Abstract
Cell membrane glycans contribute to immune recognition, signaling, and cellular adhesion and migration, and altered membrane glycosylation is a feature of cancer cells that contributes to cancer progression. The uptake and metabolism of glucose and other nutrients essential for glycan synthesis could underlie altered membrane glycosylation, but the relationship between shifts in nutrient metabolism and the effects on glycans have not been directly examined. We developed a method that combines stable isotope tracing with metabolomics to enable direct observations of glucose allocation to nucleotide sugars and cell-membrane glycans. We compared the glucose allocation to membrane glycans of two pancreatic cancer cell lines that are genetically identical but have differing energy requirements. The 8988-S cells had higher glucose allocation to membrane glycans and intracellular pathways relating to glycan synthesis, but the 8988-T cells had higher glucose uptake and commitment of glucose to non-glycosylation pathways. The cell lines differed in the requirements of glucose for energy production, resulting in differences in glucose bioavailability for glycan synthesis. The workflow demonstrated here enables studies on the effects of metabolic shifts on the commitment of nutrients to cell-membrane glycans. The results suggest that cell-membrane glycans are remodeled through shifts in glucose commitment to non-glycosylation pathways.
Subject terms: Biological techniques, Metabolomics
Stable isotope tracing reveals distinct glucose allocation to membrane glycans in genetically identical pancreatic cancer cells, showing how metabolic shifts impact glycosylation. This approach highlights the role of nutrient commitment in cancer cell glycan remodeling.
Introduction
The glycosylation of membrane glycoconjugates of protein, lipid, or nucleic acids affects tissue organization, cell surface receptor modulation, cellular immune responses, and cellular adhesion and migration. Cancer cell membrane glycans are typically altered when compared with those observed on non-cancer cells1, and altered cell membrane glycosylation can affect many aspects of cellular behavior including altered cellular adhesion and migration with implications for cancer progression. The mechanisms by which membrane glycans become altered in cancer are not well understood. However, cancer cells have been reported to shift their nutrient preferences and utilization, such as by increased uptake and use of glucose for energy and anabolism2,3. Glucose and other nutrients such as fructose, glutamine, acetate, and nucleotides, are required for cell membrane glycan synthesis and therefore alterations in metabolism and energy usage could significantly affect the composition and structure of those glycans.
Previous studies have shown associations between modulations in metabolism and alterations to cell-surface glycans4–6. For example, cancer cells undergoing epithelial-mesenchymal transition (EMT) increased both their glucose metabolism and the glycosylation of a fibronectin variant7. Activated T cells in vivo show increased flow of glucose toward anabolic pathways, accompanied by an increase in nucleotide-sugar metabolites that are used for glycosylation8. Related to this, the further differentiation of activated T cells into T helper cells induces a shift of glucose use towards aerobic glycolysis, potentially constraining glucose availability for N-glycosylation4. These observations taken together suggest that metabolic shifts can manifest as altered patterns of cell membrane glycan synthesis. However, a direct mechanistic connection between metabolic shifts and altered cell membrane glycans has not been made.
The incorporation of the stable isotopes 13C or 15N into cellular metabolites such as glucose and glutamine enables the tracing by mass-spectrometry metabolomics of the labeled carbon or nitrogen atoms through their incorporation into downstream molecules such as cell membrane glycans9,10. This approach is widely used in studies of cellular metabolic states11–13, and may provide direct measurements of glucose routing to monosaccharide synthesis and cell membrane glycan production. However, the combination of stable isotope labeling, and metabolomics has not previously been reported in the study of cell membrane glycans.
Here, we combine stable isotope labeling and metabolomics to enable direct observations of glucose allocation into cell membrane glycans. The method uses the growth of cells in media containing 13C labeled glucose followed by the metabolomic analysis of 13C labeled monosaccharides of cell-membrane glycans. We used this method to ask whether alterations in the cellular metabolism of glucose result in changes in glucose allocation to monosaccharide production and changes to the cell membrane glycans. We report that cancer-associated metabolic shifts directly affect glucose allocation to membrane glycan synthesis.
Results
Derivatization and Identification of Monosaccharides by LC-MS
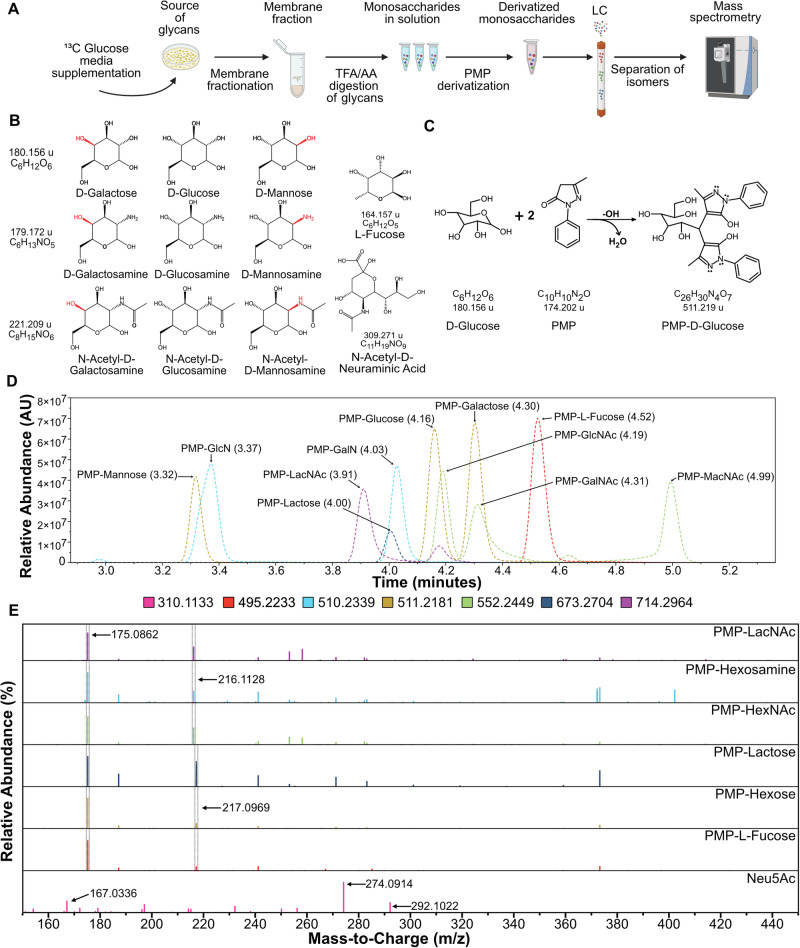
We developed a strategy to quantify the incorporation of glucose-derived 13C carbons into the monosaccharides in cell-membrane glycans (Fig. 1A). We analyzed the monosaccharides instead of the intact glycans to enable determination of glucose allocation to each of the monosaccharide types within the glycans. To carry out this strategy, we first had to establish the ability to resolve isomeric monosaccharides (Fig. 1B) that cannot be distinguished by retention time or mass-to-charge ratio in LC-MS. We therefore derivatized the neutral monosaccharides with 1-phenyl-3-methyl-5-pyrazolone (PMP) (Fig. 1C)14–16 to enable their resolution in liquid chromatography (Supplementary Fig. 1). We used three unique mixtures of monosaccharides containing one monosaccharide from each isomeric group as standards, and we included the di-saccharides lactose and N-acetyl-lactosamine (LacNAc) (Table 1 and Supplementary Table 1) to test for cleavage of the di-saccharide junctions in subsequent steps. N-acetyl neuraminic acid (Neu5Ac) could not be derivatized by PMP due to its negative charge16,17 and was added to a mixture after PMP derivatization.
Fig. 1. Specific detection of monosaccharide isomers.
