Abstract
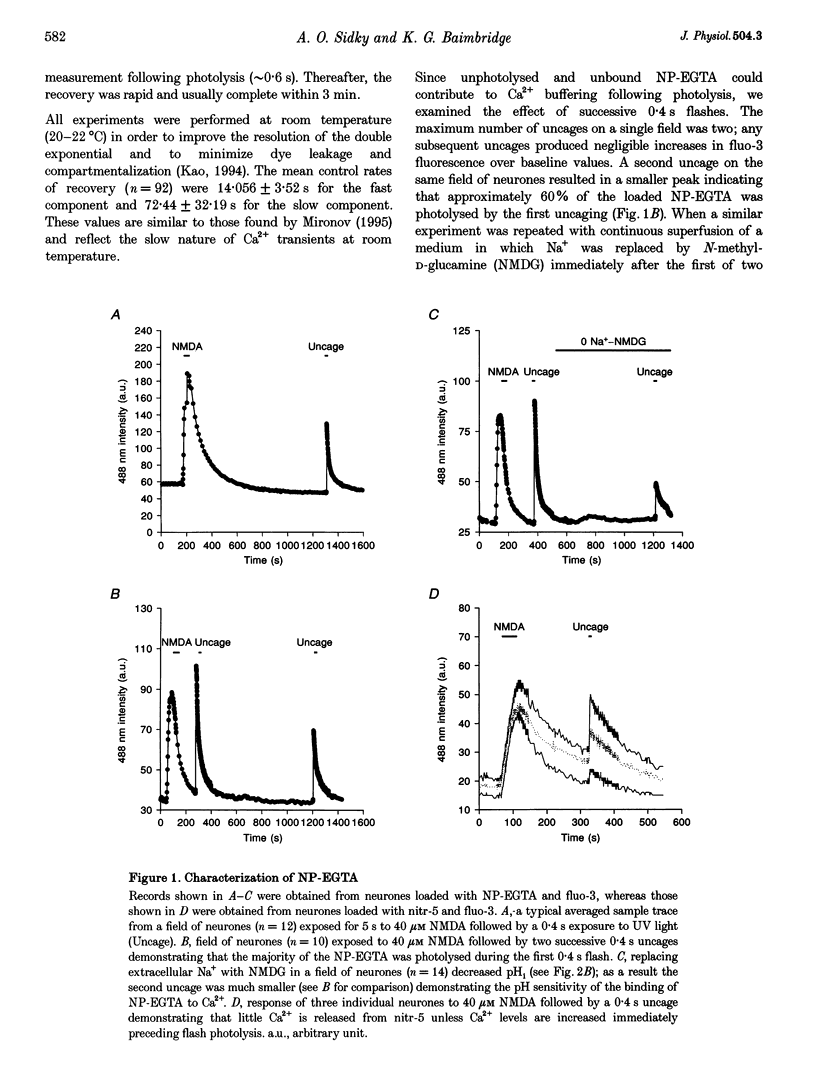
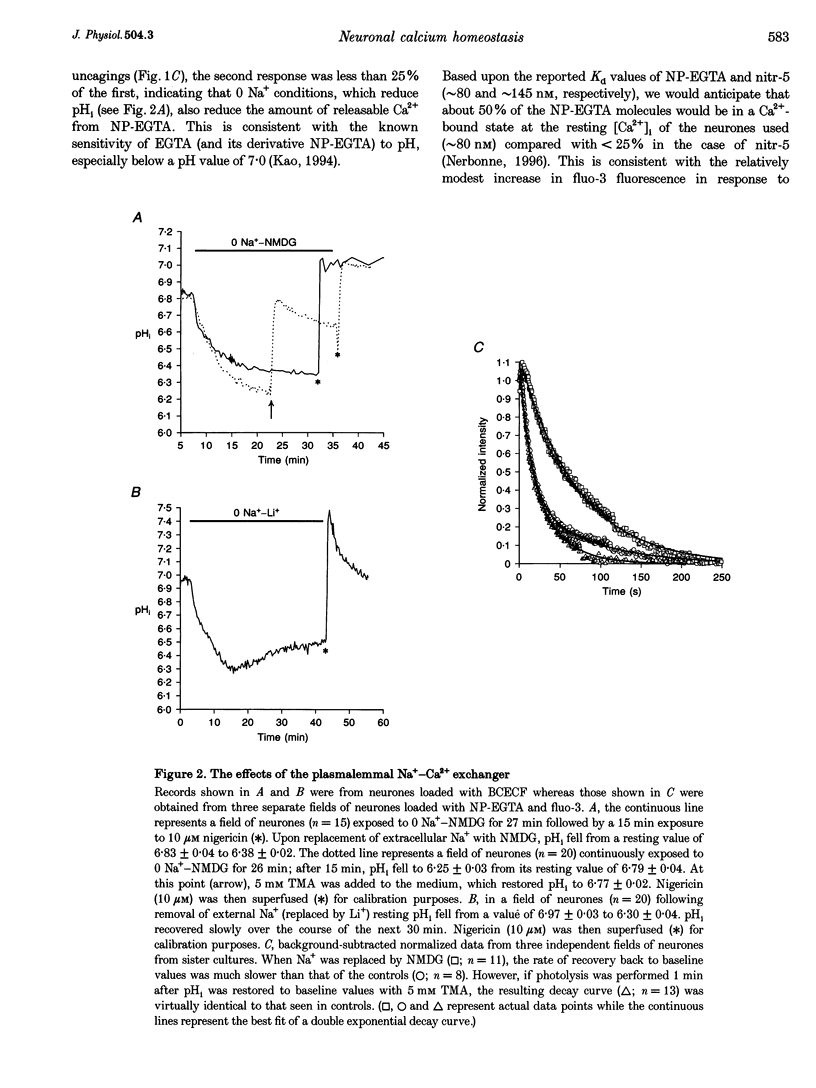
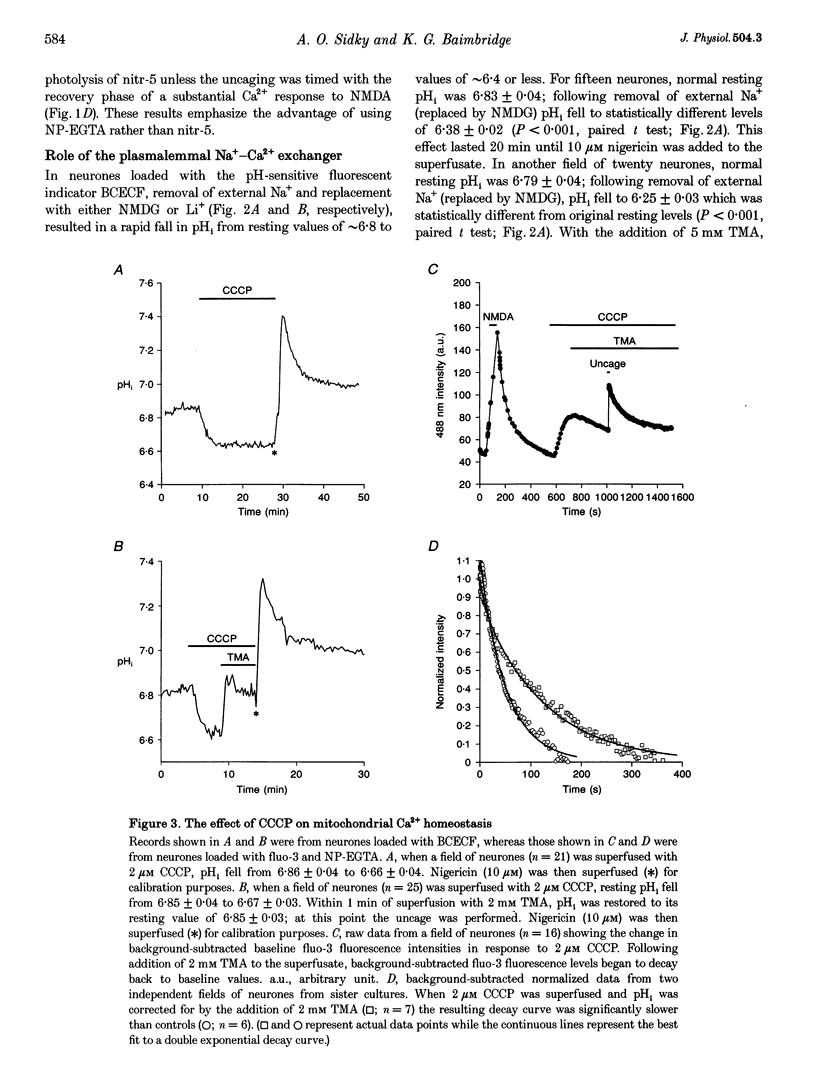
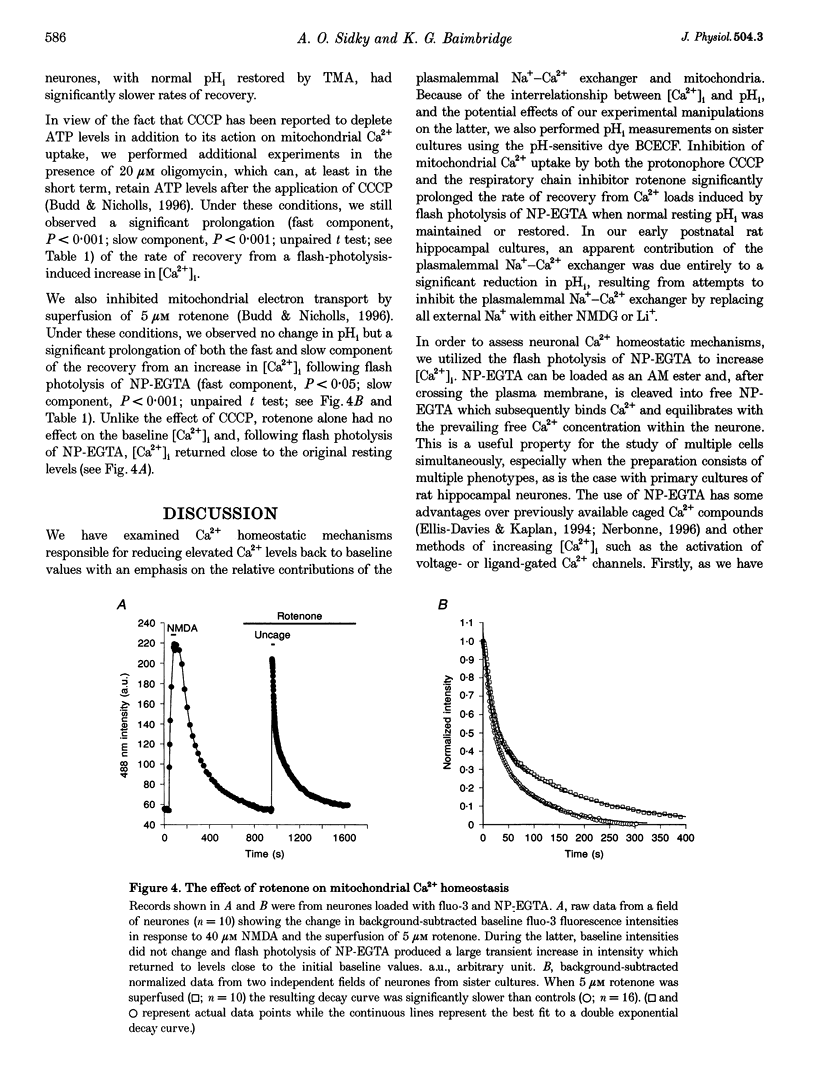
1. We examined Ca2+ homeostatic mechanisms in cultured postnatal rat hippocampal neurones by monitoring the recovery of background-subtracted fluo-3 fluorescence levels at 20-22 degrees C immediately following a rapid increase in Ca2+ levels induced by flash photolysis of the caged Ca2+ compound nitrophenyl-EGTA (NP-EGTA). 2. A variety of methods or drugs were used in attempt to block specifically efflux of Ca2+ by the plasmalemmal Na(+)-Ca2+ exchanger or uptake of Ca2+ into mitochondria. 3. Many of the experimental manipulations produced a decrease in intracellular pH (pHi) measured in sister cultures using the pH-sensitive dye 2',7'-bis-(2-carboxyethyl)-5-(and-6)-carboxyfluorescein (BCECF). Accordingly, in each case, we determined the appropriate amount of the weak base trimethylamine (TMA) required to restore baseline pHi prior to flash photolysis. 4. Blockade of the plasmalemmal Na(+)-Ca2+ exchanger by replacement of external Na+ with either Li+ or N-methyl-D-glucamine (NMDG) markedly reduced pHi but did not affect the rate of recovery of fluo-3 fluorescence intensities once pHi was restored. 5. Inhibition of mitochondrial Ca2+ uptake, using the protonophore carbonyl cyanide m-chloro-phenylhydrazone (CCCP), resulted in a reduction in pHi, which could be restored by the addition of 2 mM TMA. Under these conditions the rate of recovery of Ca2+ levels was significantly slower than in the controls. Similar results were found using the respiratory chain inhibitor rotenone. 6. We conclude that, when the potential effects of changes in pHi are taken into account, mitochondria appear to sequester significant amounts of Ca2+ in the neuronal preparations used.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Calcium regulation by and buffer capacity of molluscan neurons during calcium transients. Cell Calcium. 1988 Apr;9(2):57–69. doi: 10.1016/0143-4160(88)90025-5. [DOI] [PubMed] [Google Scholar]
