Abstract
Strains of Pseudomonas aeruginosa causing keratitis can be either cytotoxic (6206) or invasive (6294), while a strain (Paer1) causing contact lens-induced acute red eye has been shown to be neither. In situ hybridization was used to examine the location and identity of cells expressing interleukin-6 (IL-6) mRNA in the murine cornea and changes in expression in response to infection with different strains of P. aeruginosa. The number of IL-6-positive cells was determined by image analysis. IL-6 protein levels were measured by an enzyme-linked immunosorbent assay. BALB/c mice were challenged by use of the wounded-cornea model with P. aeruginosa 6294, 6206, or Paer1 (2 × 106 CFU). At time intervals up to 24 h, postchallenge corneal tissue was probed for IL-6 mRNA. IL-6 mRNA expression was rapidly elevated in the epithelium in response to strains 6294 and 6206. At the conclusion of the experiments, infiltrating inflammatory cells also stained positively for IL-6 mRNA. In contrast, corneas challenged with strain Paer1 showed significant upregulation of IL-6 mRNA only at 4 h postchallenge. Three distinct patterns of IL-6 mRNA expression in the mouse cornea occur in response to these three ocular isolates of P. aeruginosa. The data obtained for mRNA expression in the cornea for all three strains of P. aeruginosa correlated well with IL-6 protein analysis of whole-eye homogenates. Differences in the cytokine responses to these strains correlate with differences in the pathology associated with each strain and may offer an opportunity to develop strategies for the improved management of ocular inflammation.
Pseudomonas aeruginosa is an opportunistic bacterial pathogen capable of causing severe corneal infection, often leading to blindness. It is not part of the normal ocular microbiota (30) but is the pathogen most commonly involved in bacterial keratitis associated with the use of contact lenses (2, 5). P. aeruginosa does not infect experimental animals with an intact corneal epithelium unless the host is otherwise compromised (13, 14) but does adhere strongly to injured corneal epithelial cells (32). The outcome of the inflammatory response to an invading pathogen is determined by multiple host-associated and microbial factors. It has been suggested by various groups that host inflammatory responses play an important role in the outcome of ocular infection with P. aeruginosa (33, 35). Regulation of these responses is important for the maintenance of the integrity and transparency of the cornea. Unlike most mucosal surfaces, the normal cornea contains no blood vessels or lymphatics; therefore, the immune mechanisms are different from those of most tissues and, consequently, the cornea is vulnerable to infection once the protective epithelial layer is damaged. Very little is known about the exact nature of the inflammatory mediators induced by P. aeruginosa in the cornea in vivo.
There are several lines of evidence indicating an important role for interleukin-6 (IL-6) in corneal infection and inflammation. IL-6 is a multifunctional cytokine sharing a number of overlapping functions with the proinflammatory cytokines, e.g., IL-1 and tumor necrosis factor (1). Its production could influence a number of immunological activities within the eye. IL-6 is produced at low levels by unstimulated corneal cells in cultures (7, 28); this fact suggests that resident corneal cells are capable of producing IL-6 constitutively. IL-6 levels become rapidly elevated in whole-eye homogenates after challenge with P. aeruginosa (20) or with herpes simplex virus (31). Further, intravitreal injection of IL-6 produces uveitis (17). We have recently demonstrated that IL-6 can be found in the tears of subjects experiencing corneal inflammation during contact lens wear (34). On the other hand, IL-6 may also play a regulatory role, as it inhibits IL-1 and tumor necrosis factor production, dampens the inflammatory response, and possibly reduces damage to the ocular surface (1). IL-6 is also a regulator of epithelial cell growth and cell-cell adhesion (21). Topical application of IL-6 to wounded rabbit corneas has been shown to facilitate epithelial wound closure, possibly by upregulating the expression of integrins (24). However, it is still unclear whether resident corneal cells or infiltrating inflammatory cells contribute to the upregulation of IL-6.
In this study, in situ hybridization was used to provide information on the kinetics of IL-6 expression and on the location and identity of cells expressing IL-6 mRNA in the cornea of the mouse. The expression of IL-6 mRNA was examined during the inflammatory response to corneal wounding and challenge with P. aeruginosa strains reported to be invasive (6294) or cytotoxic (6206) (11) or neither cytotoxic nor invasive (Paer1) (6) over a 24-h period. These strains can be distinguished genetically; only invasive strains possess the gene encoding 49-kDa exoenzyme S (12), whereas only cytotoxic strains possess the gene encoding an approximately 70-kDa protein, ExoU (10).
MATERIALS AND METHODS
Bacterial cultures.
Stock cultures of P. aeruginosa 6294 and 6206, originally isolated from human corneal ulcers, were kindly supplied by Suzanne Fleiszig (University of California, Berkeley). Strain Paer1 was originally isolated from a case of contact lens-induced acute red eye (CLARE) (18). Cultures stored in 30% glycerol at −70°C were inoculated into 10 ml of tryptone soya broth (Oxoid Ltd., Sydney, New South Wales [NSW], Australia). Cultures were incubated at 35°C for 18 h, centrifuged at 2,000 × g for 10 min, washed three times with phosphate-buffered saline (PBS) (NaCl, 8 g/liter; KCl, 0.2 g/liter; Na2HPO4, 1.15 g/liter; KH2PO4, 0.2 g/liter [pH 7.4]), and suspended in the same buffer to a concentration of 4 × 108 CFU/ml (optical density at 660 nm, 0.5). Strain 6294 has been shown to be an invasive strain; i.e., it is able to invade a variety of epithelial cells (including corneal epithelial cells) in in vitro and in vivo experiments (6, 11, 12). Strain 6206 has been shown to cause acute cytotoxic effects in corneal epithelial cells in vitro and ex vivo (11, 12). We have recently demonstrated that strain Paer 1 is neither cytotoxic nor invasive in vitro (6).
