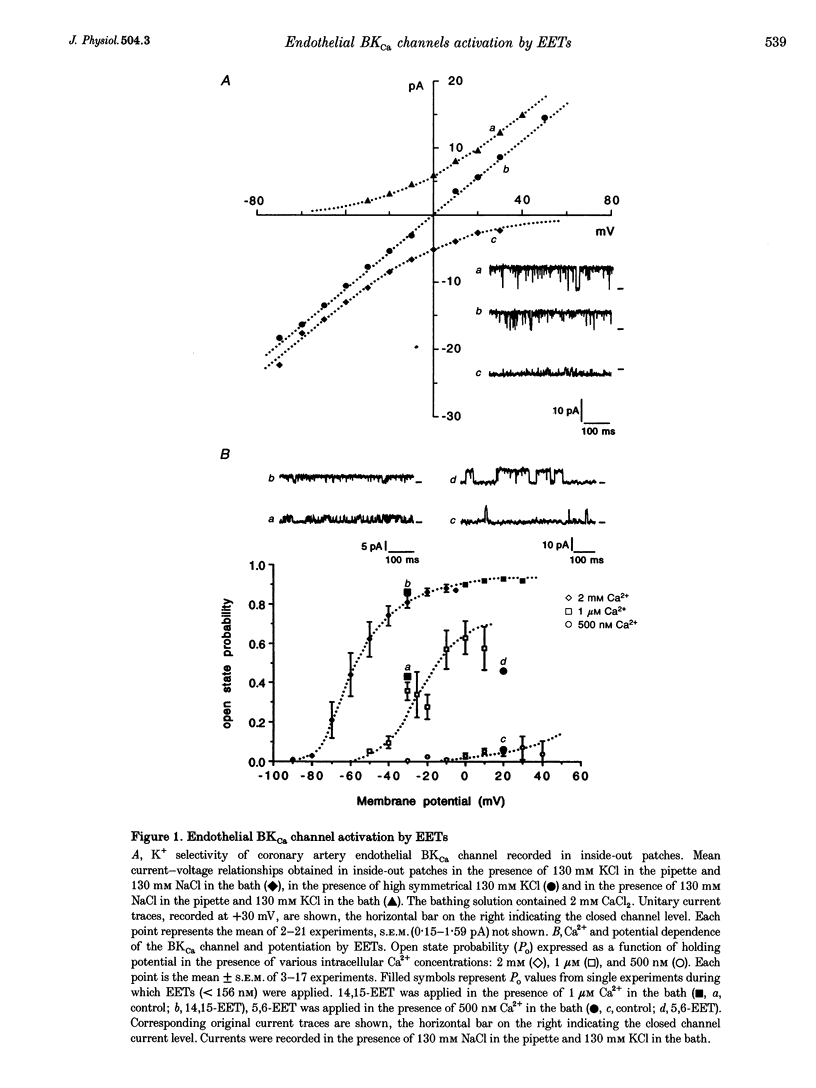
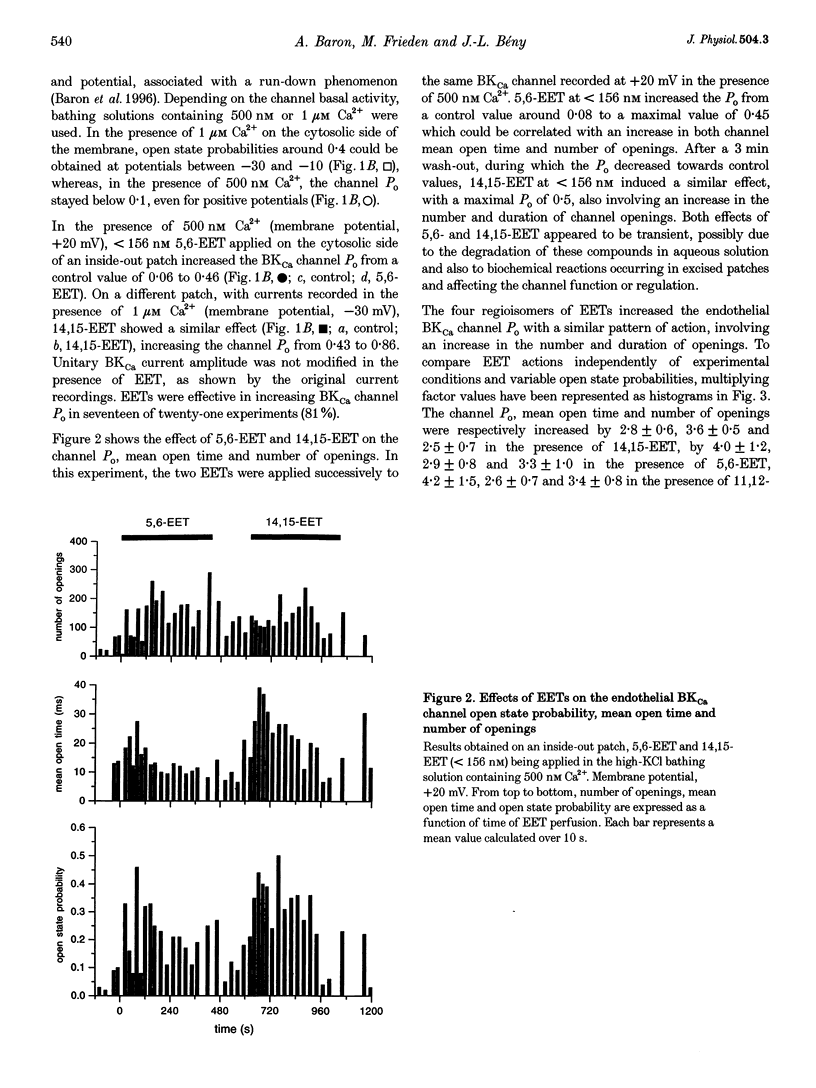
Abstract
1. Epoxyeicosatrienoic acids (EETs) have been described as endothelium-derived hyperpolarizing factors (EDHFs), based on their stimulatory effects on smooth muscle K+ channels. In order to reveal a putative autocrine effect of EETs on endothelial channels, we have studied the effects of the four EET regioisomers (5,6-EET, 8,9-EET, 11,12-EET and 14,15-EET) on the high-conductance, Ca(2+)-dependent K+ (BKCa) channel recorded in inside-out patches of primary cultured pig coronary artery endothelial cells. Currents were recorded in the presence of either 500 nm or 1 microM free Ca2+ on the cytosolic side of the membrane. 2. In 81% of experiments, EETs at < 156 nM, applied on the cytosolic side of the membrane, transiently increased BKCa channel open state probability (PO) without affecting its unitary conductance, thus providing evidence for direct action of EETs, without involvement of a cytosolic transduction pathway. 3. The four EET regioisomers appeared to be equally active, multiplying the BKCa channel PO by a mean factor of 4.3 +/- 0.6 (n = 15), and involving an increase in the number and duration of openings. 4. The EET-induced increase in BKCa channel activity was more pronounced with low initial PO. When the BKCa channel was activated by 500 nM Ca2+, application of EETs increased the initial PO value of below 0.1 by a factor of 5. When the channel was activated by 1 microM Ca2+, application of EETs increased the initial PO value by a factor of 3. 5. Our results show that EETs potentiate endothelial BKCa channel activation by Ca2+. The autocrine action of EETs on endothelial cells, which occurs in the same concentration range as their action on muscle cells, should therefore fully participate in the vasoactive effects of EETs, and thus be taken into account when considering their putative EDHF function.
Full text
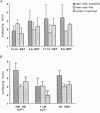
PDF

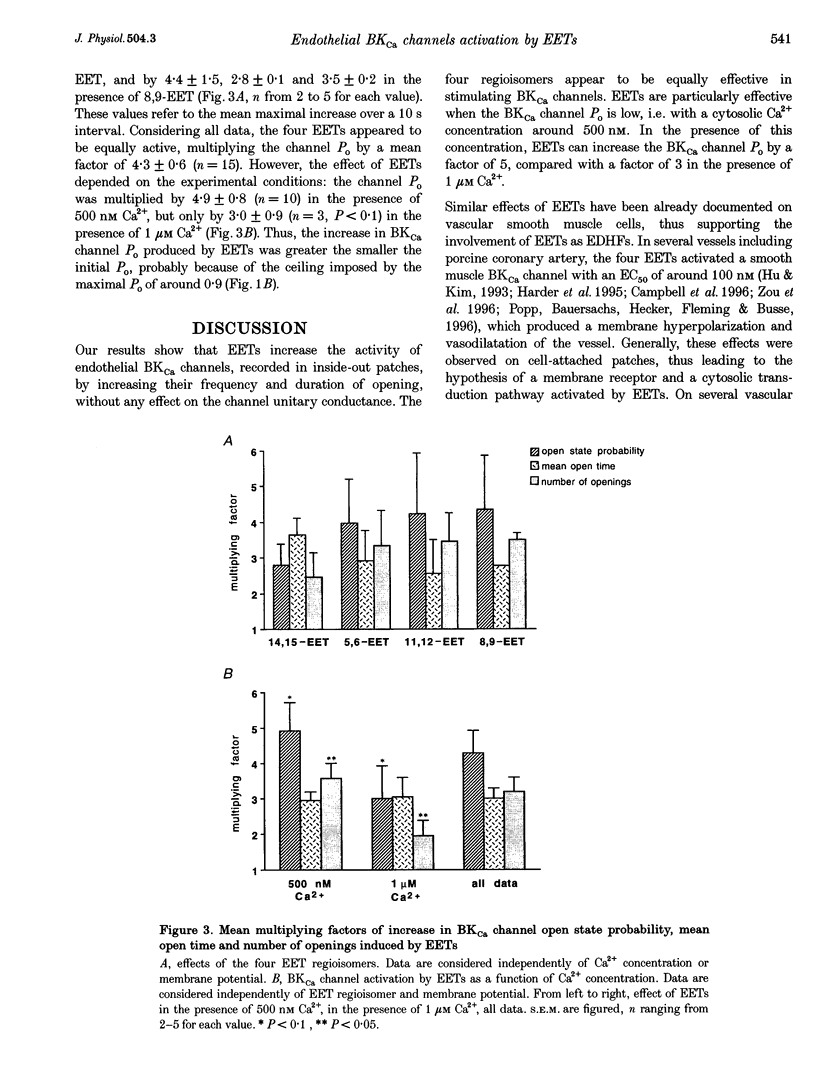