- Baimbridge K. G., Celio M. R., Rogers J. H. Calcium-binding proteins in the nervous system. Trends Neurosci. 1992 Aug;15(8):303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Baxter K. A., Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J Physiol. 1996 Jun 1;493(Pt 2):457–470. doi: 10.1113/jphysiol.1996.sp021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benham C. D., Evans M. L., McBain C. J. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurones. J Physiol. 1992 Sep;455:567–583. doi: 10.1113/jphysiol.1992.sp019316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevensee M. O., Cummins T. R., Haddad G. G., Boron W. F., Boyarsky G. pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol. 1996 Jul 15;494(Pt 2):315–328. doi: 10.1113/jphysiol.1996.sp021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleakman D., Roback J. D., Wainer B. H., Miller R. J., Harrison N. L. Calcium homeostasis in rat septal neurons in tissue culture. Brain Res. 1993 Jan 15;600(2):257–267. doi: 10.1016/0006-8993(93)91381-2. [DOI] [PubMed] [Google Scholar]
- Budd S. L., Nicholls D. G. A reevaluation of the role of mitochondria in neuronal Ca2+ homeostasis. J Neurochem. 1996 Jan;66(1):403–411. doi: 10.1046/j.1471-4159.1996.66010403.x. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chaillet J. R., Boron W. F. Intracellular calibration of a pH-sensitive dye in isolated, perfused salamander proximal tubules. J Gen Physiol. 1985 Dec;86(6):765–794. doi: 10.1085/jgp.86.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Beaugé L. Physiological role of ATP-driven calcium pump in squid axon. Nature. 1979 Mar 15;278(5701):271–273. doi: 10.1038/278271a0. [DOI] [PubMed] [Google Scholar]
