsal heads, 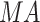 medial arch,
medial arch,  lateral arch, and
lateral arch, and  represents the heel. The selection of these ROIs is based on the Semmes-Weinstein monofilament examination of plantar areas for predicting the prognosis of diabetic foot patients [31].
represents the heel. The selection of these ROIs is based on the Semmes-Weinstein monofilament examination of plantar areas for predicting the prognosis of diabetic foot patients [31].
Temperature Change Index
A comparison of temperature differences between plantar regions could be useful in identifying signs of diabetic ulceration or inflammation. Measuring a regional division of plantar foot based on the concept of angiosomes can provide a better analysis. An angiosome is defined as a three-dimensional block of tissue supplied by a specific artery and its branches, along with the accompanying veins and lymphatics. It is considered that the foot has five angiosomes: the medial plantar angiosome (MPA), primarily supplied by the medial plantar artery and encompassing the medial aspect of the foot, including the great toe; the lateral plantar angiosome (LPA) supplied by the lateral plantar artery on the lateral aspect of the foot, including the great toe and adjacent toes; the medial calcaneal angiosome (MCA), which supplies blood to the medial aspect of the heel; the lateral calcaneal angiosome (LCA), irrigated by the posterior tibial artery that supplies flow to the lateral aspect the heel; and the dorsalis pedis angiosome, supplied by the dorsalis pedis artery and covering the dorsum of the foot toward the toes [32]. For the present study, only the four plantar angiosomes were considered. Temperature Change Index (TCI) was calculated using the Equation (3):
 |
3 |
Where  and
and 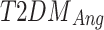 represent the temperature values of the angiosome for the control and participants with Type 2 diabetes mellitus, respectively. The four angiosomes segmented from the thermograms include the MPA, LPA, MCA, and LCA [33], and are depicted in panels (c) and (d) of Fig. 3. These angiosomes provide valuable information about the damage caused by DM in specific arteries and the associated ischemia in the tissue territory irrigated by it since they are used to compute the local temperature distribution. To explore the correlation between thermographic data and reference HbA1c values, the Pearson correlation coefficient was computed between the TCI and HbA1c values and between the MFT and HbA1c values. Also, the Pearson correlation coefficient was computed between the MFT and BMI and between the TCI and BMI. The conventional threshold of p-value < 0.05 was considered statistically significant.
represent the temperature values of the angiosome for the control and participants with Type 2 diabetes mellitus, respectively. The four angiosomes segmented from the thermograms include the MPA, LPA, MCA, and LCA [33], and are depicted in panels (c) and (d) of Fig. 3. These angiosomes provide valuable information about the damage caused by DM in specific arteries and the associated ischemia in the tissue territory irrigated by it since they are used to compute the local temperature distribution. To explore the correlation between thermographic data and reference HbA1c values, the Pearson correlation coefficient was computed between the TCI and HbA1c values and between the MFT and HbA1c values. Also, the Pearson correlation coefficient was computed between the MFT and BMI and between the TCI and BMI. The conventional threshold of p-value < 0.05 was considered statistically significant.
Fig. 3.