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron A., Frieden M., Chabaud F., Bény J. L. Ca(2+)-dependent non-selective cation and potassium channels activated by bradykinin in pig coronary artery endothelial cells. J Physiol. 1996 Jun 15;493(Pt 3):691–706. doi: 10.1113/jphysiol.1996.sp021415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauersachs J., Hecker M., Busse R. Display of the characteristics of endothelium-derived hyperpolarizing factor by a cytochrome P450-derived arachidonic acid metabolite in the coronary microcirculation. Br J Pharmacol. 1994 Dec;113(4):1548–1553. doi: 10.1111/j.1476-5381.1994.tb17172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley B. J., Barchowsky A., Dolor R. J., Whorton A. R. Regulation of arachidonic acid release in vascular endothelium. Ca(2+)-dependent and -independent pathways. Biochem J. 1991 Dec 1;280(Pt 2):281–287. doi: 10.1042/bj2800281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bény J. L., Brunet P. C. Neither nitric oxide nor nitroglycerin accounts for all the characteristics of endothelially mediated vasodilatation of pig coronary arteries. Blood Vessels. 1988;25(6):308–311. [PubMed] [Google Scholar]
- Campbell W. B., Gebremedhin D., Pratt P. F., Harder D. R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996 Mar;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Gebremedhin D., Ma Y. H., Falck J. R., Roman R. J., VanRollins M., Harder D. R. Mechanism of action of cerebral epoxyeicosatrienoic acids on cerebral arterial smooth muscle. Am J Physiol. 1992 Aug;263(2 Pt 2):H519–H525. doi: 10.1152/ajpheart.1992.263.2.H519. [DOI] [PubMed] [Google Scholar]
- Graier W. F., Simecek S., Sturek M. Cytochrome P450 mono-oxygenase-regulated signalling of Ca2+ entry in human and bovine endothelial cells. J Physiol. 1995 Jan 15;482(Pt 2):259–274. doi: 10.1113/jphysiol.1995.sp020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Campbell W. B., Roman R. J. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. J Vasc Res. 1995 Mar-Apr;32(2):79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- Hecker M., Bara A. T., Bauersachs J., Busse R. Characterization of endothelium-derived hyperpolarizing factor as a cytochrome P450-derived arachidonic acid metabolite in mammals. J Physiol. 