A Overall strategy for the tracing of 13C glucose from the media to the glycans in cellular membranes. Created in BioRender. Reyes Oliveras, A. (2024) https://BioRender.com/i42k626B. Structures of monosaccharides found in glycans in humans showing isomer groups, hexoses, hexosamines, N-acetyl-hexosamines, and L-fucose and N-acetyl-neuraminic acid. C The chemical reaction of neutral carbohydrates with PMP to form derivatized PMP-carbohydrates. D Chromatogram showing retention peaks of standard solutions of monosaccharides derivatized with PMP. E MS2 spectra of all detected carbohydrates.
Table 1.
Mono- and di-saccharides used as standards
| Name | Chemical Formula | PMP Derivatized Formula | Retention Time | Parent Ion [M + H] | Ion Fragments [M + H] |
|---|---|---|---|---|---|
| GlcNAc | C8H15NO6 | C28H33N5O7 | 4.19 | 552.2453 | 175.0862, 216.1128 |
| GalNAc | C8H15NO6 | C28H33N5O7 | 4.31 | 552.2453 | 175.0862, 216.1128 |
| MacNAc | C8H15NO6 | C28H33N5O7 | 4.99 | 552.2453 | 175.0862, 216.1128 |
| Mannose | C6H12O6 | C26H30N4O7 | 3.32 | 511.2187 | 175.0862, 217.0969 |
| Glucose | C6H12O6 | C26H30N4O7 | 4.16 | 511.2187 | 175.0862, 217.0969 |
| Galactose | C6H12O6 | C26H30N4O7 | 4.3 | 511.2187 | 175.0862, 217.0969 |
| GlcN | C6H13NO5 | C26H31N5O6 | 3.37 | 510.2347 | 175.0862, 216.1128 |
| GalN | C6H13NO5 | C26H31N5O6 | 4.03 | 510.2347 | 175.0862, 216.1128 |
| L-Fucose | C26H30N4O7 | C26H30N4O6 | 4.52 | 495.2238 | 175.0862, 217.0969 |
| Neu5Ac | C11H19NO9 | - | 1.07 | 310.1133 | 292.1022, 274.0914, 167.0336 |
| Lactose | C12H22O11 | C32H40O12N4 | 4.00 | 673.2715 | 175.0862, 217.0969 |
| LacNAc | C14H25NO11 | C34H43N5O12 | 3.91/4.18 | 717.2981 | 175.0862, 216.1128 |
N-acetyl-glucosamine (GlcNAc), N-acetyl-galactosamine (GalNAc), N-acetyl-mannosamine (ManNAc), glucosamine (GlcN), galactosamine (GalN)), N-acetyl-neuraminic acid (Neu5Ac)), N-acetyl-lactosamine (LacNAc).
This approach enabled rapid (10-minute) LC-MS separations of all PMP-derivatized and underivatized carbohydrates (Fig. 1D). The mass spectra of the parent ions (Supplementary Fig. 1) and MS2 fragmentation spectra (Fig. 1E), in conjunction with unique retention times (Fig. 1D), allowed the identification of each carbohydrate in each isomeric grouping. The peak at 175.0862 m/z is the common PMP fragment from all derivatized PMP-sugars. For all amine-containing carbohydrates (e.g., hexosamines and N-acetyl-hexosamines), the second most abundant fragment ion was the peak at 216.1128 m/z, and for the other carbohydrates except for Neu5Ac, the peak at 217.0969 m/z. These peaks arise from the cleavage of C2-C3 and C1-PMP bonds (Supplementary Fig. 1). For Neu5Ac, we detected peaks at 292.1022, 274.0914, and 167.0336 m/z, coming from the loss of one or two molecules of H2O and cross-ring cleavage, respectively (Supplementary Fig. 1C). These masses were further confirmed in complete, high-resolution MS2 spectra for each PMP-carbohydrate using collision-induced dissociation (CID) fragmentation (Supplementary Fig. 2)16. These results demonstrate unambiguous identification and chromatographic resolution of each monosaccharide.
Detection and quantification of cell membrane monosaccharides
To further establish the strategy outlined above (Fig. 1A), we sought to confirm the accurate determination of the relative abundances of the monosaccharides derived from cell-membrane glycans. We collected the membrane fractions from cultured cells by subcellular fractionation and confirmed by western blot that the membrane fractions showed high amounts of N-glycans (detected by concanavalin A) and low amounts of the cytosolic marker GAPDH, in contrast to the cytosolic fractions (Fig. 2A). We hydrolyzed the membrane fraction with trifluoroacetic acid (TFA) or acetic acid to cleave the glycosidic bonds between the glycan monosaccharides, after which we derivatized the purified monosaccharides with PMP.
Fig. 2. Detection and quantification of cell membrane monosaccharides.
A Western Blot showing isolation of cells in cytosolic fraction or membrane fraction. E-Cadherin (membrane marker), GAPDH (cytosolic marker), and Con-A for N-Glycan marker. B Liquid chromatography showing PMP-derivatized carbohydrates isolated from glycans after acid hydrolysis. C Monosaccharide distribution across cell lines and glycoprotein fetuin. D Monosaccharide abundance of OVCAR4-EV and ST6Gal1-OE. E Validation of ST6Gal1 overexpression by flow cytometry using lectins SNA and MAL I. MFI, mean fluorescence intensity. n = 3, Asterisks with * indicating p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
To account for a matrix effect caused by the cell membrane matrix in comparison to the external standard curve prepared in water (Supplementary Fig. 3), we added internal standards (13C-glucose, 13C-galactose, and 13C-mannose) at 0.1 µg/mL to both the cell lysate samples and the external standards prior to acid hydrolysis. The matrix effect, which refers to potential ion suppression due to interference from the sample matrix affecting the accuracy of the quantification, was evaluated by comparing HEK 293-T cell lysate samples with and without acid hydrolysis to the signal of the internal standards in water. This comparison showed no significant matrix effect in the HEK 293-T samples (Supplementary Fig. 3). However, when we compared the internal standard signal from the cell lysates to that of the external standard curve, a statistically significant matrix effect was observed. We therefore corrected for this effect by using the internal standards to adjust the quantification (Supplementary Fig. 3).
LC-MS analysis revealed seven monosaccharides: PMP-glucose, PMP-galactose, PMP-mannose, PMP-L-fucose, PMP-glucosamine, PMP-galactosamine, and Neu5Ac (Fig. 2B). Mammalian glycans do not contain hexosamines1, but because reaction with TFA removes the acetyl group in N-acetyl hexosamines leading to the formation of hexosamines18,19, we interpreted the presence of hexosamines as a surrogate for N-acetyl-hexosamines. These observations confirmed the unique identification of the primary monosaccharide components of membrane-bound glycans.
We asked whether the distributions of monosaccharides in the cell lines and the purified glycoprotein fetuin corresponded to predicted abundances based on previous studies20,21. We developed external standard curves of the derivatized monosaccharides normalized by 13C PMP-hexoses internal standards to enable comparisons of the absolute levels of each monosaccharide (Supplementary Fig. 3). The standard curves showed high precision and linearity in the measurements (R2 > 0.994) and similar detection limits across the standards, confirming applicability to each of the monosaccharides measured in this study. Comparisons of the absolute levels of monosaccharides (Fig. 2C) showed that galactose, N-acetyl-glucosamine, and mannose were the predominant monosaccharides. This finding concords with their relative usage: galactose is a key component found in most glycans, including N-glycans, O-glycans, glycosphingolipids (GSLs), and less common proteoglycans like alpha-dystroglycans, collagen-Hyl-Gal, and proteoglycan-O-Xyl22; N-Acetylglucosamine (GlcNAc) is essential for N-glycan branching and the LacNAc extensions; and mannose is essential for the synthesis of N-glycans and glycosylphosphatidylinositol (GPI) anchored proteins. The least abundant were fucose and Neu5Ac, consistent with their positions as capping features of glycans. The cell line OVCAR4 was an exception, in which glucose was most abundant. This finding could correspond to the elevation in ovarian cancer cells such as OVCAR4 in the production of glycosphingolipids23, which contain glucose as an essential component.