Infection of animals.
Inbred 7-week-old BALB/c mice were challenged with P. aeruginosa. They were anesthetized with Avertin (125 mg/kg intraperitoneally), and under a stereomicroscope the corneal surface of only the left eye was incised (two parallel incisions penetrating only the epithelium) with a sterile 27.5-gauge needle. Five microliters of the above bacterial suspension (2.0 × 106 CFU) was pipetted directly onto the wounded cornea, or the eye was mock challenged with PBS only. The right eye of each animal served as a control and was neither scratched nor infected. The animals were monitored during each experiment, and all protocols for animal use were approved by the Animal Care and Ethics Committee, University of New South Wales. Three mice per time point and three mice for each control were used. All experiments were repeated three times.
Preparation of corneal tissue.
Mice were sacrificed at 0, 1.5, 4, and 24 h postchallenge by an overdose of ketamine and/or cervical dislocation. Three replicate experiments were carried out, and at least three mice were used at each time point. During corneal infection with cytotoxic and invasive strains of P. aeruginosa, rapid progression to perforation occurs within 24 h, so the experiments were terminated at this time. For investigation into the pathology associated with the disease, the eyes were immediately removed and whole eyes were fixed in 2.5% (vol/vol) glutaraldehyde in 0.1 M sodium cacodylate (pH 7.2) for 4 h at 4°C. After fixation, the corneas were bisected and the anterior segment was collected. The excised corneas were rinsed in buffer, dehydrated in three changes of cold absolute acetone, and embedded in Historesin Plus (Leica, Sydney, NSW, Australia). Sections were then collected at a 3-μm thickness with a Reichert-Jung Ultracut microtome (C. Reichert Optische Werk AG, Vienna, Austria), stained with hematoxylin and eosin, and examined by light microscopy. Sections were examined for the presence of bacteria and polymorphonuclear leukocytes, the morphology of the corneal layers (epithelium, stroma, and endothelium), and the degree of ulceration.
For examination of sections by in situ hybridization, the eyes were immediately enucleated, fixed in neutral buffered formalin, and embedded in paraffin. Five-micrometer sections were cut, mounted onto RNase-free glass slides coated with 3-aminopropyl-triethoxy silane (Sigma Aldrich, Sydney, NSW, Australia), and air dried at 37°C for 2 h. The sections were rehydrated through a graded series of ethanol with diethyl pyrocarbonate (DEPC; Sigma Aldrich)-treated water. Target mRNA was exposed by digestion of tissue sections on slides with 10 μg of proteinase K (Sigma Aldrich) per ml in 50 mM Tris (pH 7.5) at 37°C for 20 min. The digestion was stopped by two 3-min washes in DEPC-treated water. The sections were then dehydrated through a graded series of ethanol and air dried at 37°C for 1 h.
Synthesis of Dig-labelled cRNA probes.
A cDNA clone for a murine IL-6 probe was generously provided by J. Van Snick (Ludwig Institute for Cancer Research, Brussels, Belgium). For transcription of the IL-6 riboprobe, plasmid pBSK(+) was initially linearized by restriction with BamHI (sense orientation, control probe) or EcoRI (antisense orientation, detection probe) followed by transcription with either T3 or T7 RNA polymerase, respectively, and digoxigenin (Dig)-RNA labelling mixture (Boehringer Mannheim, Sydney, NSW, Australia) according to the method outlined by the manufacturer. The synthesis of cRNA was confirmed by electrophoresis. Template DNA was removed by digestion with 20 U of RNase-free DNase I (Promega Biotech, Sydney, NSW, Australia) at 37°C for 15 min. The reaction was stopped by the addition of EDTA (pH 8.0, to 0.02 M), and the Dig-labelled RNA was precipitated in the presence of 0.3 M sodium acetate (pH 6.0) and 2.2 volumes of ethanol at −20°C overnight. The precipitated RNA was recovered by centrifugation, resuspended in 20 μL of DEPC-treated water containing 20 U of RNase inhibitor (Promega Biotech), and stored at −20°C until required.
In situ hybridization.
For in situ hybridization, all buffers were prepared with DEPC-treated water. Prior to hybridization, sections were heated to 60°C for 10 min in a Hybaid slide thermal cycler (Integrated Sciences, Sydney, NSW, Australia) to inhibit endogenous alkaline phosphatases and then were returned to 37°C before incubation with the riboprobe. Sections were hybridized with either the sense (control) or the antisense (detection) Dig-labelled riboprobe in a buffer comprising 0.05 M Tris (pH 7.5), 0.6 M NaCl, 20% (wt/vol) dextran sulfate (molecular weight [MW], 500,000; Sigma Aldrich), 30% (vol/vol) formamide, 0.1% (wt/vol) sodium pyrophosphate, 0.2% (vol/vol) polyvinylpyrrolidone (MW, 40,000), 0.2% (vol/vol) Ficoll (MW, 400,000), and 5 mM EDTA. Sections were overlaid with coverslips and incubated in a humidified slide thermal cycler at 37°C (26) for 18 h.
