Abstract
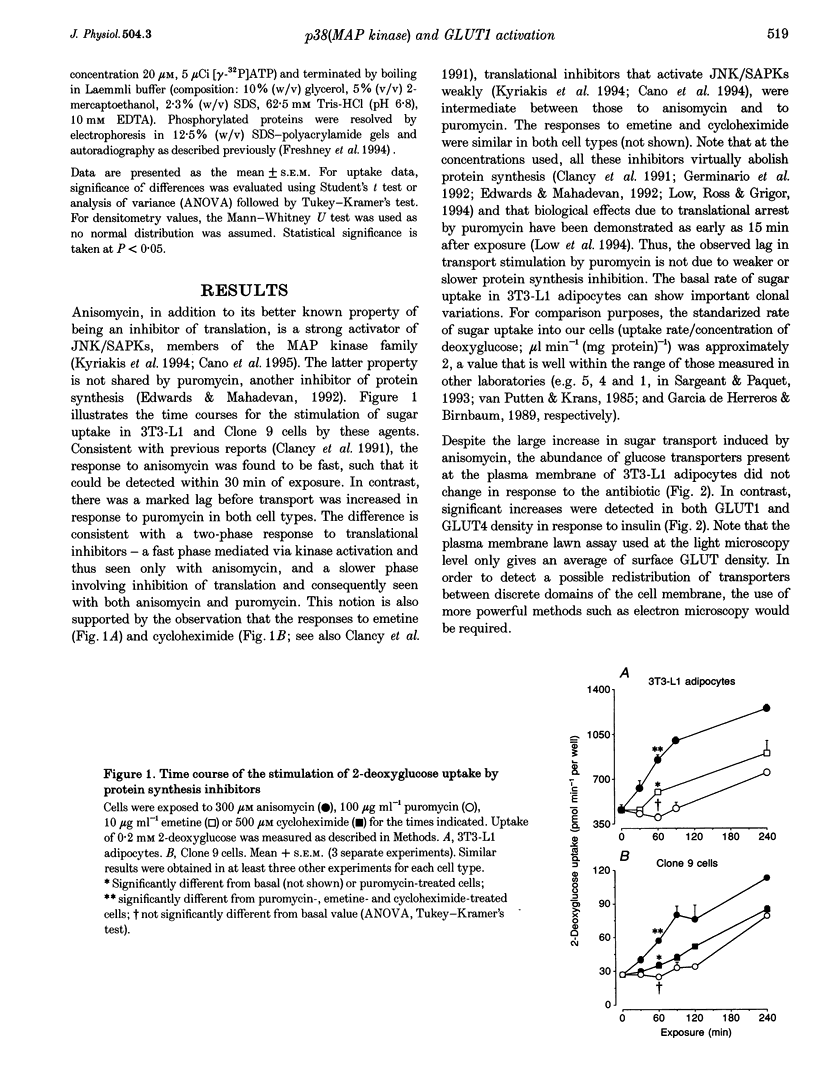
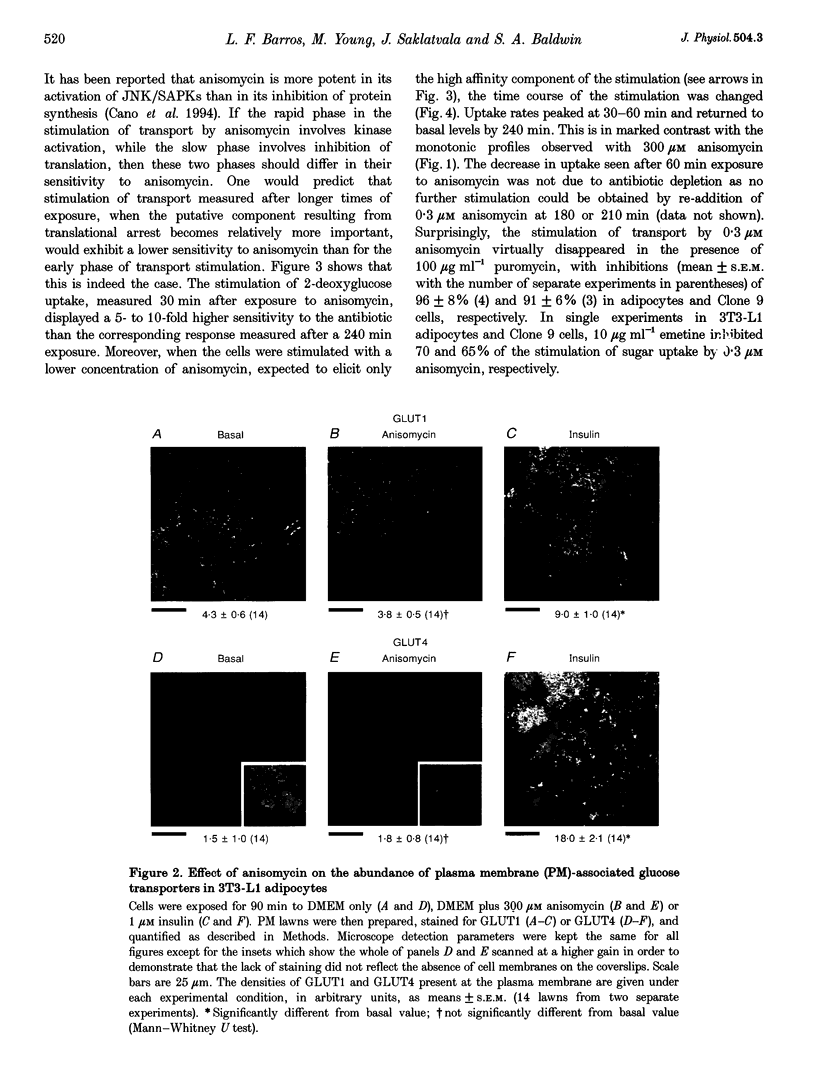
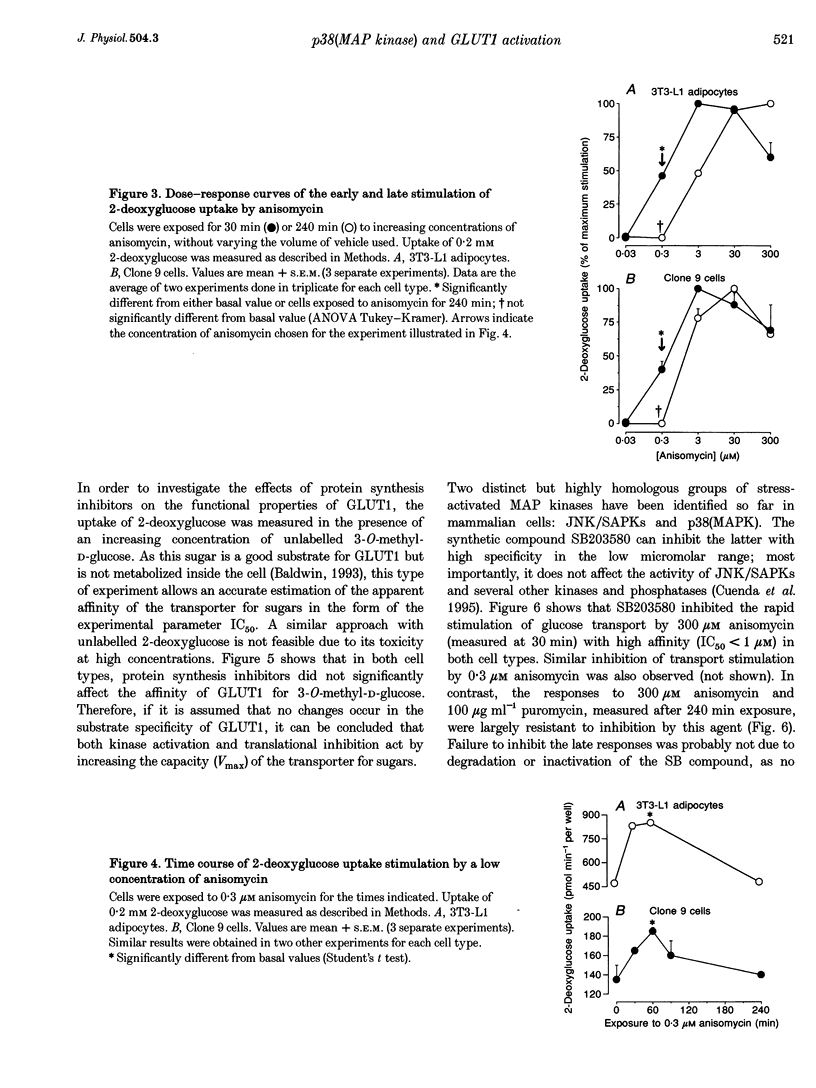
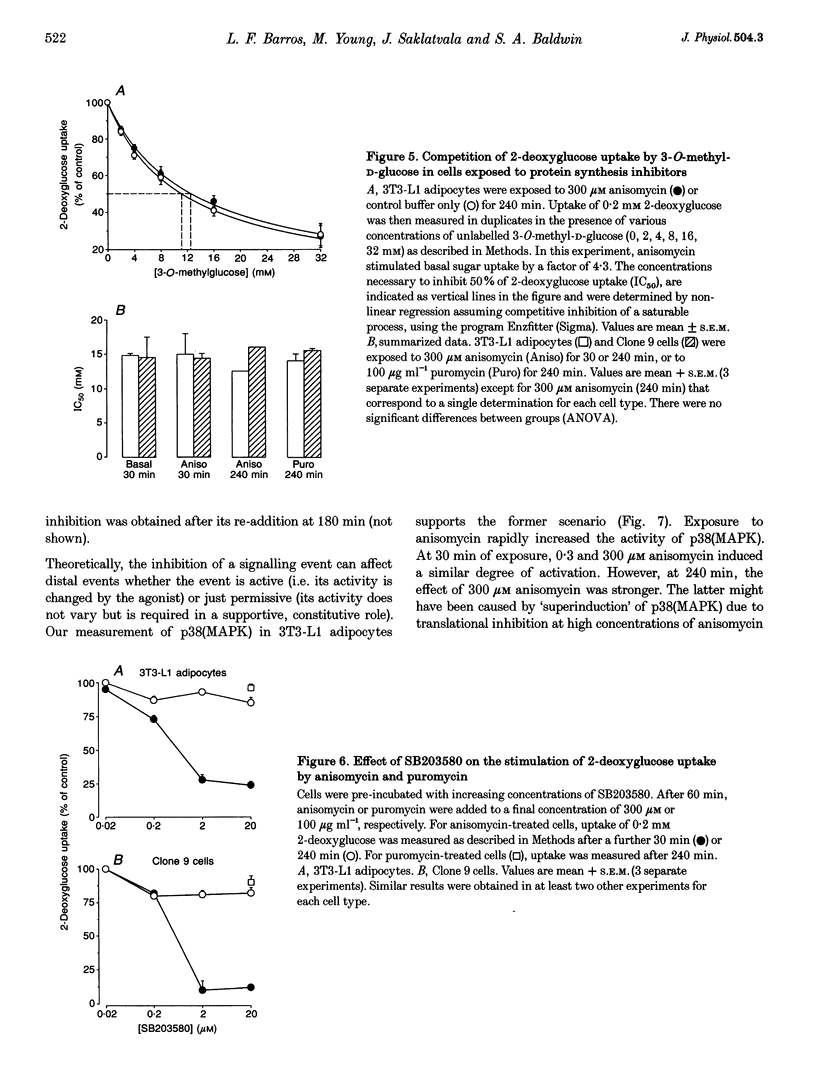
1. Inhibitors of protein synthesis stimulate sugar transport in mammalian cells through activation of plasma membrane GLUT1, the housekeeping isoform of the glucose transporter. However, it has been reported that some of these compounds, in addition to their effect on protein synthesis, also activate protein kinases. 2. In the present study we have explored the role of these two effects on GLUT1 activation. In 3T3-L1 adipocytes and Clone 9 cells, stimulation of sugar transport by puromycin, a translational inhibitor that does not activate kinases, was not detectable until 90 min after exposure. In contrast, stimulation by anisomycin, a potent Jun-NH2-terminal kinase (JNK) agonist, exhibited no lag phase. An intermediate response was observed to emetine and cycloheximide, weak activators of JNK. 3. The potency of anisomycin to stimulate transport acutely (30 min of exposure) was 5- to 10-fold greater than for its chronic stimulation of transport, measured after 4 h of exposure. The stimulation of transport by a low concentration of anisomycin (0.3 microM) was transient, peaked at 30-60 min and it was inhibited (IC50 < 1 microM) by SB203580, which indicates that its mediator is not JNK, but the homologous p38(MAP kinase) (p38(MAPK)). In contrast, the responses to 4 h exposure to 300 microM anisomycin or puromycin were refractory to SB203580. 4. Exposure to anisomycin resulted in rapid activation of p38(MAPK). Activation of both p38(MAPK) and GLUT1 by 0.3 microM anisomycin was cancelled by puromycin. 