We further tested the accuracy and sensitivity of our method using OVCAR4 cell lines that either overexpress the ST6GAL1 glycosyltransferase (OE) or carry an empty vector (EV). ST6GAL1 catalyzes the transfer of Neu5Ac from CMP-Neu5Ac to galactose via an α-2,6-glycosidic bond. The OE cell line showed a ~4-fold higher Neu5Ac content than the EV version with no differences in the other monosaccharides (Fig. 2D). This result agrees with the expected increase in attachment of sialic acid to the membrane glycans in the OE cell line than in the EV cell line. We tested whether the increased sialic acid was in the expected α-2,6 linkage produced by ST6GAL1 by flow cytometry with two lectins, SNA or MAL I, that respectively bind to α-2,6-Neu5Ac and α-2,3-Neu5Ac. The SNA lectin, but not MAL I, showed significantly increased mean fluorescence intensity (MFI) in the OE cell line. Taken together, these results confirm unbiased and accurate comparisons of the monosaccharide compositions of cell-membrane glycans.
Detection of 13C incorporation into membrane-bound glycans
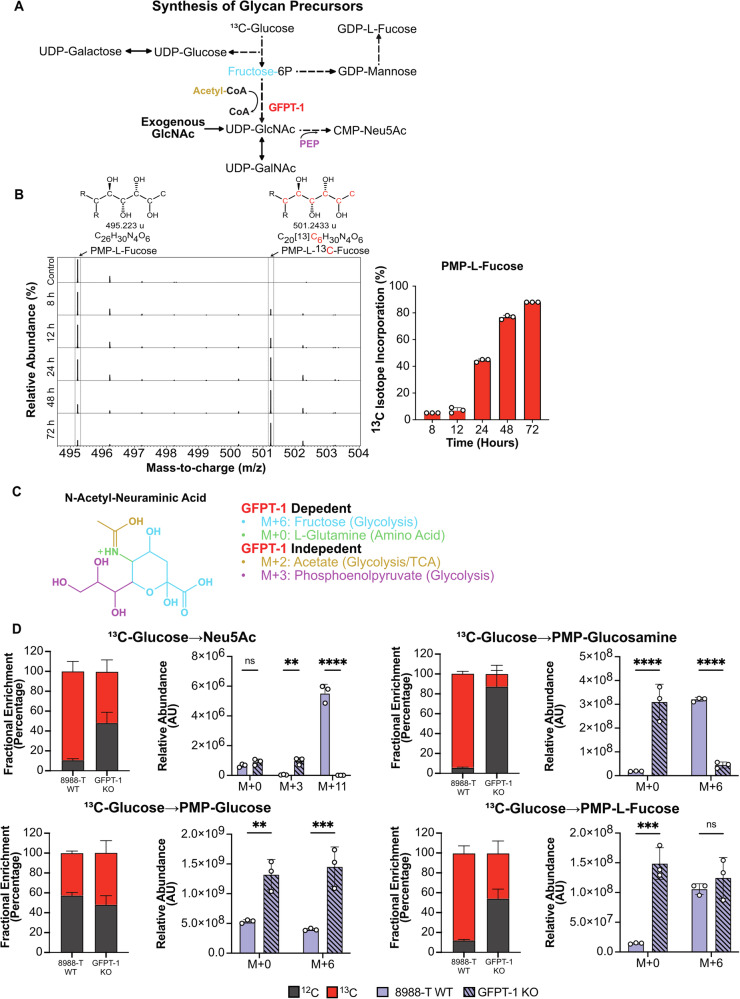
We then tested the ability to detect stable isotopes that were incorporated into the monosaccharides of cell-membrane glycans. We cultured the 8988-T and 8988-S cell lines in media containing 13C-glucose (all six carbons with the stable isotope) over three days. Imported glucose undergoes phosphorylation to enter glycolysis and is then processed to form the nucleotide sugars that serve as monosaccharide donors in glycan synthesis (Fig. 3A). The phosphorylated glucose can be directed towards various metabolic pathways (Supplementary Fig. 4), including the pentose phosphate pathway (PPP) for nucleotide synthesis, the Leloir pathway for UDP-hexose synthesis, or isomerization to fructose-6P. Fructose-6P can undergo further oxidation through glycolysis, pass to the hexosamine biosynthesis pathway (HBP) for nucleotide amino-sugar synthesis, or pass to the fructose-mannose pathway for GDP-hexose synthesis (Fig. 3A).
Fig. 3. Detection of 13C isotope incorporation from 13C-glucose to cell-membrane glycans.
A Metabolic pathways involved in nucleotide sugar synthesis derived from glycolysis. GFPT-1 in red represents the knockout of the rate-limiting enzyme of the HBP. Phosphoenolpyruvate (PEP). B Mass spectra of PMP-L-Fucose showing 13C isotope enrichment after 72 h of cells growing in 13C glucose. R represents the PMP molecule. C Molecular representation of Neu5Ac, providing a visual representation of the metabolites essential for its synthesis and the pathways from which they are derived. D Fractional enrichment and mass isotopologue distribution (MID) showing 13C incorporation in derivatized monosaccharides after knockout of GFPT-1, n = 3. Asterisks with * indicating p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
We isolated the membrane-bound glycans over several time points and analyzed the isotope abundances in the monosaccharides. The mass shift expected for galactose, mannose, and fucose produced from the incorporation of 13C carbons from isotope-labeled glucose is M + 6. We observed a consistent increase over 3 days in the M + 6 fucose peak at 501.243 m/z, the peak corresponding to PMP-13C6-fucose, confirming a steady incorporation of 13C carbons into L-fucose cleaved from membrane-bound glycoconjugates (Fig. 3B).
We then sought to confirm that the 13C-labeled glucose used for the synthesis of membrane glycans was processed through the nucleotide sugar synthesis pathways. We compared the monosaccharides obtained from membrane-bound glycoconjugates in the wildtype (WT) 8988-T cell line and in a version with knockout (KO) of the GFPT-1 gene (Supplementary Fig. 5)24. GFPT-1 catalyzes the rate-limiting step of the hexosamine biosynthesis pathway (HBP) that leads to UDP-GlcNAc synthesis (Fig. 3A)24,25, the core monosaccharide for N-linked glycosylation. UDP-GlcNAc is also used in the synthesis of UDP-GalNAc and CMP-Neu5Ac, with the latter used in the production of sialic acids. We supplemented the media of the KO cells with GlcNAc due to the growth-inhibitory consequences of GFPT-1 loss24. The expected mass shift of Neu5Ac is M + 11, based on M + 6 from N-acetyl-mannosamine (an isomer from GlcNAc), M + 3 from phosphoenol pyruvate (PEP) arising from glucose metabolism through glycolysis, and M + 2 from acetate from glycolysis (Fig. 3C). We observed a shift in the fractional labeling in the GFPT-1 KO cells with a decrease of molecular 13C labeling and an increase of 12C. This shift is consistent with a loss of glucose routing through the HBP (Fig. 3D). M + 6 13C labeling of PMP-glucosamine and M + 11 labeling of N-acetyl-neuraminic acid were significantly reduced in the KO cells relative to the WT cell. M + 3 labeling of Neu5Ac was higher in the GFPT-1 KO cells, consistent with the use of 3 carbons of glucose in the incorporation of PEP into N-acetyl-mannosamine derived from GlcNAc supplementation (Fig. 3A, C).
Interestingly, we observed an increase in total PMP-L-fucose and PMP-glucose and a decrease in total Neu5Ac in the GFPT-1 knockout cell line (Fig. 3D). A decrease in total Neu5Ac was not expected because of the supplementation with GlcNAc to supply the production of UDP-GlcNAc and CMP-Neu5Ac. The change in the abundance of monosaccharides was also observed in PMP-Mannose and PMP-Galactose and was validated by flow cytometry using lectins that bind to epitopes with these monosaccharides (Supplementary Fig. 5). These changes suggest that upon loss of GFPT-1 the cells potentially shift glucose toward the Leloir pathway, which produces UDP-glucose and UDP-galactose, and the fructose-mannose pathway, which produces GDP-mannose and GDP-L-fucose (Fig. 3A). These findings need further exploration to confirm changes in the intracellular pool of nucleotide sugars. In sum, these results confirm that we are able to detect isotope labels from media 13C-glucose that are processed through glycolysis to monosaccharide metabolism and glycan synthesis.