After hybridization, the coverslips were removed, and the sections were washed in Tris-buffered saline (50 mM Tris, 150 mM NaCl [pH 7.5] containing 0.1% Triton X-100 [TBS-T] filtered twice through a 0.22-μm-pore-size filter) in a Hybaid wash module at 40°C for 40 min each time with regular, gentle agitation. The sections were incubated for 2 h at room temperature in TBS-T containing 20% (vol/vol) fetal calf serum filtered through a 0.22-μm-pore-size filter. Sections were then incubated for 1 h at room temperature in a 1:200 dilution of Fab fragments from a sheep anti-Dig antibody conjugated with alkaline phosphatase (Boehringer Mannheim). Sections were then washed three times in TBS-T for 5 min each time, followed by two washes for 5 min each time in alkaline phosphatase substrate buffer, comprising 100 mM Tris (pH 9.5), 100 mM NaCl, and 50 mM MgCl2. Sections were incubated with 175 μg of 5-bromo-4-chloro-3-indolyl phosphate per ml, 337 μg of nitroblue tetrazolium per ml, and 3 mM Levamisole for 1 h at ambient temperature. When color development was complete, the reaction was stopped by two 5-min washes of sections with water.
Image analysis.
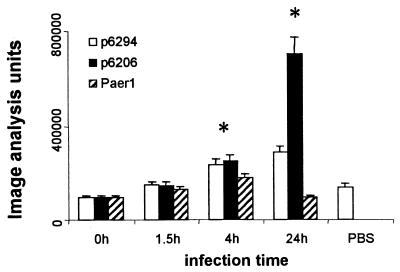
The noncounterstained corneal sections from in situ hybridization experiments were analysed with a Leica Q500 MC image processing and analysis system for the light microscope. Positive targets on the corneal tissues were identified and designated visually on the displayed image. This definition of positive was used for the analysis of all subsequent samples. Sections from a minimum of three corneas per time point were analyzed. The image analysis results are represented as a sum of total IL-6 mRNA-positive areas per corneal cross section from 0 to 24 h after challenge with P. aeruginosa or mock challenge with PBS only (see Fig. 2). The data obtained from the image analysis were examined for significance with the two-tailed, unpaired Student t test.
FIG. 2.
Changes in IL-6 mRNA expression in the corneas of mice after challenge with three strains of P. aeruginosa, 6294, 6206, and Paer1, over time. The amount of IL-6 mRNA was semiquantitated by image analysis and is expressed as image analysis units per section. An asterisk indicates that the value was significantly different from control and 0-h values. Data represent mean image analysis units for three eyes per time point, and error bars represent standard errors of the mean.
Cytokine ELISA.
Samples for the cytokine enzyme-linked immunosorbent assay (ELISA) were collected by sacrificing the mice and enucleating three eyes per sample at intervals of 0, 1, 4, and 24 h. The eyes were homogenized in 1.0 ml of sterile PBS containing 0.05% (vol/vol) Triton X-100 in a sterile tissue grinder. The supernatant was collected by centrifugation at 1,000 × g and 4°C and immediately frozen at −70°C until required for the assay. ELISA kits for murine IL-6 were purchased from R & D Systems (catalog no. M6000; Bioscientific, Sydney, NSW, Australia) and used according to the manufacturer’s directions. Supplied standards were used to generate a standard curve. The absorbance at 450 nm was converted to picograms per milliliter for each cytokine. Data were examined statistically with an unpaired Student t test.
RESULTS
For all experiments, only sections hybridized with the IL-6 antisense riboprobe showed positive hybridization. Consistent patterns of IL-6 mRNA distribution were observed in three replicate experiments, and representative photographs are shown in Fig. 1. Sections hybridized with the IL-6 sense (control) riboprobe showed no color development. Figure 1P shows a typical section hybridized with the control (sense) Dig-labelled probe with procedures identical to those used for the antisense probe but with the control (sense) probe substituted at the hybridization step. Both IL-6 mRNA and protein production in the unchallenged right eye and at the 0-h time point were comparable (data not shown). The quality of the morphology of sections used for in situ hybridization was compromised, due to the necessity to expose mRNA on the sections to proteinase K digestion for in situ hybridization. Moreover, these sections could not be counterstained, as this procedure may obscure the in situ signal and prevent image analysis.
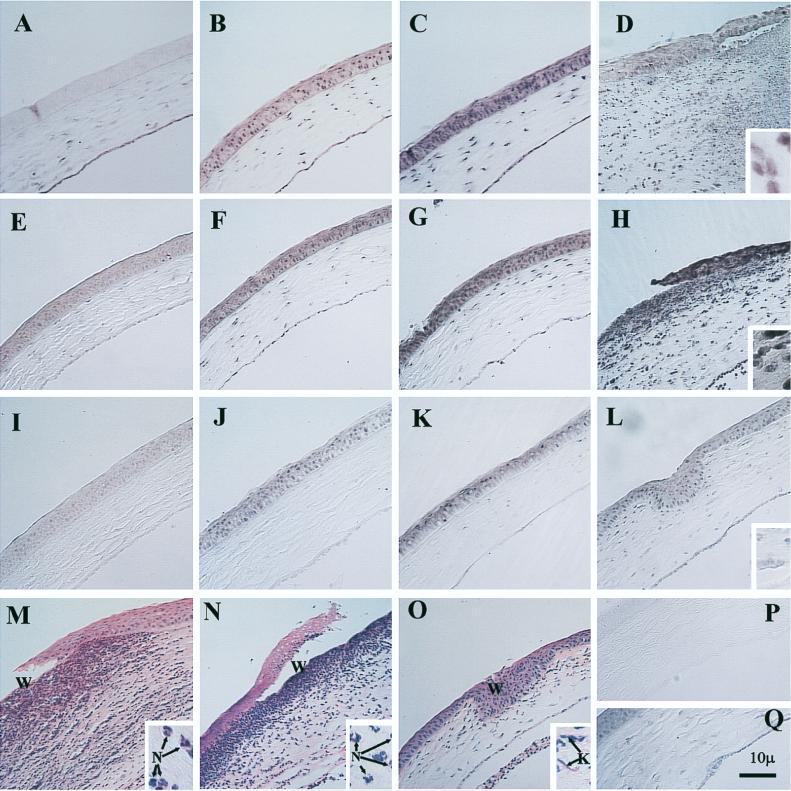
FIG. 1.