5. We conclude that the activation of GLUT1 in response to anisomycin includes two components: a delayed component involving translational inhibition and a fast, puromycin-inhibitable component that is secondary to activation of p38(MAPK).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin S. A. Mammalian passive glucose transporters: members of an ubiquitous family of active and passive transport proteins. Biochim Biophys Acta. 1993 Jun 8;1154(1):17–49. doi: 10.1016/0304-4157(93)90015-g. [DOI] [PubMed] [Google Scholar]
- Barros L. F., Marchant R. B., Baldwin S. A. Dissection of stress-activated glucose transport from insulin-induced glucose transport in mammalian cells using wortmannin and ML-9. Biochem J. 1995 Aug 1;309(Pt 3):731–736. doi: 10.1042/bj3090731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E., Hazzalin C. A., Mahadevan L. C. Anisomycin-activated protein kinases p45 and p55 but not mitogen-activated protein kinases ERK-1 and -2 are implicated in the induction of c-fos and c-jun. Mol Cell Biol. 1994 Nov;14(11):7352–7362. doi: 10.1128/mcb.14.11.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano E., Mahadevan L. C. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995 Mar;20(3):117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- Clancy B. M., Harrison S. A., Buxton J. M., Czech M. P. Protein synthesis inhibitors activate glucose transport without increasing plasma membrane glucose transporters in 3T3-L1 adipocytes. J Biol Chem. 1991 Jun 5;266(16):10122–10130. [PubMed] [Google Scholar]
- Clark A. E., Holman G. D., Kozka I. J. Determination of the rates of appearance and loss of glucose transporters at the cell surface of rat adipose cells. Biochem J. 1991 Aug 15;278(Pt 1):235–241. doi: 10.1042/bj2780235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A., Rouse J., Doza Y. N., Meier R., Cohen P., Gallagher T. F., Young P. R., Lee J. C. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995 May 8;364(2):229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Clancy B. M., Pessino A., Woon C. W., Harrison S. A. Complex regulation of simple sugar transport in insulin-responsive cells. Trends Biochem Sci. 1992 May;17(5):197–201. doi: 10.1016/0968-0004(92)90266-c. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Mahadevan L. C. Protein synthesis inhibitors differentially superinduce c-fos and c-jun by three distinct mechanisms: lack of evidence for labile repressors. EMBO J. 1992 Jul;11(7):2415–2424. doi: 10.1002/j.1460-2075.1992.tb05306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer Y., Thomas J., Rose H., Kammermeier H. Alanine and hyperosmolarity are responsible for the stimulation of cardiomyocyte glucose transport by samples containing a glucose tolerance factor. Life Sci. 1992;50(25):1963–1972. doi: 10.1016/0024-3205(92)90526-u. [DOI] [PubMed] [Google Scholar]
- Freshney N. W., Rawlinson L., Guesdon F., Jones E., Cowley S., Hsuan J., Saklatvala J. Interleukin-1 activates a novel protein kinase cascade that results in the phosphorylation of Hsp27. Cell. 1994 Sep 23;78(6):1039–1049. doi: 10.1016/0092-8674(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Garcia de Herreros A., Birnbaum M. J. The acquisition of increased insulin-responsive hexose transport in 3T3-L1 adipocytes correlates with expression of a novel transporter gene. J Biol Chem. 1989 Nov 25;264(33):19994–19999. [PubMed] [Google Scholar]
- Germinario R. J., Manuel S., Chang Z., Leckett B. Inhibitors of protein synthesis cause increased hexose transport in cultured human fibroblasts by a mechanism other than transporter translocation. J Cell Physiol. 1992 Apr;151(1):156–163. doi: 10.1002/jcp.1041510120. [DOI] [PubMed] [Google Scholar]