Box plot representation and Wilcoxon test results from the age-matched control and diabetic groups: (a) No significant difference in age was found between the two groups; (b) A significant difference in BMI was found between the two groups; (c) A significant difference in HbA1c was found between the two groups
Classification model and validation
Nested cross-validation model
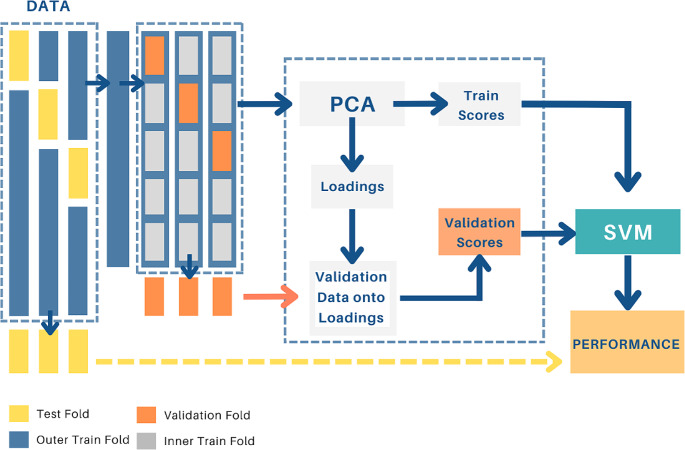
The main approach to tackle the collinearity of measurements is principal component analysis (PCA), a statistical technique that reduces data dimensionality by transforming the original variables into uncorrelated variables known as principal components [34]. PCA does not require any prior information from the data and is based on the decomposition of the covariance matrix of the training data. Temperature data from the ROIs depicted in Fig. 4b and the temperature difference from each ROI and its contralateral homolog were chosen as the variables to reduce with PCA. The first few principal components explain the highest variance in the data. Combined with PCA, we used support vector machines (SVMs) for classification. SVMs are binary classifiers that find a linear decision boundary between samples [35]. If the classes are not linearly separable, a kernel function is used to transform the data into a space where linear classification can be applied [36]. This study used the radial basis function (RBF) kernel. The SVM model is trained on samples from each class, with a positive class labeled + 1 and a negative class labeled − 1. The hyperparameters of the SVM, such as the weight, bias, and kernel width, are optimized using a Bayesian optimization process [37]. The optimization aims to maximize the expected improvement in the classifier performance. To avoid overfitting and overestimation of the classifier performance, a dual-layer cross-validation scheme was implemented [38, 39]. The data was divided into multiple folds, with one as the test set and the remaining for training and validation. Principal component analysis was applied to the training data, and the validation data was projected onto the loadings obtained from the training data, thus ensuring that the training data did not pollute the validation set. This process was then repeated for different numbers of principal components, and the model’s performance was assessed using accuracy measures on the test set, as depicted in Fig. 2.
Fig. 2.
Flow diagram of the two-layer cross-validation PCA-SVM model
Performance Metrics
A true negative (TN) was defined as a sample from the control group that was correctly labeled, a true positive (TP) as a sample from the T2DM class that was correctly classified as positive, a false negative (FN) as a sample from the T2DM group that was incorrectly labeled as control and a false positive (FP) is a sample from the control group incorrectly labeled as T2DM. These Equations (4–9) determine all the performance metrics described previously.
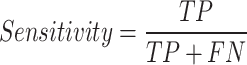 |
4 |
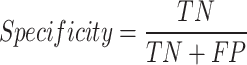 |
5 |
 |
6 |
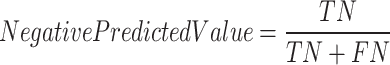 |
7 |
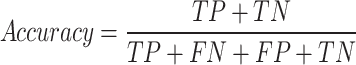 |
8 |
 |
9 |
Using the bootstrap resampling method, 95% confidence intervals were calculated, and averages of the ROC curves were taken over 500 times [41]. Using the entirely independent test dataset, all metrics reported in this study were calculated over the layered cross-validation procedure, as schematized in Fig. 2.
Results
Clinical evaluation
Figure 3 summarizes the clinical and demographic characteristics of the studied groups. No significant differences were found in age. However, the body mass index (BMI) and glycated hemoglobin (HbA1C) of T2DM patients were significantly higher than that of control participants, as expected and shown by previous studies [42, 43].
Thermal pattern
The mean plantar temperature of the control group was 28.3 °C ± 3.3, while the T2DM group presented a mean temperature of 30.5 °C ± 3.6. The spatial distribution of the mean temperatures is displayed in Fig. 4(a).