IL-6 mRNA expression in the corneas of mice during experimental infection with P. aeruginosa 6294, 6206, or Paer1 at various times. All of the micrographs are at the same magnification, except for the insets, which are at ×1,620. Not all sections are counterstained. Bar, 10 μm. (A) Cornea immediately after challenge with P. aeruginosa 6294, showing IL-6 mRNA expression. (B) Increased IL-6 mRNA expression in the corneal epithelium 1.5 h after challenge with P. aeruginosa 6294. (C) High levels of IL-6 mRNA expression in the cornea 4 h after challenge with P. aeruginosa 6294. (D) Significantly edematous cornea 24 h after infection with P. aeruginosa 6294. Large numbers of inflammatory cells have infiltrated the cornea, with destruction. IL-6 mRNA expression is significantly induced. The inset shows a higher magnification of IL-6 mRNA-positive cells in the stroma at the wound site. (E) Cornea immediately after challenge with strain 6206, showing IL-6 mRNA expression. (F) IL-6 mRNA expression in the corneal epithelium 1.5 h after challenge with strain 6206. (G) Higher levels of IL-6 mRNA expression in the cornea 4 h after challenge with strain 6206. (H) Cornea at 24 h after challenge with strain 6206. Large numbers of inflammatory cells have infiltrated the cornea. There is a loss of epithelial cells at the wound site. Inflammatory cells have also infiltrated the anterior chamber. The inset shows a higher magnification of IL-6 mRNA-positive cells in the stroma at the wound site. (I) Cornea immediately after challenge with Paer1, showing IL-6 mRNA expression. (J) mRNA expression 1.5 h after challenge with Paer1. (K) Induction of IL-6 mRNA expression at 4 h after challenge with Paer1. (L) At 24 h after challenge with Paer1, the cornea shows levels of IL-6 induction comparable to those elicited by PBS. The healed wound site can be seen. The inset shows a higher magnification of keratocytes staining positively for IL-6 mRNA. (M) Hematoxylin-eosin staining of the cornea 24 h after infection with 6294, showing sloughing off of the epithelium at the site of wounding (W) and infiltrating inflammatory cells. The inset shows a higher magnification of infiltrating cells, confirming that they are predominantly neutrophils (N) and that the IL-6 mRNA-positive cells in panel D are largely infiltrating inflammatory cells. (N) Hematoxylin-eosin staining of the cornea 24 h after infection with 6206, showing sloughing off of the epithelium at the site of wounding (W) and infiltrating inflammatory cells. The inset shows a higher magnification of infiltrating cells, confirming that they are predominantly neutrophils (N) and that the IL-6 mRNA-positive cells in panel H are largely infiltrating inflammatory cells. (O) Hematoxylin-eosin staining of the cornea 24 h after challenge with Paer1. The scratch site is fully reepithelialized. The inset shows a higher magnification of keratocytes (K) and occasional neutrophils associated with small focal infiltrates at the wound site. (P) Typical cornea hybridized with Dig-labelled control probe (sense), showing no positive staining in any part of the cornea. (Q) Cornea 24 h after wounding and topical application of PBS, showing levels of IL-6 mRNA induction comparable to those seen at 0 h for all strains.
IL-6 mRNA expression in the cornea during infection with invasive P. aeruginosa 6294.
Low constitutive levels of IL-6 mRNA were detected in the keratocytes and the endothelium of the cornea immediately after scarification and challenge with P. aeruginosa 6294. The central corneal epithelium showed little positive staining (Fig. 1A), although some faint staining of epithelial cells could be seen in the periphery of the cornea.
At 1.5 h after challenge with P. aeruginosa 6294, the expression of IL-6 mRNA in the cornea increased by approximately 1.5-fold, but this change was not statistically significant (Fig. 1B and 2A). At 4 h after challenge (Fig. 1C), there was a further, significant (P, <0.01) increase in IL-6 mRNA staining of approximately 2.5 times above the baseline (Fig. 2). IL-6 mRNA-positive cells were detected throughout the epithelium and showed an increased intensity of staining in comparison to that seen at the start of the experiment. Keratocyte and endothelial staining intensity also increased compared to that at the zero time point.