- Gould G. W., Cuenda A., Thomson F. J., Cohen P. The activation of distinct mitogen-activated protein kinase cascades is required for the stimulation of 2-deoxyglucose uptake by interleukin-1 and insulin-like growth factor-1 in KB cells. Biochem J. 1995 Nov 1;311(Pt 3):735–738. doi: 10.1042/bj3110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. A., Clancy B. M., Pessino A., Czech M. P. Activation of cell surface glucose transporters measured by photoaffinity labeling of insulin-sensitive 3T3-L1 adipocytes. J Biol Chem. 1992 Feb 25;267(6):3783–3788. [PubMed] [Google Scholar]
- Hayes N., Biswas C., Strout H. V., Berger J. Activation by protein synthesis inhibitors of glucose transport into L6 muscle cells. Biochem Biophys Res Commun. 1993 Feb 15;190(3):881–887. doi: 10.1006/bbrc.1993.1131. [DOI] [PubMed] [Google Scholar]
- Ismail-Beigi F. Metabolic regulation of glucose transport. J Membr Biol. 1993 Jul;135(1):1–10. doi: 10.1007/BF00234646. [DOI] [PubMed] [Google Scholar]
- Kyriakis J. M., Banerjee P., Nikolakaki E., Dai T., Rubie E. A., Ahmad M. F., Avruch J., Woodgett J. R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994 May 12;369(6476):156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Low B. C., Ross I. K., Grigor M. R. Glucose deprivation and acute cycloheximide treatment stimulate system L amino acid transport in cultured vascular smooth muscle cells. J Biol Chem. 1994 Dec 23;269(51):32098–32103. [PubMed] [Google Scholar]
- Mahadevan L. C., Edwards D. R. Signalling and superinduction. Nature. 1991 Feb 28;349(6312):747–748. doi: 10.1038/349747c0. [DOI] [PubMed] [Google Scholar]