1994 Dec 1;481(Pt 2):407–414. doi: 10.1113/jphysiol.1994.sp020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Kim H. S. Activation of K+ channel in vascular smooth muscles by cytochrome P450 metabolites of arachidonic acid. Eur J Pharmacol. 1993 Jan 12;230(2):215–221. doi: 10.1016/0014-2999(93)90805-r. [DOI] [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Popp R., Bauersachs J., Hecker M., Fleming I., Busse R. A transferable, beta-naphthoflavone-inducible, hyperpolarizing factor is synthesized by native and cultured porcine coronary endothelial cells. J Physiol. 1996 Dec 15;497(Pt 3):699–709. doi: 10.1113/jphysiol.1996.sp021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor K. G., Falck J. R., Capdevila J. Intestinal vasodilation by epoxyeicosatrienoic acids: arachidonic acid metabolites produced by a cytochrome P450 monooxygenase. Circ Res. 1987 Jan;60(1):50–59. doi: 10.1161/01.res.60.1.50. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M., Campbell W. B. Role of PGI2 and epoxyeicosatrienoic acids in relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993 Feb;264(2 Pt 2):H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- Rosolowsky M., Falck J. R., Campbell W. B. Synthesis and biological activity of epoxyeicosatrienoic acids (EETs) by cultured bovine coronary artery endothelial cells. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:213–216. [PubMed] [Google Scholar]
- Rosolowsky M., Falck J. R., Willerson J. T., Campbell W. B. Synthesis of lipoxygenase and epoxygenase products of arachidonic acid by normal and stenosed canine coronary arteries. Circ Res. 1990 Mar;66(3):608–621. doi: 10.1161/01.res.66.3.608. [DOI] [PubMed] [Google Scholar]
- Zhu Y., Schieber E. B., McGiff J. C., Balazy M. Identification of arachidonate P-450 metabolites in human platelet phospholipids. Hypertension. 1995 Apr;25(4 Pt 2):854–859. doi: 10.1161/01.hyp.25.4.854. [DOI] [PubMed] [Google Scholar]
- Zou A. P., Fleming J. T., Falck J. R., Jacobs E. R., Gebremedhin D., Harder D. R., Roman R. J. Stereospecific effects of epoxyeicosatrienoic acids on renal vascular tone and K(+)-channel activity. Am J Physiol. 1996 May;270(5 Pt 2):F822–F832. doi: 10.1152/ajprenal.1996.270.5.F822. [DOI] [PubMed] [Google Scholar]