By 24 h after challenge, there was extensive edema of the cornea, loss of epithelial cells at and beyond the site of wounding, and massive infiltration of the stroma with inflammatory cells. Figure 1D shows these pathological changes as well as a shift in the cells producing IL-6 mRNA from corneal cells to inflammatory cells infiltrating the infected corneal stroma. IL-6 mRNA-producing inflammatory cells could be seen in the corneal stroma (Fig. 1D, inset). There was also a reduction in the intensity of staining in the epithelium at this time. The infiltrating cells in the cornea were confirmed histologically by hematoxylin-eosin staining as predominantly neutrophils (Fig. 1M, inset), in agreement with the observations of others (13, 14). Image analysis of sections probed for IL-6 mRNA showed that both the number and the intensity of cells staining positively for IL-6 mRNA increased during the course of the experiment (Fig. 2). At 24 h postchallenge, there was an overall increase in total positive staining of approximately threefold above the baseline (Fig. 2), and this increase was significant (P, <0.01).
The contribution of IL-6 mRNA production from corneal wounding was estimated in these experiments by use of a mock-challenged cornea. Figure 1Q shows IL-6 mRNA-positive staining in a cornea which was wounded but given a topical application of PBS only. This cornea showed a healed wound site by 24 h, in contrast to those in Fig. 1D and H. The expression of IL-6 mRNA was increased in the epithelium, and the level was similar to that seen between 0 and 1.5 h during infection (Fig. 2A) but was not significantly above the baseline.
IL-6 mRNA expression in the cornea during infection with cytotoxic P. aeruginosa 6206.
Low constitutive levels of IL-6 mRNA were detected in the epithelium, endothelium, and keratocytes of the cornea immediately after challenge with P. aeruginosa 6206 (Fig. 1E).
At 1.5 h after challenge, the expression of IL-6 mRNA in the cornea appeared slightly increased (Fig. 1F), but this change was not statistically significant (Fig. 2). At 4 h postchallenge, IL-6 mRNA-positive cells were detected throughout the epithelium and showed an increased intensity of staining in comparison to that seen at the start of the experiment. Keratocyte and endothelial staining intensity also increased compared to that at the zero time point (Fig. 1G). This induction was confirmed by image analysis, showing a significant increase in IL-6 mRNA staining of greater than 2.5 times (P, <0.01) (Fig. 2).
At 24 h after challenge, the cornea was grossly edematous and showed dense infiltration with inflammatory cells. There was a loss of epithelial cells in the region of the scratch (Fig. 1N, W). IL-6 mRNA expression was observed in the epithelium and in the large numbers of infiltrating inflammatory cells (predominantly neutrophils) (Fig. 1H). Inflammatory cells infiltrating the anterior chamber also stained positively (Fig. 1H). The inset in Fig. 1H shows IL-6 mRNA-positive cells in the stroma at the wound site at a higher magnification. There was a further, significant (P, <0.01) increase in IL-6 mRNA expression of greater than sevenfold above baseline expression, as determined by image analysis (Fig. 2). Cells in the anterior chamber were not included in the image analysis. The inset in Fig. 1N confirms histologically that the infiltrating inflammatory cells were predominantly neutrophils.
IL-6 mRNA expression in the cornea during challenge with P. aeruginosa Paer1.
In situ hybridization studies of the mouse cornea challenged with Paer1 (Fig. 1I to L) showed low levels of IL-6 mRNA production in the corneal epithelium, stromal keratocytes, and endothelium at all the observed time points. Image analysis (Fig. 2) confirmed that there was a significant increase (P, <0.01) in positive staining after 0 h only at the 4-h time point. These results also show that this level of induction was similar to that observed with wounding and application of PBS only to the cornea (Fig. 1Q and 2). The results for this strain of P. aeruginosa contrast with the strong induction of IL-6 mRNA in the corneal epithelium during the early stages of infection with either strain 6294 or strain 6206.
Histology of the cornea during infection.
The hematoxylin-eosin-stained sections demonstrated distinct pathologies for each strain or type of P. aeruginosa examined. For invasive strain 6294, bacteria initially could be seen adherent to the surface of the scratched epithelium (data not shown). Up to the 24-h time point, no polymorphonuclear leukocytes (PMNs) could be seen. At that time point, there was a massive infiltration of the corneal stroma with inflammatory cells that were almost exclusively PMNs. Bacteria could be seen throughout the stroma, and some bacteria were associated with epithelial cells. The epithelium was sloughing off at the wound site (Fig. 1M). For strain 6206 (Fig. 1N), there were more PMNs at the periphery of the cornea than in the central area, and most bacteria were concentrated toward the anterior stroma near the wound site. The epithelial defect in eyes challenged with strain 6206 was not as great as that in eyes challenged with strain 6294. Again, no PMNs were seen up to the 24-h time point. For strain Paer1 (Fig. 1O), a low level of infiltration of PMNs was observed at 24 h, and the epithelium at the initial scratch site had healed. At a higher magnification, no bacteria could be seen in any area of the cornea.
Quantitation of ocular IL-6 protein levels.
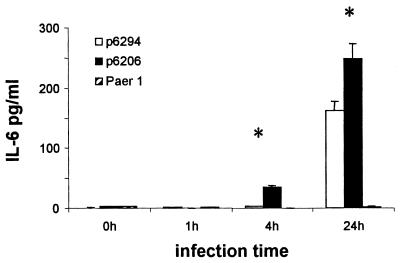
IL-6 protein levels in the eye in response to strain Paer1 were below the limits of detection of the assay used and did not change during the course of the experiment (Fig. 3). In response to strain 6294, there was an approximately 60-fold increase in IL-6 expression at 24 h postchallenge compared to control levels (P, <0.05); levels at earlier time points were below the limits of detection for the assay. IL-6 protein was upregulated by approximately 10-fold at 4 h postchallenge and by approximately 90-fold at 24 h postchallenge in response to infection with the cytotoxic strain 6206. Again, this increase was significant compared to baseline control levels (P, <0.05). These changes correlated well with changes in the expression of IL-6 mRNA (Fig. 2).