Differential commitment of glucose to membrane glycans
The ability to track the incorporation of 13C carbons from supplemented 13C-glucose to membrane glycans allowed us to test whether the commitment of glucose toward monosaccharide metabolism and membrane glycosylation is affected by cancer-associated shifts in glucose uptake or metabolism. We compared two pancreatic cancer cell lines, 8988-S and 8988-T, that provide an ideal model to study the effects of glucose metabolism on glycosylation because they are genetically identical but have differences in phenotype, metabolism, and glycosylation. The 8988-S cells are epithelial, lipogenic, and weakly metastatic, and the 8988-T cells are mesenchymal, glycolytic, highly metastatic, and high in sialylated glycans and gangliosides26–28.
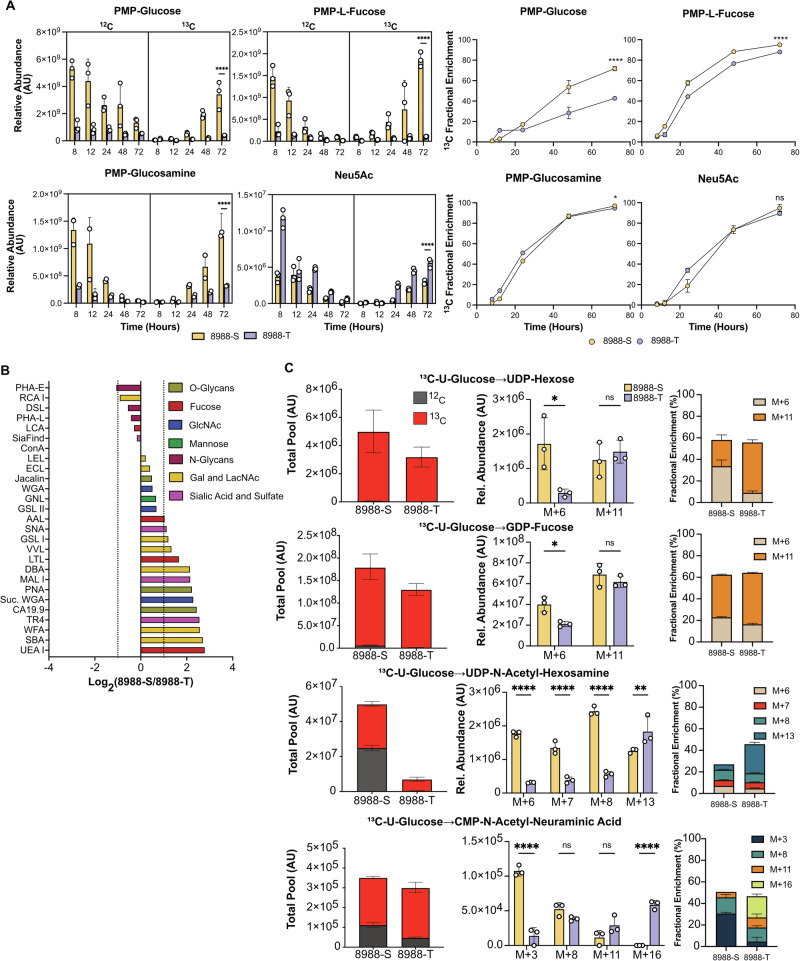
We monitored 13C isotope incorporation from media 13C-glucose into the membrane-bound glycoconjugates of the cell lines over 3 days, measuring both total pool size and fractional enrichment to determine the glucose allocation into glycans. The 8988-S exhibited higher levels and higher 13C fractional enrichment of most monosaccharides, except for Neu5Ac (Fig. 4A and Supplementary Fig. 6). The greater depletion of 12C together with higher total pools in the 8988-S cells suggested a faster turnover of membrane glycans plus a higher allocation of glucose toward glycan synthesis in the 8988-S cells.
Fig. 4. Differential commitment of glucose to membrane glycans.
A Fractional abundance and enrichment of 13C and 12C across derivatized monosaccharides after labeling with 13C glucose for 72 h. B Fold change analysis of glycosylation between 8988-S and 8988-T. C Fractional abundance of 13C and 12C and MID of nucleotide sugars. n = 3, Asterisks with * indicating p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
To assess whether the increased abundance of cell-membrane monosaccharides in the 8988-S cells translated to discernable changes in the cell-membrane glycans, we used flow cytometry of 27 lectins and glycan-binding antibodies to determine the relative quantifications of multiple glycan motifs on the cell surfaces (Fig. 4B). The 8988-S cells showed higher levels than the 8988-T cells in the targets of most lectins, with 2-4-fold higher levels of fucosylation (AAL, LTL, UEA I) and extensions containing HexNAc and galactose (SBA, WFA, DBA, VVL, GSL I). These results are consistent with the increased intracellular glucose commitment to monosaccharide production in 8988-S cells.
We further tested for differences between the cell lines in glucose commitment to glycan production by monitoring the 13C-glucose labeling in the intracellular pathways related to nucleotide-sugar synthesis (Fig. 4C). The total pools and the 13C-glucose incorporation to UDP-hexose (representing UDP-glucose and UDP-galactose) and GDP-fucose were substantially higher in the 8988-S cells, mirroring the higher amounts observed in the membrane glycans. The other monosaccharides stemming from the HBP, UDP-GlcNAc, and UDP-GalNAc (Fig. 4C) (measured jointly by UDP-HexNAc), also were more abundant in the 8988-S cells, with ~7-fold higher total pool and ~3 fold more 13C label incorporation. The total and 13C-labeled CMP-Neu5Ac was similar between the 8988-S cells and the 8988-T cells, whereas cell-membrane Neu5Ac was higher in the 8988-T cells. The difference in cell-membrane levels between 8988-T and 8988-S potentially stem from differential sialyltransferase activity. Nevertheless, this result confirms that 8988-T cells maintain relatively higher Neu5Ac production than the other monosaccharides. In sum, the intracellular levels of the nucleotide sugar precursors generally match the membrane-bound levels of the corresponding monosaccharides. The results support the conclusion that the 8988-S cells have higher glucose allocation towards monosaccharide metabolism related to nucleotide sugar synthesis and glycan synthesis.
To determine whether the difference in 13C incorporation and abundance was from the carbohydrate moiety of the nucleotide sugars or the ribose nucleotide portion, we analyzed the mass isotopologue distribution (MID) of the nucleotide sugars (Fig. 4C). The ribose arises from the pentose phosphate pathway (PPP), an offshoot of the second step of glycolysis. The 8988-S cells showed a higher fractional enrichment of the carbohydrate moiety arising from the incorporation of 13C carbons coming from the Leloir pathway for UDP-hexose (M + 6) and fructose-mannose metabolism for GDP-fucose (M + 6). UDP-HexNAc showed higher fractional enrichment in M + 6 arising from 6 carbons of glucose through the first step of HBP, M + 7 arising from 6 carbons of glucose through the first step of HBP and one carbon allocated in the ring of the uridine and M + 8 arising from the HexNAc completely labeled. CMP-Neu5Ac showed enrichment in M + 3 arising from PEP synthesis through glycolysis (Fig. 3A). The 8988-T cells, on the other hand, showed higher enrichment of isotopes from ribose arising from the PPP combined with the monosaccharide moiety arising from nucleotide sugar synthesis. The enriched isotopologues were M + 11 (13C5-ribose-containing UDP/GDP combining with 13C6-hexose) in UDP-hexose and GDP-fucose, M + 13 (13C5-ribose-containing UDP combining with 13C8-N-HexNAc) in UDP-HexNAc, and M + 16 (13C5-ribose-containing CMP combining with 13C11-Neu5Ac). Thus, the amount of glucose committed to monosaccharide portion of the nucleotide sugars was lower in the 8988-T cells, but the glucose commitment to the pentose phosphate pathway that produces the ribose for nucleotide synthesis was higher. Because ribose is also used for nucleic acid production, the higher ribose production in the 8988-T cells is consistent with its higher proliferation rate (Supplementary Fig. 6). In sum, these results demonstrate that glucose commitment to the monosaccharide portion of the nucleotide sugars was lower in the 8988-T cells, leading to lower monosaccharide usage in membrane glycans.