Correlation analysis
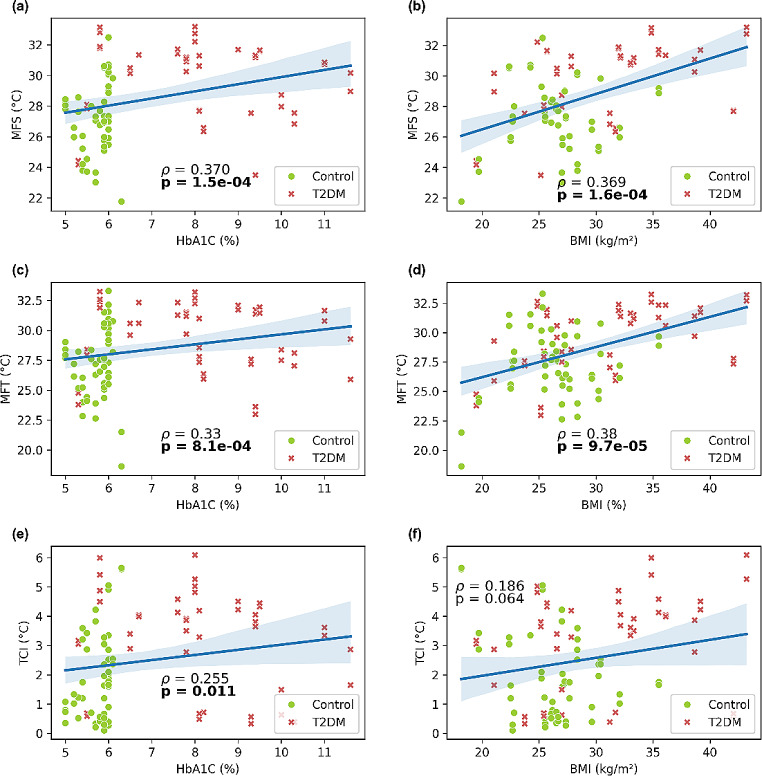
Our findings suggest significant correlations between various temperature measures using thermography and different clinical variables, as shown in Fig. 5. The correlation between MFS and BhA1c is positive, moderate (ρ = 0.37), and significant (p = 0.00015), as well as the correlation between MFS and BMI, which is also positive (ρ = 0.369) and significant (p = 0.00016). Correlation between MFT and BMI is positive (ρ = 0.38) and significant (p = 0.000097), whereas correlation between MFT and HbA1c is also positive but weaker (ρ = 0.33), albeit significant (p = 0.00081). TCI show a very weak positive correlation with HbA1c (ρ = 0.255), but significant (p = 0.011), and no correlation with BMI because of statistical significance (p = 0.064).
Fig. 5.
Correlation analysis. (a) Mean foot sole temperature and glycated hemoglobin; (b) Mean foot sole temperature and body mass index; (c) Mean foot temperature and glycated hemoglobin; (d) Mean foot temperature and body mass index; (e) Temperature change index and glycated hemoglobin; and (f) Temperature change index and body mass index
Two-layered cross-validation
Figure 6(a) shows the confusion matrix of the nested cross-validation applied to the thermography dataset using 5 PCs. In this case, the model did not make mistakes for the control group, correctly labeling all 27 samples from this group. In the T2DM group, 18 of 23 samples were correctly labeled, while 5 were mislabeled as controls. Figure 6(b) depicts the ROC curve analysis. The black line represents the ROC curve with an AUC value of 0.92 (0.77–0.98) and an optimal point of sensitivity = 0.88 and specificity = 0.90; its 95% confidence intervals are represented by gray shading.
Fig. 6.

Performance of the PCA-SVM classifier: (a) Utilizing 5 PCs, a two-layer cross-validation was applied to the dataset. Every row of the confusion matrix shows the occurrences of the predicted label, whereas every column of the matrix shows the occurrences of a real label; (b) ROC curve analysis for the proposed model
Discussion
There is a renewed interest in using infrared thermography to diagnose peripheral neuropathy and vascular disorders in diabetic subjects due to the technique’s ability to detect subtle changes in skin temperature distribution in diabetic-at-risk feet, which makes early detection possible [16].