FIG. 3.
Amounts of IL-6 protein detected in homogenates of whole eyes of mice infected with strain 6294, 6206, or Paer1. Note the increased presence of IL-6 24 h after infection with strains 6294 and 6206 only. See the legend to Fig. 2 for an explanation of asterisks. Data represent the mean for three eyes per time point, and error bars represent standard errors of the mean.
DISCUSSION
Cytotoxic (6206), invasive (6294), and CLARE (Paer1) strains of P. aeruginosa produce different pathologies after challenge of the cornea (6). The invasive strain (6294) produced extensive damage to the corneal epithelium, and large numbers of PMNs were recruited into the corneal stroma during the infection. The cytotoxic strain (6206) produced less damage to the epithelium during the infection, but again, large numbers of PMNs were recruited into the corneal stroma. During infection with the CLARE strain (Paer1), which is noncytotoxic and noninvasive in vitro (6), the corneal epithelium healed, and only small numbers of PMNs were recruited into the stroma. In situ hybridization has been used to establish the site of IL-6 mRNA production and kinetics of IL-6 mRNA expression in the eye, and such studies have demonstrated that mouse corneas show three patterns of IL-6 mRNA expression in response to these different strains of P. aeruginosa.
Resident ocular cells, i.e., epithelial cells, keratocytes, and endothelial cells, are capable of producing IL-6 mRNA constitutively at a low level, consistent with findings obtained in vitro (28). Constitutive IL-6 mRNA production in the epithelium is predominantly seen in the periphery of the cornea. This observation may be a result of the involvement of IL-6 in the growth of corneal epithelial cells, which takes place in the periphery of the epithelium, or may be due to factors resulting from the proximity of these cells to ocular vasculature. Wounding and infection of the central portion of the cornea with P. aeruginosa strains causing keratitis induced a rapid IL-6 response in resident corneal cells, consistent with that reported by Kernacki and Berk (20) for whole-eye homogenates. The intensity of staining of these resident corneal cells increased locally in response to wounding of the epithelium and during the early phase of the infection, indicating that these cells play a critical role in the corneal response to wounding and bacterial challenge. More recently, Kernacki et al. (19) showed that IL-6 mRNA induction occurs in the cornea as early as 6 h after challenge with P. aeruginosa (19660) by using an RNase protection assay, supporting our findings.
IL-6 mRNA production during infection with invasive P. aeruginosa differed from that seen for keratitis induced with cytotoxic P. aeruginosa 6206 at the 24-h hour time point, and this difference was significant (P, <0.05). Although the increases in positive staining for IL-6 mRNA at 4 h were the same for both strains 6206 and 6294, the increase at 24 h was approximately threefold greater than that seen at baseline in response to the invasive strain, and inflammatory cells were the predominant source. During infection with the cytotoxic strain, the increase above baseline at 24 h postchallenge was greater than sevenfold, and there was little loss of IL-6 mRNA staining in the epithelium, with inflammatory cells representing an additional source of IL-6 mRNA. This finding may be a reflection of differences in the integrity of the corneal epithelium during these infections.
The increase in IL-6 mRNA induction upon challenge with Paer1 was generally similar to that observed with wounding and application of PBS, although there was a significant increase above the control at 4 h after wounding. This finding suggests that in this model, induction of IL-6 mRNA in the presence of Paer1 occurs principally as a result of wounding and wound healing. This notion is supported by the finding that mucosal epithelial cells produce cytokines in response to bacterial adhesion (23), as in a CLARE response, bacteria are found associated only with the contact lens and not with corneal cells (29). The effects of the presence of bacteria on the production of cytokines under these conditions are not known, but bacterial lipopolysaccharide poorly stimulates cytokine production by epithelial cells (9, 16) and the addition of lipopolysaccharide to corneal epithelial cells in vitro does not induce the production of IL-6 (28). However, it has been shown that IL-6 induction by wounding of epithelial cells occurs via a mechanism different from that of infection (15); it is possible that the mechanism for the transitory low-level induction of IL-6 mRNA in response to CLARE challenge principally results from wounding and so may be different from that for IL-6 mRNA induction during keratitis in this model.
Cytokines are very tightly regulated in vivo, and gene transcription does not ensure the production of cytokine proteins. IL-6 protein levels in whole-eye homogenates were determined for all three strains and correlated well with increases in mRNA levels in the corneal tissue, the greatest increase of approximately 90-fold being seen at 24 h in response to strain 6206. No increases in protein levels in response to challenge with Paer1, the CLARE strain, were observed with this assay. The increase in IL-6 protein levels for strain 6206 was significantly different from baseline 4 h after infection, at which time epithelial cells were the predominant source of mRNA for IL-6. These findings may differ slightly from those for IL-6 levels in the cornea alone, which will be determined in future experiments.