High energy demand limits glucose bioavailability for glycan synthesis
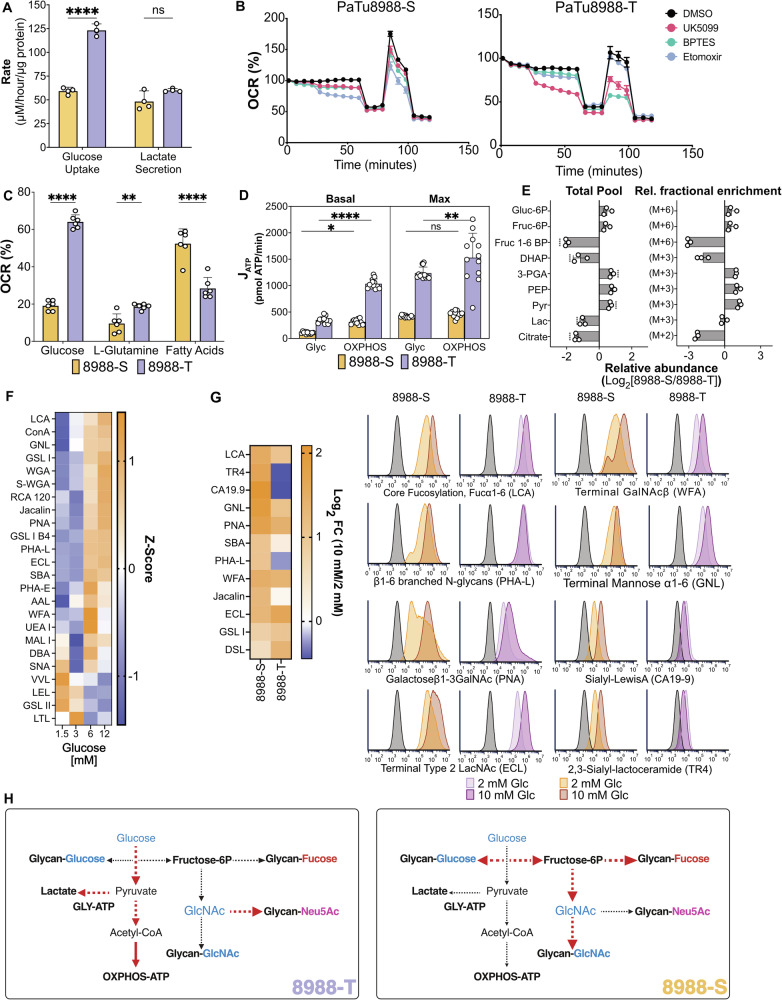
The higher glucose flow through glycolysis combined with the lower glucose allocation to glycan production in the 8988-T cells suggested that the 8988-T cells have higher glucose allocation to non-glycosylation pathways in addition to the PPP. The lactate secretion is similar between the 8988-T and 8988-S cells despite the ~2-fold higher glucose uptake in the 8988-T cells (Fig. 5A), consistent with higher internal glucose usage in 8988-T. Based on Seahorse analysis after acute treatments of the cells with mitochondrial transport inhibitors of pyruvate (UK5099), L-glutamine (BPTES), and fatty acids (etomoxir) (Fig. 5B), the 8988-T cells depended primarily on glucose for energy production by oxidative phosphorylation (OXPHOS) using oxygen consumption rate (OCR) as a surrogate, while the 8988-S cells depended primarily on fatty acids (Fig. 5C). The cell lines had similar apportionments of ATP production between glycolysis and OXPHOS (Fig. 5D), as determined by transformations of the extracellular acidification rate (ECAR) and OCR measurements (Supplementary Fig. 7) to glycolytic and oxidative ATP production rates (JATP)29. However, the overall production of ATP was approximately 3 times higher in 8988-T cells than in 8988-S cells (Fig. 5D). This higher ATP production by both glycolysis and OXPHOS in 8988-T cells is consistent with the 3.2-fold higher proliferation rate of the 8988-T cells (Supplementary Fig. 6). Therefore, the 8988-T cells are using more glucose to produce ATP than the 8988-S cells and have a greater reliance on glucose for mitochondrial ATP production.
Fig. 5. Differential glucose availability constrains glucose commitment to glycosylation.
A Measurement of glucose uptake and lactate secretion in 8988-S and 8988-T. B Nutrient dependency measured by Seahorse analysis using inhibitors that target the transporters UK5099 (MPC), BPTES (GLS1), and Etomoxir (CPT1), n = 6. C ATP production by 8988-S and 8988-T cells measured by MitoStress test and MitoFuel Flex by Seahorse analysis, n = 6. D JATP quantification from glycolysis and OXPHOS, n = 12. E Examination of fold changes in metabolite pools related to glycolysis and 13C fractional enrichment related to glycolysis and citrate. F Heatmap illustrating variations in glycan abundances influenced by glucose concentration in 8988-T cells. G Flow cytometry analysis of glycan motifs between high and low glucose conditions for 8988-S and 8988-T cells. The plot shows capping motifs with a direct relationship between motif abundance and lectin/antibody binding. H Summary elucidating the impact of metabolic differences between 8988-S and 8988-T cells on glycosylation. n = 3, Asterisks with * indicating p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
We asked whether the metabolites associated with the products of glycolysis also showed differences between the cell lines in both total pools and fractional enrichment of 13C (Fig. 5E). The total pool of fructose-1,6-BP was significantly higher in the 8988-T cells with a higher M + 6 fold change, indicating a greater commitment of glucose to glycolysis, and intracellular lactate was higher with similar lactate secretion (Fig. 5A), consistent with greater intracellular use of glucose. The total citrate pool (Fig. 5E) and the M + 2 13C enrichment from glucose to citrate (Supplementary Fig. 7) were also higher in the 8988-T cells, indicating a potentially greater glycolytic flux of carbons from glucose to the TCA cycle. Therefore, these findings support the conclusion that the 8988-T cells have greater glucose flow in glycolysis and greater glucose allocation to non-glycosylation pathways such as the ribose production in the PPP, lactate production, the TCA cycle, and ATP production by glycolysis and OXPHOS.
The higher glucose uptake and flow in glycolysis in the 8988-T cells combined with the lower allocation of glucose to all monosaccharide production pathways except CMP-Neu5Ac suggest that allocation of glucose to glycosylation depends on residual availability: the 8988-T cells have low glucose availability due to increased reliance upon glucose for energy and non-glycosylation needs, whereas the 8988-S cells have greater glucose availability due to their use of fatty acids for energy. Thus, we asked whether changes in the amount of externally available glucose likewise modulated the amount of glucose committed to glycosylation. We cultured the 8988-T cells in a range of glucose concentrations for 5 days and quantified overall glycosylation using flow cytometry. The majority of glycans were substantially increased at glucose concentrations above 6 mM (Fig. 5F), including N-glycans (Con-A), branching (PHA-L), and O-glycans (PNA and Jacalin). The decreased binding of certain lectins to single HexNAc moieties, such as VVL, LEL, and GSL II, is consistent with the exposure of short, truncated glycans upon the limitation of glycan extension. The 8988-S cells showed higher sensitivity to glucose concentration than the 8988T cells (Fig. 5G), consistent with a lower requirement for glucose usage in non-glycosylation pathways.
These results support a model of regulation of glucose commitment glycosylation by glucose availability, where glucose demands in energy and non-glycosylation pathways determine glucose availability for glycosylation (Fig. 5H). The 8988-T cells had higher glucose uptake but increased glucose flow to non-glycosylation pathways, which reduces the availability of fructose-6P for the nucleotide sugar synthesis essential to glycosylation (Fig. 5H). The 8988-S cells, on the other hand, owing to their use of fatty acids for energy, required less glucose for non-glycosylation pathways and therefore had a higher glucose allocation to glycosylation.