Here, we demonstrate that when paired with a machine-learning classificator, digital infrared thermography can be used with high precision to predict the diabetic status of an individual. These differences in the mean foot sole temperature between T2DM patients and the control group are in line with a previous study that found that the average difference in temperature between the feet of people with diabetes mellitus and those without diabetes is between 3.39 °C and 4.27 °C [44]. Figure 4 clearly shows this difference between subject groups in our study. The proposed reason for this difference is that normal individuals are capable of thermoregulating the temperature in their feet, meaning that their physiological processes to maintain or dissipate heat are performed correctly, as opposed to diabetic subjects with varying degrees of neuropathy [19]. Furthermore, the positive and significant correlations between MFS and MFT with HbA1C or BMI found in our study, suggest that changes in foot temperature may be an important early indicator of diabetic foot complications.
Elevated HbA1c levels are one of the main ways to identify diabetes, and it has been shown that increased glucose in the body can affect skin temperature and healing [45]. However, although our results in correlating foot temperatures metrics with HbA1c were positive and significant, the correlation values were not as high as expected, probably because once diabetes is established, the physiological processes of thermoregulation of the diabetic foot skin is damaged and not reversible. If we consider that HbA1c only reflects glycemia over two to three months ago, it is very likely that vascular damage is already present, regardless of whether the diabetic already has controlled blood glucose levels. Additionally, we did not consider the glycaemic control in our study, so, there is a chance that some individuals may have had low Hb1Ac levels at the moment of the thermographic measurements, without it reflecting their past medical history.
A recent study by Yeung et al. [46] found that elevated HbA1c levels likely cause coronary artery disease, highlighting the critical role of HbA1c as a biomarker for both diabetes management and cardiovascular risk assessment. Our study extends this understanding by demonstrating significant correlations between foot temperature metrics and HbA1c levels, suggesting that thermography could serve as a non-invasive screening tool for diabetic complications, thus emphasizing the importance of comprehensive monitoring of HbA1c in various clinical applications.
Results obtained in correlations of temperature metrics with BMI, denote that overweight also affects the physiological processes to thermoregulate the soles of their feet. For example, peripheral effectors, such as vasoconstrictors, vasodilators and sweat glands, could be altered by this condition. Additionally, hypothalamic changes and excess of adipose tissue in the foot sole, which isolates thermally the human body, can explain these differences [47]. Yazdanpanah et al. [48]. explored the prevalence of diabetic foot ulcers and associated risk factors, including BMI. They found that a higher BMI significantly increases the risk of developing diabetic foot ulcers, demonstrating the critical role of obesity in exacerbating diabetic foot complications. Our research builds on these findings by employing thermographic imaging and machine learning to further elucidate the relationship between BMI and foot health in diabetic patients, emphasizing the potential for innovative diagnostic approaches.
The results obtained with the PCA-SVM algorithm suggests that the model has a high level of accuracy in classifying the control and T2DM groups using thermography data. While the model has high specificity, it has only moderate sensitivity. The high accuracy (90%) and specificity (100%) indicate that the model is very good at identifying true negatives. This is ideal for screening, where one wants to minimize false positives while still detecting as many true positives as possible.
Overall, these findings are consistent with the existing literature on diabetic foot and suggest that thermography and machine learning can be useful tools for assessing foot temperature in patients with diabetes, particularly in resource constrained and remote settings, when paired with smart-phone telemedicine adjuncts [49].
While the current study does have certain limitations, particularly in relation to the size of the populations examined, the tests conducted on the correlations have nonetheless demonstrated a high level of statistical significance. Due to the nature of the study, efforts were directed at finding temperature patterns in the sole of the foot, but other measures could have improved the results in the study. For instance, ankle brachial index ratio, can provide us with more conclusive information to determine peripheral artery disease and its relationship to blood flow to the foot. Furthermore, a study on resting blood pressure or heart rate would confirm the normal thermal status of people with a healthy hemodynamic stability. Finally, the physical activity levels of the groups and their direct effect on limb circulatory health may be a confounding factor.