The contribution of wounding to IL-6 mRNA production was estimated in this study by the application of PBS to the wounded cornea; the findings were in agreement with low levels of IL-6 induction reported in response to mechanical (22) or chemical (25) trauma of the cornea. The level of IL-6 mRNA production was shown to be significantly different from the level of IL-6 mRNA induction during corneal infection with either invasive or cytotoxic strains at 4 and 24 h postchallenge but not from that induced in response to CLARE strain challenge. It has been reported from in vitro studies that the IL-6 response to trauma and infection in kidney and bladder epithelial cell lines is significantly higher and more prolonged than the response to infection alone (15). However, in in vivo studies, corneal infection does not occur unless the corneal epithelium is damaged (13, 14). There was a low-level induction of IL-6 mRNA expression in the cornea in response to wounding alone. However, the contribution of wounding to the upregulation of IL-6 mRNA expression during bacterial challenge with strains causing keratitis was approximately 20% of the total induction. A mechanism involving the upregulation of integrins on the epithelial cell surface has been proposed for the involvement of IL-6 in corneal wound repair (24).
The precise role played by IL-6 in the cornea in response to P. aeruginosa infection is still unclear. Bacterial infection at mucosal sites, including the eye, is characterized by a rapid influx of predominantly neutrophils into the sites. IL-6 has been reported to be involved in their recruitment via upregulation of ICAM-1 (36) and via priming (4) and regulation (3) of neutrophils at sites of inflammation in other systems. High mortality rates during microbial challenge and increased bacterial loads in IL-6-deficient mice have been correlated with defective neutrophilia (8, 27). These findings suggest that IL-6 may be necessary for optimal neutrophil function. The ability of IL-6 to modulate apoptosis of neutrophils (3) may govern neutrophil-mediated cytotoxicity and organ dysfunction (corneal transparency or opacity) in infectious and inflammatory states. These data and the rapid induction of IL-6 mRNA expression in both corneal tissues and infiltrating inflammatory cells demonstrated here suggest that IL-6 is closely involved in the host corneal response during bacterial challenge and that the mechanism of action may involve the regulation of neutrophil recruitment and neutrophil effector functions.
ACKNOWLEDGMENTS
This study was supported in part by a grant from the Australian Federal Government under the Cooperative Research Centres Programme; Vistakon, a division of Johnson and Johnson Vision Products Inc. (Jacksonville, Fla.); and the Ramacioti Foundation (Sydney, Australia).
REFERENCES
- 1.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 2.Alfonso E, Mandelbaum S, Fox M J, Forster R K. Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol. 1986;101:429–433. doi: 10.1016/0002-9394(86)90641-0. [DOI] [PubMed] [Google Scholar]
- 3.Biffl W L, Moore E E, Moore F A, Barnett C C., Jr Interleukin-6 suppression of neutrophil apoptosis is neutrophil concentration dependent. J Leukoc Biol. 1995;58:582–584. doi: 10.1002/jlb.58.5.582. [DOI] [PubMed] [Google Scholar]
- 4.Borish L, Rosenbaum R, Albury L, Clark S. Activation of neutrophils by recombinant interleukin-6. Cell Immunol. 1989;121:280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- 5.Cohen E J, Laibson P R, Arentsen J J, Clemons C S. Corneal ulcers associated with cosmetic extended wear soft contact lenses. Ophthalmology. 1987;94:109–114. doi: 10.1016/s0161-6420(87)33491-8. [DOI] [PubMed] [Google Scholar]
- 6.Cole N, Willcox M D P, Fleiszig S M J, Stapleton F, Bao S, Tout S, Husband A J. Different strains of Pseudomonas aeruginosa isolated from ocular infections or inflammation display distinct corneal pathologies in an animal model. Exp Eye Res. 1998;18:730–735. [PubMed] [Google Scholar]
- 7.Cubitt C L, Lausch R N, Oakes J E. Differences in interleukin-6 gene expression between cultured human corneal epithelial cells and keratocytes. Investig Ophthalmol Visual Sci. 1995;36:330–336. [PubMed] [Google Scholar]
- 8.Dalrymple S A, Lucian L A, Slattery R, McNeil T, Aud D M, Fuchino S, Lee F, Murray F. Interleukin-6-deficient mice are highly susceptible to Listeria monocytogenes infection: correlation with inefficient neutrophilia. Infect Immun. 1995;63:2262–2268. doi: 10.1128/iai.63.6.2262-2268.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M J, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 11.Fleiszig S M J, Zaidi T S, Preston M J, Grout M, Evans D J, Pier G B. Relationship between cytotoxicity and corneal epithelial cell invasion by clinical isolates of Pseudomonas aeruginosa. Infect Immun. 1996;64:2288–2294. doi: 10.1128/iai.64.6.2288-2294.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiszig S M J, Wiener-Kronish J P, Miyazaki H, Vallas V, Mostov K E, Kanada D, Sawa T, Yen T S B, Frank D W. Pseudomonas aeruginosa-mediated cytotoxicity and invasion correlate with distinct genotypes at the loci encoding exoenzyme S. Infect Immun. 1997;65:579–586. doi: 10.1128/iai.65.2.579-586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerke J R, Magliocco M V. Experimental Pseudomonas aeruginosa infection of the mouse cornea. Infect Immun. 1971;3:209–216. doi: 10.1128/iai.3.2.209-216.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazlett L D, Rosen D, Berk R S. Experimental eye infections caused by Pseudomonas aeruginosa. Ophthalmol Res. 1976;8:311–318. [Google Scholar]