Discussion
Here, we report a method that combines stable isotope tracing with metabolomics to enable direct observations of glucose allocation to nucleotide sugars and cell-membrane glycans. Previous studies have shown associations between alterations in metabolism and altered glycosylation in glycoconjugates in cell membrane glycans. However, a direct link between glucose metabolism and altered glycan synthesis expression has not previously been established. We enabled unambiguous identification of isotope incorporation into cell membrane glycans by isolating monosaccharides from membrane-bound glycoconjugates and then resolving the monosaccharide isomers in high-performance liquid chromatography (HPLC) coupled with mass spectrometry. This method has overcome the challenge of distinguishing between multiple types of isomeric monosaccharides that have only minor structural differences. Here, we found that competing demands for glucose utilization for energy or other anabolic pathways can constrain glucose commitment to glycosylation. We have demonstrated that the synthesis of nucleotide sugars and cell membrane glycans was limited by the availability of glucose-6P and fructose-6P, rather than the quantity of glucose processed through glycolysis.
These findings suggest that metabolic shifts observed in cancer and immune cell activation alter nutrient flux to monosaccharide production and may therefore underlie the major changes observed in cell-membrane glycans. Such metabolic shifts occur, for example, in cancer EMT30 and the differentiation of naïve CD8+ T cells into effector T cells8,31, both of which use more glucose to meet anabolic and energy requirements. Furthermore, steady-state differences in such metabolic phenotypes exist between T cells: cytotoxic and helper T cells primarily rely on aerobic glycolysis for their differentiation32,33, whereas regulatory T and memory T cells predominantly utilize oxidative phosphorylation and fatty acid oxidation34,35. Therefore, metabolic shifts that potentially affect glycosylation are prominent in multiple cellular processes. Our carbon tracing metabolomic method enables investigations into how the metabolic shifts affect cell-membrane glycosylation.
Our observation that altered glucose carbon usage regulates cell membrane glycan expression could further inform our understanding of tissue organization, cell surface receptor modulation, cellular immune responses, and cellular adhesion and migration. For example, increased complex N-glycosylation increases the retention, stability, clustering, and/or activation of cell-surface receptors36,37. Increases in glycosphingolipids, including GM3 and SM4, help maintain KRAS plasma membrane localization38, and changes in glycosylation of adhesion molecules like E-cadherin and integrins promote cell adhesion and migration39. Elevations of sialic acid in cell-membrane glycans could contribute to cancer progression by dampening the immune response40,41 or could enable migration after epithelial-mesenchymal transition (EMT)23,42. In the immune system, changes in the glycosylation of membrane proteins and lipid rafts upon activation and differentiation of lymphocytes enable antigen recognition43 and homing of immune cells44–46. Further investigations could show how metabolic shifts affect function through alterations in cell-membrane glycosylation. A key experiment then would be in vivo stable isotope tracing in mouse models of cancer8, which would directly examine how altered cancer cell nutrient metabolism leads to altered membrane glycosylation and cancer progression.
The current study used cell lines derived from the same patient, which provided comparisons between genetically identical cells with differing metabolic requirements, but we do not yet know the generalizability of the study to other settings. In addition, we only demonstrated differences in total amounts of monosaccharides rather than specific glycan motifs. The structures of glycans are regulated in part by the expression levels of ~200 glycosyltransferases, many of which are redundant22, and specific motifs are tightly controlled by key glycosyltransferases. For example, the ABO blood type glycans are controlled by the expression and genotype of the ABO glycosyltransferase47, and the production of the Lewis X glycan comprising the CD15 epitope on granulocytes is controlled by the FUT4 and FUT9 glycosyltransferases48. Further details could be probed using inhibitors of specific glycosylation pathways, such as N- and O-glycans and glycosphingolipids, or by presorting various types of intact glycans to localize isotope incorporation into specific features. Also, stable isotope detection and metabolomics on cell-surface glycans readily applies to other nutrients, such as glutamine, lactate, or galactose. The method presented here could be further developed for such studies.
In summary, we have developed a method for stable-isotope tracing and metabolomics to determine the commitment of media nutrients to cell-membrane glycans. The workflow enables studies of the connection between the flux of nutrients to non-glycosylation and glycosylation pathways and the effects of metabolic shifts upon cell-membrane glycosylation. In the system studied here, the commitment of glucose to membrane glycosylation was determined primarily by the availability of glucose relative to the use of glucose in non-glycosylation pathways. This relationship suggests a mode by which metabolism affects cell functions such signaling, immune recognition, and adhesion and migration.
Methods
Cell culture and subcellular fractionation
The PaTu8988-S and PaTu8988-T cell lines (referred to as 8988-S and 8988-T) were purchased from ATCC (Manassas, VA); the OVCAR4-EV and OVCAR4-OE cell lines were provided by Dr. Susan Bellis at The University of Alabama at Birmingham; and the 8988-T GFPT-1 knockout was contributed by Dr. Costas Lyssiotis at the University of Michigan. All cell lines were cultured in RPMI-1640 media supplemented with 5% FBS and Penicillin-Streptomycin in a 75 mm dish until they achieved 80% confluency. The cells were detached from the plate surface using a cell scraper and were resuspended in growth media. The resulting cell suspension was centrifuged at 300 × g for 5 min, and the pellets were washed twice with 3 mL of Cell Wash Solution from the MemPer kit (Thermofisher 89842) followed by centrifugation at 300 × g for 5 min. To extract cytosolic proteins, the cells were permeabilized with 0.75 mL of permeabilization buffer from the MemPer kit and incubated with constant mixing at 4 °C for 10 min. Subsequently, the cells were centrifuged at 16,000 g for 15 min, and the cytosolic fraction in the supernatant was collected and transferred to a new tube. To obtain a membrane fraction containing glycoconjugates, the pellet was mixed with 0.5 ml of solubilization buffer. The resulting cytosolic and membrane fractions were either stored on ice for immediate use or aliquoted and stored at −80 °C for future use.
Western blot
The cytosolic and membrane fractions were subjected to electrophoresis on a Novex™ WedgeWell™ 4 to 20%, Tris-Glycine, 1.0 mm, Mini Protein Gel (Thermofisher, XP04200BOX) for 1.5 hours at 125 V to separate the proteins. The proteins were then transferred onto a PVDF membrane and probed for cytosolic, membrane, and glycan markers. Specifically, GAPDH (ThermoFisher, # MA5-15738) was detected as the cytosolic marker, E-Cadherin (Cell Signal, #3195) as the membrane marker, and ConA (Vector Laboratories, FL-1001-25) as the N-glycan marker. A GFPT-1 (MA5-31739) antibody was used to confirm the absence of GFPT-1 in the 8988-T GFPT-1 knockout cells.
Glycan cleavage by acid hydrolysis
To release monosaccharides from the isolated glycans, the samples underwent digestion with either trifluoroacetic acid (TFA) or acetic acid for acid hydrolysis. To assess the matrix effect, we compared the signal output of external standard curves prepared in water with those of the cell lysate samples. Internal standards (13C-glucose, 13C-galactose, and 13C-mannose) at a concentration of 0.1 µg/mL were added to both the lysates and the standard curves to correct for any discrepancies arising from the sample matrix. Quantification was adjusted using the signal ratios between the internal standards and the external standards. TFA was used to cleave any glycosidic bond between neutral monosaccharides in the glycans, while acetic acid was used to cleave only the bonds with N-acetyl neuraminic acid. Briefly, 50 µg of protein from the samples were exposed to either 2 M TFA or acetic acid for three hours at 100 °C or two hours at 80 °C, respectively. The resulting samples were then dried using rotatory evaporation and derivatized with PMP.