- Ellis-Davies G. C., Kaplan J. H. Nitrophenyl-EGTA, a photolabile chelator that selectively binds Ca2+ with high affinity and releases it rapidly upon photolysis. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):187–191. doi: 10.1073/pnas.91.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambassi G., Hansford R. G., Sollott S. J., Hogue B. A., Lakatta E. G., Capogrossi M. C. Effects of acidosis on resting cytosolic and mitochondrial Ca2+ in mammalian myocardium. J Gen Physiol. 1993 Sep;102(3):575–597. doi: 10.1085/jgp.102.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J., Park Y. B., Babcock D. F., Hille B. Dominant role of mitochondria in clearance of large Ca2+ loads from rat adrenal chromaffin cells. Neuron. 1996 Jan;16(1):219–228. doi: 10.1016/s0896-6273(00)80038-0. [DOI] [PubMed] [Google Scholar]
- Huettner J. E., Baughman R. W. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986 Oct;6(10):3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingersoll R. J., Wasserman R. H. Vitamin D3-induced calcium-binding protein. Binding characteristics, conformational effects, and other properties. J Biol Chem. 1971 May 10;246(9):2808–2814. [PubMed] [Google Scholar]
- Jensen J. R., Rehder V. FCCP releases Ca2+ from a non-mitochondrial store in an identified Helisoma neuron. Brain Res. 1991 Jun 14;551(1-2):311–314. doi: 10.1016/0006-8993(91)90947-t. [DOI] [PubMed] [Google Scholar]
- Kao J. P. Practical aspects of measuring [Ca2+] with fluorescent indicators. Methods Cell Biol. 1994;40:155–181. doi: 10.1016/s0091-679x(08)61114-0. [DOI] [PubMed] [Google Scholar]