Conclusions
Our results suggest that accurate detection of diabetic people may help identify those subjects likely to develop diabetic foot ulcer and accelerate the use of preventive treatments. Further research is needed to explore the underlying mechanisms behind the temperature patterns and to determine the clinical utility of thermography in the diagnosis and management of diabetic foot complications.
Acknowledgements
We would like to thank all the volunteers that participated in the study and made it possible. R. Castillo-Morquecho acknowledges financial support from Mexican National Council of Humanities, Science and Technology.
(CONAHCYT) through national scholarship program (CVU 662217).
Author contributions
Conceptualization, E.S. K-M and M.A. M.J.; methodology, M.G. M-R; software, E.G. and R. C-M; validation, J.L. R-GL, M.A. M-J and E.S. K-M; formal analysis, E.G. and R. C.M.; investigation, X.X.; resources, M.A. M-J, M.G. M-R and E.S. K-M; data curation, R. C-M and E.G.; writing—original draft preparation, R. C-M and E.G.; writing—review and editing, J.L. R-GL and E.S. K-M; visualization, E.G.; supervision, E.S. K-M; project administration, E.S. K-M; funding acquisition, E.S. K-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported in part [E.G.] by the “Cátedras CONACYT” program, project 528.
Data availability
The data that support the findings of this study are available from the corresponding author, [E.S.K-M], upon reasonable request.
Declarations
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the local Ethics Committee (registration number R-2019-2402-043).
Informed consent
Informed consent was obtained from all subjects involved in the study.
Conflict of interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ramirez-GarciaLuna JL, Bartlett R, Arriaga-Caballero JE, Fraser RDJ, Saiko G. Infrared thermography in Wound Care, surgery, and Sports Medicine: a review. Front Physiol. 2022;13:838528. 10.3389/fphys.2022.838528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilo A, Romsi P, Mäkelä J. Infrared thermography and vascular disorders in Diabetic feet. J Diabetes Sci Technol. 2020;14:28–36. 10.1177/1932296819871270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hobizal KB, Wukich DK. Diabetic foot infections: current concept review. Diabet Foot Ankle. 2012;3:18409. 10.3402/dfa.v3i0.18409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Amri AM, Shahrani IM, Almaker YA, Alshehri DM, Argabi MA, Alghamidi FA, Alqahtani YZ. (2021) Knowledge, attitude and practice regarding risk of Diabetic Foot among Diabetic patients in Aseer Region, Saudi Arabia. Cureus. 10.7759/cureus.18791. [DOI] [PMC free article] [PubMed]
- 5.Bowling FL, Rashid ST, Boulton AJM. Preventing and treating foot complications associated with diabetes mellitus. Nat Rev Endocrinol. 2015;11:606–16. 10.1038/nrendo.2015.130. [DOI] [PubMed] [Google Scholar]
- 6.Peregrina-Barreto H, Morales-Hernandez LA, Rangel-Magdaleno JJ, Avina-Cervantes JG, Ramirez-Cortes JM, Morales-Caporal R. Quantitative estimation of temperature variations in Plantar angiosomes: a Study Case for Diabetic Foot. Comput Math Methods Med. 2014;2014:1–10. 10.1155/2014/585306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatchawan U, Narkto P, Damri T, Yamauchi J. An exploration of the relationship between foot skin temperature and blood flow in type 2 diabetes mellitus patients: a cross-sectional study. J Phys Therapy Sci. 2018;30:1359–63. 10.1589/jpts.30.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hazenberg CEVB, Van Netten JJ, Van Baal SG, Bus SA. Assessment of signs of Foot infection in diabetes patients using photographic Foot Imaging and Infrared Thermography. Diabetes Technol Ther. 2014;16:370–7. 10.1089/dia.2013.0251. [DOI] [PubMed] [Google Scholar]