Derivatization of monosaccharides with 1-Phenyl-3-Methyl-5-Pyrazolone (PMP)
To enable the separation of the carbohydrate isomers, PMP was used to derivatize the cleaved glycans and standard solutions of monosaccharides as controls. The mixed standard solutions containing 0.5 mg/mL of glucose, galactose, mannose, N-acetyl-glucosamine, N-acetyl-galactosamine, glucosamine, galactosamine, or L-fucose were prepared in sterile water. 50 µL of a standard solution or a sample-derived monosaccharide solution were mixed with a PMP solution to a final concentration of 0.1 M PMP in 15% ammonia and 50% methanol. The mixtures were incubated at 70 °C for 1 h, dried by vacuum centrifugation, reconstituted in 500 µL of water, and washed three times with chloroform. The mixtures were transferred to a new tube containing either underivatized standard of N-acetyl neuraminic acid or samples treated with acetic acid to obtain N-acetyl neuraminic acid from glycans.
13C carbon tracing
The cell lines were cultured in glucose-free RPMI-1640 media supplemented with 5% dialyzed FBS and P/S and 10 mM of 13C-U-Glucose (CLM-1396, Cambridge Isotope Laboratories). The cells were collected at 8, 12, 24, 48, and 72 hours, to examine the contribution of glucose towards glycosylation. The collected samples were subjected to membrane isolation, acid hydrolysis, and PMP derivatization to quantify the amount of 13C labeled monosaccharides in the glycans. This method allowed for the measurement of glycosylation changes over time, and the data obtained helped to elucidate the dynamics of glycosylation in response to changes in glucose metabolism.
Liquid chromatography-mass spectrometry
PMP-derivatized standards and samples were analyzed with a Vanquish liquid chromatography system coupled to an Orbitrap ID-X (Thermo Fisher Scientific) using an H-ESI (heated electrospray ionization) source in positive mode. 2 μL of each standard and sample was injected and run through a 10-min reversed-phase chromatography CORTECS T3 column (1.6 μm, 2.1 mm × 150 mm, 186008500, Waters, Eschborn, Germany) combined with a VanGuard pre-column (1.6 μm, 2.1 mm × 5 mm, 186008508, Waters). Buffer A consisted of 100% LC/MS grade water (W6-4, Fisher), 0.01% ammonium hydroxide (A470, Fisher), 5 mM ammonium acetate (73594, Sigma), and buffer B consisted of 99% LC/MS grade acetonitrile (A955, Fisher). Column temperature was kept at 30 °C, flow rate was held at 0.3 mL/min, and the chromatography gradient was as follows: 0-1 min from 0% B to 20% B, 1–4 min from 20% B to 30% B, 4–7.5 min from 30% B to 45% B, 7.5–8.5 min from 45% B to 100% B, and 8.5–10 min held at 100% B. A 5 min wash gradient was run between every injection to flush the column and to re-equilibrate solvent conditions as follows: 0–2 min held at 100% B, 2–3 min from 100% B to 0% B, and 3–5 min held at 0% B. Mass spectrometer parameters were: source voltage 3500 V, sheath gas 70, aux gas 25, sweep gas 1, ion transfer tube temperature 300 °C, and vaporizer temperature 250 °C. Full scan data were collected using the orbitrap with a scan range of 105–1200 m/z at a resolution of 120,000. Fragmentation was induced in the orbitrap with assisted higher-energy collisional dissociation (HCD) collision energies at 20, 40, 60, 80, and 100%. The collision-induced dissociation (CID) energy was fixed at 35%, and resolution was set at 30,000. Data were analyzed using Compound Discoverer (version 3.3), FreeStyle (version 1.8), and TraceFinder (version 5.1) from Thermo Fisher. To correct for natural isotope abundances in labeled samples, a set of unlabeled samples was processed using 12C-glucose in place of 13C-labeled glucose. FluxFix Isotopologue Analysis Tool49 (version 0.1) was used to calculate mass isotopologue distributions.
Glycan profiling by flow cytometry
Glycan-binding proteins, consisting of lectins (Vector Laboratories), antibodies, and the SiaFind engineered protein (Lectenz), were prepared in BD Pharmingen Stain Buffer. A total of 100,000 cells were incubated with 2 µg/mL of FITC-conjugated glycan-binding proteins or FITC-conjugated streptavidin for 1 h at 4 °C. Subsequently, samples were run in BD Accuri C6 at a medium flow rate (35 µL/min), and 5000 events were collected in the singlets gate. The data analysis was carried out in FCS Express and are presented as the geometric mean and standard error/deviation.
Bioenergetics analyses
The Seahorse Analysis was conducted using the MitoStress test according to the manufacturer’s instructions. For the determination of nutrient dependence on glucose, L-glutamine, or fatty acids, acute injections of 2 µM UK5099 (mitochondrial pyruvate carrier inhibitor), 3 µM BPTES (glutaminase inhibitor), or 4 µM Etomoxir (carnitine-palmitoyl transferase inhibitor) were administered after six basal measurements. A fourth injection of 30 µM monensin was introduced29,50 to assess ATP production from glycolysis and oxidative phosphorylation. A DMSO injection served as the control.
Statistical analysis
All data are presented as mean ± SD, and all analyses were performed in GraphPad Prism 10. We used two-tailed Student’s t tests to assess the differences between two groups. In instances involving multiple groups, we used a two-way ANOVA with Fisher’s LSD test or Bonferroni post-hoc tests.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the Catalytic Fund for Metabolism Research at the Van Andel Institute. We would like to thank the Mass Spectrometry Core members for their expertise. We also want to thank Alfredo’s thesis advisory committee members, Dr. Russel Jones, Dr. Carrie Graveel, and Dr. Costas Lyissiotis for their guidance throughout the project. Lastly, we want to thank Dr. David M Brass for his assistance in the preparation of this manuscript. Figure 1A, Supplementary Fig. 5, and Supplementary Fig. 7A were created at https://BioRender.com.
Author contributions
Alfredo Reyes-Oliveras: Writing – Original Draft, Conceptualization, Visualization, Methodology, Investigation, Validation, Formal analysis, Data Curation, Writing – Review & Editing. Abigail E. Ellis: Data curation, Validation, Resources. Ryan D. Sheldon: Validation, Supervision, Visualization, Resources. Brian Haab: Review & editing, Supervision, Funding acquisition, Conceptualization.
Peer review
Peer review information
Communications Biology thanks Ruohong Wang, Masamitsu Maekawa and Shyam Lal Mudavath for their contribution to the peer review of this work. Primary Handling Editors: Zheng-Jiang Zhu and Tobias Goris. A peer review file is available.
Data availability
The numerical source data behind the graphs can be found in the Supplementary Datafile. Original uncropped blots are in Supplementary Fig. 5A-B. All other data are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-07277-0.