- Kennedy H. J., Thomas R. C. Intracellular calcium and its sodium-independent regulation in voltage-clamped snail neurones. J Physiol. 1995 May 1;484(Pt 3):533–548. doi: 10.1113/jphysiol.1995.sp020684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch R. A., Barish M. E. Perturbation of intracellular calcium and hydrogen ion regulation in cultured mouse hippocampal neurons by reduction of the sodium ion concentration gradient. J Neurosci. 1994 May;14(5 Pt 1):2585–2593. doi: 10.1523/JNEUROSCI.14-05-02585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraig R. P., Petito C. K., Plum F., Pulsinelli W. A. Hydrogen ions kill brain at concentrations reached in ischemia. J Cereb Blood Flow Metab. 1987 Aug;7(4):379–386. doi: 10.1038/jcbfm.1987.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Zaguilán R., Parnami G., Lynch R. M. Selection of fluorescent ion indicators for simultaneous measurements of pH and Ca2+. Cell Calcium. 1996 Apr;19(4):337–349. doi: 10.1016/s0143-4160(96)90074-3. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. J. The control of neuronal Ca2+ homeostasis. Prog Neurobiol. 1991;37(3):255–285. doi: 10.1016/0301-0082(91)90028-y. [DOI] [PubMed] [Google Scholar]
- Mironov S. L. Plasmalemmal and intracellular Ca2+ pumps as main determinants of slow Ca2+ buffering in rat hippocampal neurones. Neuropharmacology. 1995 Sep;34(9):1123–1132. doi: 10.1016/0028-3908(95)00080-p. [DOI] [PubMed] [Google Scholar]
- Mironov S. L., Usachev YuM, Lux H. D. Spatial and temporal control of intracellular free Ca2+ in chick sensory neurons. Pflugers Arch. 1993 Jul;424(2):183–191. doi: 10.1007/BF00374610. [DOI] [PubMed] [Google Scholar]
- Nerbonne J. M. Caged compounds: tools for illuminating neuronal responses and connections. Curr Opin Neurobiol. 1996 Jun;6(3):379–386. doi: 10.1016/s0959-4388(96)80123-1. [DOI] [PubMed] [Google Scholar]