- Merrall N. W., Plevin R. J., Stokoe D., Cohen P., Nebreda A. R., Gould G. W. Mitogen-activated protein kinase (MAP kinase), MAP kinase kinase and c-Mos stimulate glucose transport in Xenopus oocytes. Biochem J. 1993 Oct 15;295(Pt 2):351–355. doi: 10.1042/bj2950351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant R. J., Pâquet M. R. Effect of insulin on the rates of synthesis and degradation of GLUT1 and GLUT4 glucose transporters in 3T3-L1 adipocytes. Biochem J. 1993 Mar 15;290(Pt 3):913–919. doi: 10.1042/bj2900913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty M., Loeb J. N., Vikstrom K., Ismail-Beigi F. Rapid activation of GLUT-1 glucose transporter following inhibition of oxidative phosphorylation in clone 9 cells. J Biol Chem. 1993 Aug 15;268(23):17225–17232. [PubMed] [Google Scholar]
- Tsakiridis T., Vranic M., Klip A. Phosphatidylinositol 3-kinase and the actin network are not required for the stimulation of glucose transport caused by mitochondrial uncoupling: comparison with insulin action. Biochem J. 1995 Jul 1;309(Pt 1):1–5. doi: 10.1042/bj3090001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widnell C. C., Baldwin S. A., Davies A., Martin S., Pasternak C. A. Cellular stress induces a redistribution of the glucose transporter. FASEB J. 1990 Apr 1;4(6):1634–1637. doi: 10.1096/fasebj.4.6.2156742. [DOI] [PubMed] [Google Scholar]
- van Putten J. P., Krans H. M. Glucose as a regulator of insulin-sensitive hexose uptake in 3T3 adipocytes. J Biol Chem. 1985 Jul 5;260(13):7996–8001. [PubMed] [Google Scholar]
- van den Berghe N., Barros L. F., van Mackelenbergh M. G., Krans H. M. Clostridium botulinum C3 exoenzyme stimulates GLUT4-mediated glucose transport, but not glycogen synthesis, in 3T3-L1 adipocytes--a potential role of rho? Biochem Biophys Res Commun. 1996 Dec 13;229(2):430–439. doi: 10.1006/bbrc.1996.1821. [DOI] [PubMed] [Google Scholar]