- 9.Ramirez-GarciaLuna JL, Martinez-Jimenez MA, Fraser RDJ, Bartlett R, Lorincz A, Liu Z, Saiko G, Berry GK. Is my wound infected? A study on the use of hyperspectral imaging to assess wound infection. Front Med. 2023;10:1165281. 10.3389/fmed.2023.1165281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bharara M, Schoess J, Armstrong DG. Coming events cast their shadows before: detecting inflammation in the acute diabetic foot and the foot in remission. Diabetes Metab Res Rev. 2012;28:15–20. 10.1002/dmrr.2231. [DOI] [PubMed] [Google Scholar]
- 11.Staffa E, Bernard V, Kubicek L, Vlachovsky R, Vlk D, Mornstein V, Bourek A, Staffa R. Infrared thermography as option for evaluating the treatment effect of percutaneous transluminal angioplasty by patients with peripheral arterial disease. Vascular. 2017;25:42–9. 10.1177/1708538116640444. [DOI] [PubMed] [Google Scholar]
- 12.Ali SS, Khan AY, Michael SG, Tankha P, Tokuno H. (2019) Use of Digital Infrared Thermal Imaging in the Electromyography Clinic: a Case Series. Cureus. 10.7759/cureus.4087. [DOI] [PMC free article] [PubMed]
- 13.Reyzelman AM, Shih C-D, Tovmassian G, Nathan M, Ma R, Scholten HJ, Malhotra K, Armstrong DG. An evaluation of real-world Smart sock–based temperature Monitoring Data as a physiological Indicator of Early Diabetic Foot Injury: case-control study. JMIR Form Res. 2022;6:e31870. 10.2196/31870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khandakar A, Mahmud S, Chowdhury MEH, Reaz MBI, Kiranyaz S, Mahbub ZB, Ali SH, Bakar AAA, Ayari MA, Alhatou M, Abdul-Moniem M, Faisal MAA. Design and implementation of a Smart Insole System to measure Plantar pressure and temperature. Sensors. 2022;22:7599. 10.3390/s22197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bus SA. Innovations in plantar pressure and foot temperature measurements in diabetes. Diabetes Metab Res Rev. 2016;32:221–6. 10.1002/dmrr.2760. [DOI] [PubMed] [Google Scholar]
- 16.Lahiri BB, Bagavathiappan S, Raj B, Philip J. Infrared thermography for detection of Diabetic Neuropathy and vascular disorder. In: Ng EY, Etehadtavakol M, editors. Application of Infrared to Biomedical sciences. Singapore: Springer Singapore; 2017. pp. 217–47. 10.1007/978-981-10-3147-2_13. [Google Scholar]
- 17.Hernandez-Contreras D, Peregrina-Barreto H, Rangel-Magdaleno J, Ramirez-Cortes J, Renero-Carrillo F. Automatic classification of thermal patterns in diabetic foot based on morphological pattern spectrum. Infrared Phys Technol. 2015;73:149–57. 10.1016/j.infrared.2015.09.022. [Google Scholar]
- 18.Todd C, Salvetti P, Naylor K, Albatat M. Towards non-invasive extraction and determination of blood glucose levels. Bioengineering. 2017;4:82. 10.3390/bioengineering4040082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagavathiappan S, Philip J, Jayakumar T, Raj B, Rao PNS, Varalakshmi M, Mohan V. Correlation between Plantar Foot temperature and Diabetic Neuropathy: a case study by using an infrared thermal imaging technique. J Diabetes Sci Technol. 2010;4:1386–92. 10.1177/193229681000400613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kazune S, Vasiljevs E, Caica-Rinca A, Marcinkevics Z, Grabovskis A. Infrared thermography imaging for Assessment of Peripheral Perfusion in patients with septic shock. Bioengineering. 2023;10:729. 10.3390/bioengineering10060729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.(2002) Standards and protocols in Clinical Thermographic Imaging. International Academy of Clinical Thermology Quality Assurance Guidelines, Foster City, CA.