- 15.Hedges S, Agace W, Svensson M, Svanborg C. Cyclosporin A does not inhibit IL-1 alpha-induced epithelial cell IL-6 secretion. Scand J Immunol. 1993;37:581–586. doi: 10.1111/j.1365-3083.1993.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 16.Hedges S R, Bjarnadottir M, Agace W, Hang L, Svanborg C. Immunoregulatory cytokines modify Escherichia coli induced uroepithelial cell IL-6 and IL-8 responses. Cytokine. 1996;8:686–697. doi: 10.1006/cyto.1996.0091. [DOI] [PubMed] [Google Scholar]
- 17.Hoekzema R, Murray P I, van Haren M A, Helle M, Kijlstra A. Analysis of interleukin-6 in endotoxin-induced uveitis. Investig Ophthalmol Visual Sci. 1991;32:88–95. [PubMed] [Google Scholar]
- 18.Holden B A, Grant T, La Hood D, Baleriola-Lucas C, Newton-Howes J, Willcox M D P, Sweeney D F. Gram negative bacteria can induce contact lens related acute red eye (CLARE) CLAO J. 1996;22:47–52. [PubMed] [Google Scholar]
- 19.Kernacki K A, Goebel D J, Poosch M S, Hazlett L D. Early cytokine and chemokine expression during Pseudomonas aeruginosa corneal infection in mice. Infect Immun. 1998;66:376–379. doi: 10.1128/iai.66.1.376-379.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kernacki K A, Berk R S. Characterization of the inflammatory response induced by corneal infection with Pseudomonas aeruginosa. J Ocular Pharmacol. 1994;10:281–288. doi: 10.1089/jop.1994.10.281. [DOI] [PubMed] [Google Scholar]
- 21.Krueger J, Ray A, Tamm I, Sehgal P B. Expression and function of interleukin-6 in epithelial cells. J Cell Biochem. 1991;45:327–334. doi: 10.1002/jcb.240450404. [DOI] [PubMed] [Google Scholar]
- 22.Lausch R N, Chen S-H, Tumpey T M, Su Y-H, Oakes J E. Early cytokine synthesis in the excised mouse cornea. J Interferon Cytokine Res. 1996;16:35–40. doi: 10.1089/jir.1996.16.35. [DOI] [PubMed] [Google Scholar]
- 23.Linder H, Engberg I, Hoschutzky H, Mattsby-Baltzer I, Svanborg C. Adhesion-dependent activation of mucosal interleukin-6 production. Infect Immun. 1991;59:4357–4362. doi: 10.1128/iai.59.12.4357-4362.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishida T, Nakamura M, Mishima H, Otori T, Hikida M. Interleukin 6 facilitates corneal epithelial wound closure in vivo. Arch Ophthalmol. 1992;110:1292–1294. doi: 10.1001/archopht.1992.01080210110036. [DOI] [PubMed] [Google Scholar]
- 25.Planck S R, Rich L F, Ansel J C, Huang X N, Rosenbaum J T. Trauma and alkali burns induce distinct patterns of gene expression in the rat cornea. Ocular Immunol Inflammation. 1997;5:95–100. doi: 10.3109/09273949709085057. [DOI] [PubMed] [Google Scholar]
- 26.Ramsay A J, Husband A J, Ramshaw I A, Bao S, Matthaei K I, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- 27.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in IL-6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakamoto S, Inada K. Human corneal epithelial, stromal and endothelial cells produce interleukin-6. Nippon Ganka Gakkai Zasshi. 1992;96:702–709. [PubMed] [Google Scholar]
- 29.Sankaridurg P R, Willcox M D P, Sharma S, Gopinathan U, Janakiraman D, Hickson S, Vuppala N, Sweeney D F, Rao G N, Holden B A. Haemophilus influenzae adherent to contact lenses is associated with the production of acute ocular inflammation. J Clin Microbiol. 1996;34:2426–2431. doi: 10.1128/jcm.34.10.2426-2431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smolin G, Okumoto M, Nozik R A. The microbial flora in extended-wear soft contact-lens wearers. Am J Ophthalmol. 1979;88:543–547. doi: 10.1016/0002-9394(79)90512-9. [DOI] [PubMed] [Google Scholar]
- 31.Staats H F, Lausch R N. Cytokine expression in vivo during murine herpetic stromal keratitis. Effect of protective antibody therapy. J Immunol. 1993;151:277–283. [PubMed] [Google Scholar]
- 32.Stern G A, Weitzenkorn D, Valenti J. Adherence of Pseudomonas aeruginosa to the mouse cornea. Epithelial v stromal adherence. Arch Ophthalmol. 1982;100:1956–1958. doi: 10.1001/archopht.1982.01030040936014. [DOI] [PubMed] [Google Scholar]
- 33.Steuhl K P, Doring G, Henni A, Thiel H J, Botzenhart K. Relevance of host-derived and bacterial factors in Pseudomonas aeruginosa corneal infections. Investig Ophthalmol Visual Sci. 1987;28:1559–1568. [PubMed] [Google Scholar]
- 34.Thakur A, Willcox M D P. Cytokines and lipid inflammatory mediator profiles of human tears during contact lens associated diseases. Exp Eye Res. 1998;67:9–19. doi: 10.1006/exer.1998.0480. [DOI] [PubMed] [Google Scholar]
- 35.Twining S S, Lohr K M, Moulder J E. The immune system in experimental Pseudomonas keratitis. Investig Ophthalmol Visual Sci. 1986;27:507–515. [PubMed] [Google Scholar]
- 36.Youker K, Smith C W, Anderson D C, Miller D, Michael L H, Rossen R D, Entman M L. Neutrophil adherence to isolated adult cardiac myocytes. J Clin Investig. 1992;89:602–609. doi: 10.1172/JCI115626. [DOI] [PMC free article] [PubMed] [Google Scholar]