References
- 1.Reily, C., Stewart, T. J., Renfrow, M. B. & Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol.15, 346–366 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen, X., Niu, N. & Xue, J. Oncogenic KRAS triggers metabolic reprogramming in pancreatic ductal adenocarcinoma. J. Transl. Intern. Med.11, 322–329 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li, J.-T., Wang, Y.-P., Yin, M. & Lei, Q.-Y. Metabolism remodeling in pancreatic ductal adenocarcinoma. Cell Stress3, 361 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araujo, L., Khim, P., Mkhikian, H., Mortales, C.-L. & Demetriou, M. Glycolysis and glutaminolysis cooperatively control T cell function by limiting metabolite supply to N-glycosylation. eLife6, e21330 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucena, M. C. et al. Epithelial Mesenchymal Transition Induces Aberrant Glycosylation through Hexosamine Biosynthetic Pathway Activation*. J. Biol. Chem.291, 12917–12929 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rossi, M. et al. PHGDH heterogeneity potentiates cancer cell dissemination and metastasis. Nature605, 747–753 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alisson-Silva, F. et al. Increase of O-Glycosylated Oncofetal Fibronectin in High Glucose-Induced Epithelial-Mesenchymal Transition of Cultured Human Epithelial Cells. PLoS ONE8, e60471 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma, E. H. et al. Metabolic Profiling Using Stable Isotope Tracing Reveals Distinct Patterns of Glucose Utilization by Physiologically Activated CD8+ T Cells. Immunity51, 856–870.e5 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and Isotope Tracing. Cell173, 822–837 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheldon, R. D., Ma, E. H., DeCamp, L. M., Williams, K. S. & Jones, R. G. Interrogating in vivo T-cell metabolism in mice using stable isotope labeling metabolomics and rapid cell sorting. Nat. Protoc.16, 4494–4521 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Kim, J. et al. The hexosamine biosynthesis pathway is a targetable liability in KRAS/LKB1 mutant lung cancer. Nat. Metab.2, 1401–1412 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scherpenzeel, M. et al. Dynamic tracing of sugar metabolism reveals the mechanisms of action of synthetic sugar analogs. Glycobiology32, cwab106 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conte, F. et al. Isotopic Tracing of Nucleotide Sugar Metabolism in Human Pluripotent Stem Cells. Cells12, 1765 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cebin, A. V., Komes, D. & Ralet, M.-C. Development and Validation of HPLC-DAD Method with Pre-Column PMP Derivatization for Monomeric Profile Analysis of Polysaccharides from Agro-Industrial Wastes. Polym.-basel14, 544 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang, W. et al. Optimization of reactions between reducing sugars and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology. Food Chem.254, 158–164 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Xu, G., Amicucci, M. J., Cheng, Z., Galermo, A. G. & Lebrilla, C. B. Revisiting monosaccharide analysis – quantitation of a comprehensive set of monosaccharides using dynamic multiple reaction monitoring. Analyst143, 200–207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Honda, S. et al. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem.180, 351–357 (1989). [DOI] [PubMed] [Google Scholar]
- 18.Harazono, A. et al. A comparative study of monosaccharide composition analysis as a carbohydrate test for biopharmaceuticals. Biologicals39, 171–180 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Kim, S., Kim, S. I., Ha, K.-S. & Leem, S.-H. An improved method for quantitative sugar analysis of glycoproteins. Exp. Mol. Med.32, 141–145 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Klamer, Z., Hsueh, P., Ayala-Talavera, D. & Haab, B. Deciphering Protein Glycosylation by Computational Integration of On-chip Profiling, Glycan-array Data, and Mass Spectrometry* [S]. Mol. Cell Proteom.18, 28–40 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spiro, R. G. & Bhoyroo, V. D. Structure of the O-glycosidically linked carbohydrate units of fetuin. J. Biol. Chem.249, 5704–5717 (1974). [PubMed] [Google Scholar]
- 22.Schjoldager, K. T., Narimatsu, Y., Joshi, H. J. & Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol.21 (2020). [DOI] [PubMed]
- 23.Cumin, C. et al. Glycosphingolipids are mediators of cancer plasticity through independent signaling pathways. Cell Rep.40, 111181 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Kim, P. K. et al. Hyaluronic acid fuels pancreatic cancer cell growth. Elife10, e62645 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akella, N. M., Ciraku, L. & Reginato, M. J. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol.17, 52 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daemen, A. et al. Metabolite profiling stratifies pancreatic ductal adenocarcinomas into subtypes with distinct sensitivities to metabolic inhibitors. Proc. Natl. Acad. Sci.112, E4410–E4417 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holst, S., Belo, A. I., Giovannetti, E., Die, Ivan & Wuhrer, M. Profiling of different pancreatic cancer cells used as models for metastatic behaviour shows large variation in their N-glycosylation. Sci. Rep.-uk7, 16623 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang, T. et al. Differential O- and Glycosphingolipid Glycosylation in Human Pancreatic Adenocarcinoma Cells With Opposite Morphology and Metastatic Behavior. Front. Oncol.10, 732 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mookerjee, S. A., Gerencser, A. A., Nicholls, D. G. & Brand, M. D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem.292, 7189–7207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jia, D. et al. Towards decoding the coupled decision-making of metabolism and epithelial-to-mesenchymal transition in cancer. Br. J. Cancer124, 1902–1911 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce, E. L., Poffenberger, M. C., Chang, C.-H. & Jones, R. G. Fueling Immunity: Insights into Metabolism and Lymphocyte Function. Science342, 1242454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gubser, P. M. et al. Rapid effector function of memory CD8+ T cells requires an immediate-early glycolytic switch. Nat. Immunol.14, 1064–1072 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Klysz, D. et al. Glutamine-dependent α-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci. Signal.8, ra97 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Michalek, R. D. et al. Cutting Edge: Distinct Glycolytic and Lipid Oxidative Metabolic Programs Are Essential for Effector and Regulatory CD4+ T Cell Subsets. J. Immunol.186, 3299–3303 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi, L. Z. et al. HIF1α–dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J. Exp. Med.208, 1367–1376 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou, Q. & Qiu, H. The Mechanistic Impact of N-Glycosylation on Stability, Pharmacokinetics, and Immunogenicity of Therapeutic Proteins. J. Pharm. Sci.108, 1366–1377 (2019). [DOI] [PubMed] [Google Scholar]
- 37.GC, S., Bellis, S. L. & Hjelmeland, A. B. ST6Gal1: Oncogenic signaling pathways and targets. Front. Mol. Biosci.9, 962908 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, J. et al. Glycolysis regulates KRAS plasma membrane localization and function through defined glycosphingolipids. Nat. Commun.14, 465 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bassagañas, S. et al. Pancreatic Cancer Cell Glycosylation Regulates Cell Adhesion and Invasion through the Modulation of α2β1 Integrin and E-Cadherin Function. Plos One9, e98595 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai, X., Thinn, A. M. M., Wang, Z., Shan, H. & Zhu, J. The importance of N-glycosylation on β3 integrin ligand binding and conformational regulation. Sci. Rep.7, 4656 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cagnoni, A. J., Sáez, J. M. P., Rabinovich, G. A. & Mariño, K. V. Turning-Off Signaling by Siglecs, Selectins, and Galectins: Chemical Inhibition of Glycan-Dependent Interactions in Cancer. Front. Oncol.6, 109 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beaman, E.-M., Carter, D. R. F. & Brooks, S. A. GALNTs: master regulators of metastasis-associated epithelial-mesenchymal transition (EMT)? Glycobiology32, 556–579 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Comelli, E. M. et al. Activation of Murine CD4+ and CD8+ T Lymphocytes Leads to Dramatic Remodeling of N-Linked Glycans. J. Immunol.177, 2431–2440 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Zeng, J. et al. Cosmc controls B cell homing. Nat. Commun.11, 3990 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagafuku, M. et al. CD4 and CD8 T cells require different membrane gangliosides for activation. Proc. Natl Acad. Sci.109, E336–E342 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Togayachi, A. et al. Polylactosamine on glycoproteins influences basal levels of lymphocyte and macrophage activation. Proc. Natl Acad. Sci.104, 15829–15834 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yamamoto, F., Clausen, H., White, T., Marken, J. & Hakomori, S. Molecular genetic basis of the histo-blood group ABO system. Nature345, 229–233 (1990). [DOI] [PubMed] [Google Scholar]
- 48.Nakayama, F. et al. CD15 Expression in Mature Granulocytes Is Determined by α1,3-Fucosyltransferase IX, but in Promyelocytes and Monocytes by α1,3-Fucosyltransferase IV*. J. Biol. Chem.276, 16100–16106 (2001). [DOI] [PubMed] [Google Scholar]
- 49.Trefely, S., Ashwell, P. & Snyder, N. W. FluxFix: automatic isotopologue normalization for metabolic tracer analysis. Bmc Bioinforma.17, 485 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mookerjee, S. A., Nicholls, D. G. & Brand, M. D. Determining Maximum Glycolytic Capacity Using Extracellular Flux Measurements. Plos One11, e0152016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The numerical source data behind the graphs can be found in the Supplementary Datafile. Original uncropped blots are in Supplementary Fig. 5A-B. All other data are available from the corresponding author upon reasonable request.