- 22.Verstockt J, Verspeek S, Thiessen F, Tjalma WA, Brochez L, Steenackers G. Skin Cancer detection using Infrared Thermography: Measurement Setup, Procedure and Equipment. Sensors. 2022;22:3327. 10.3390/s22093327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khandakar A, Chowdhury MEH, Reaz MBI, Ali SHM, Abbas TO, Alam T, Ayari MA, Mahbub ZB, Habib R, Rahman T, Tahir AM, Bakar AAA, Malik RA. Thermal Change Index-Based Diabetic Foot Thermogram Image classification using machine learning techniques. Sensors. 2022;22:1793. 10.3390/s22051793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor GI, Palmer JH. Angiosome theory. Br J Plast Surg. 1992;45:327–8. 10.1016/0007-1226(92)90063-4. [DOI] [PubMed] [Google Scholar]
- 25.Dutta A, Zisserman A. (2019) The VIA Annotation Software for Images, Audio and Video. In: Proceedings of the 27th ACM International Conference on Multimedia. pp 2276–2279.
- 26.Costa T, Coelho L, Silva MF. Automatic segmentation of Monofilament Testing sites in Plantar images for Diabetic Foot Management. Bioengineering. 2022;9:86. 10.3390/bioengineering9030086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zolet CMLS, Ulbricht L, Romaneli EFR, Neves EB. (2019) Thermal Asymmetries and Mean Foot Temperature. In: 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE, Berlin, Germany, pp 2821–2824. 10.1109/EMBC.2019.8857378. [DOI] [PubMed]
- 28.Macdonald A, Petrova N, Ainarkar S, Allen J, Plassmann P, Whittam A, Bevans J, Ring F, Kluwe B, Simpson R, Rogers L, Machin G, Edmonds M. Thermal symmetry of healthy feet: a precursor to a thermal study of diabetic feet prior to skin breakdown. Physiol Meas. 2017;38:33–44. 10.1088/1361-6579/38/1/33. [DOI] [PubMed] [Google Scholar]
- 29.Seixas A, Ammer K, Carvalho R, Vilas-Boas JP, Mendes J, Vardasca R. Relationship between skin temperature and soft tissue hardness in diabetic patients: an exploratory study. Physiol Meas. 2019;40:074007. 10.1088/1361-6579/ab2f03. [DOI] [PubMed] [Google Scholar]
- 30.Balbinot LF, Robinson CC, Achaval M, Zaro MA, Brioschi ML. Repeatability of Infrared Plantar Thermography in Diabetes patients: a pilot study. J Diabetes Sci Technol. 2013;7:1130–7. 10.1177/193229681300700505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.(2012) Monofilament Testing for loss of protective sensation (LOPS): Procedure. British Columbia Provincial Nursing Skin & Wound Committee, Victoria, BC, Canada.
- 32.Biancari F, Juvonen T. Angiosome-targeted Lower Limb revascularization for ischemic Foot wounds: systematic review and Meta-analysis. Eur J Vasc Endovasc Surg. 2014;47:517–22. 10.1016/j.ejvs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Orrapin S, Siribumrungwong B. Successful revascularization, Angiosome Concept, and Multivessel Revascularization: effects on Wound Healing: an Asian perspective. Int J Low Extrem Wounds. 2024;23:12–8. 10.1177/15347346231212330. [DOI] [PubMed] [Google Scholar]
- 34.Jolliffe IT, Cadima J. Principal component analysis: a review and recent developments. Phil Trans R Soc A. 2016;374:1–16. 10.1098/rsta.2015.0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortes C, Vapnik V. Support-vector networks. Mach Learn. 1995;20:273–97. 10.1007/BF00994018. [Google Scholar]
- 36.Brereton RG, Lloyd GR. Support Vector machines for classification and regression. Analyst. 2010;135:230–67. 10.1039/B918972F. [DOI] [PubMed] [Google Scholar]
- 37.Frazier PI. (2018) A Tutorial on Bayesian Optimization. ArXiv, abs/1807.02811.
- 38.Guo S, Bocklitz T, Neugebauer U, Popp J. Common mistakes in cross-validating classification models. Anal Methods. 2017;9:4410–7. 10.1039/C7AY01363A. [Google Scholar]
- 39.Guevara E, Torres-Galván JC, González FJ, Luevano‐Contreras C, Castillo‐Martínez CC, Ramírez‐Elías MG. Feasibility of Raman spectroscopy as a potential in vivo tool to screen for pre‐diabetes and diabetes. J Biophotonics. 2022;15. 10.1002/jbio.202200055. [DOI] [PubMed]
- 40.Dasariraju S, Huo M, McCalla S. Detection and classification of immature leukocytes for diagnosis of Acute myeloid leukemia using Random Forest Algorithm. Bioengineering. 2020;7:120. 10.3390/bioengineering7040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inui A, Nishimoto H, Mifune Y, Yoshikawa T, Shinohara I, Furukawa T, Kato T, Tanaka S, Kusunose M, Kuroda R. Screening for osteoporosis from blood Test Data in Elderly Women using a machine learning Approach. Bioengineering. 2023;10:277. 10.3390/bioengineering10030277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W-S, Lin C-H, Tsai C-Y, Wang H-T, Li S-Y, Liu T-Y, Tan AC, Tsou H-H, Tseng K-H, Lin C-C. Double filtration plasmapheresis with polyvinyl alcohol-based membrane lowers serum inflammation and toxins in patients with Hyperlipidemia. Bioengineering. 2023;10:89. 10.3390/bioengineering10010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsiao P-J, Hsiao C-J, Tsai F-R, Hou Y-L, Chiu C-C, Chiang W-F, Wu K-L, Li Y-K, Lin C, Chan J-S, Chang C-W, Chu C-M. From bench to Bedside: clinical and Biomedical investigations on Hepatitis C Virus (HCV) genotypes and risk factors for Albuminuria. Bioengineering. 2022;9:509. 10.3390/bioengineering9100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bayareh R, Maldonado H, Torres IA, Vera A, Leija L. Thermographic study of the diabetic foot of patients with diabetes mellitus and healthy patients. 2018 Global Medical Engineering Physics Exchanges/Pan American Health Care Exchanges (GMEPE/PAHCE). Porto: IEEE; 2018. pp. 1–5. 10.1109/GMEPE-PAHCE.2018.8400742. [Google Scholar]
- 45.Casadei G, Filippini M, Brognara L. Glycated hemoglobin (HbA1c) as a Biomarker for Diabetic Foot Peripheral Neuropathy. Diseases. 2021;9:16. 10.3390/diseases9010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Au Yeung SL, Luo S, Schooling CM. The impact of Glycated Hemoglobin (HbA1c) on Cardiovascular Disease Risk: a mendelian randomization study using UK Biobank. Diabetes Care. 2018;41:1991–7. 10.2337/dc18-0289. [DOI] [PubMed] [Google Scholar]
- 47.Renero-C F-J. The thermoregulation of healthy individuals, overweight–obese, and diabetic from the plantar skin thermogram: a clue to predict the diabetic foot. Diabet Foot Ankle. 2017;8:1361298. 10.1080/2000625X.2017.1361298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yazdanpanah L, Shahbazian H, Nazari I, Arti HR, Ahmadi F, Mohammadianinejad SE, Cheraghian B, Latifi SM. Prevalence and related risk factors of diabetic foot ulcer in Ahvaz, south west of Iran. Diabetes Metabolic Syndrome: Clin Res Reviews. 2018;12:519–24. 10.1016/j.dsx.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Wang SC, Au Y, Ramirez-GarciaLuna JL, Lee L, Berry GK. The Promise of Smartphone Applications in the remote monitoring of Postsurgical wounds: a Literature Review. Adv Skin Wound Care. 2020;33:489–96. 10.1097/01.ASW.0000694136.29135.02. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [E.S.K-M], upon reasonable request.