- Randall R. D., Thayer S. A. Glutamate-induced calcium transient triggers delayed calcium overload and neurotoxicity in rat hippocampal neurons. J Neurosci. 1992 May;12(5):1882–1895. doi: 10.1523/JNEUROSCI.12-05-01882.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiening C. J., Boron W. F. Regulation of intracellular pH in pyramidal neurones from the rat hippocampus by Na(+)-dependent Cl(-)-HCO3- exchange. J Physiol. 1994 Feb 15;475(1):59–67. doi: 10.1113/jphysiol.1994.sp020049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmigol A., Kirischuk S., Kostyuk P., Verkhratsky A. Different properties of caffeine-sensitive Ca2+ stores in peripheral and central mammalian neurones. Pflugers Arch. 1994 Jan;426(1-2):174–176. doi: 10.1007/BF00374686. [DOI] [PubMed] [Google Scholar]
- Stuenkel E. L. Regulation of intracellular calcium and calcium buffering properties of rat isolated neurohypophysial nerve endings. J Physiol. 1994 Dec 1;481(Pt 2):251–271. doi: 10.1113/jphysiol.1994.sp020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi H., Katayama Y. Regulation of the intracellular free calcium concentration in acutely dissociated neurones from rat nucleus basalis. J Physiol. 1993 May;464:165–181. doi: 10.1113/jphysiol.1993.sp019628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer S. A., Miller R. J. Regulation of the intracellular free calcium concentration in single rat dorsal root ganglion neurones in vitro. J Physiol. 1990 Jun;425:85–115. doi: 10.1113/jphysiol.1990.sp018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth J. L., Thayer S. A. Mitochondria buffer physiological calcium loads in cultured rat dorsal root ganglion neurons. J Neurosci. 1994 Jan;14(1):348–356. doi: 10.1523/JNEUROSCI.14-01-00348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werth J. L., Usachev Y. M., Thayer S. A. Modulation of calcium efflux from cultured rat dorsal root ganglion neurons. J Neurosci. 1996 Feb 1;16(3):1008–1015. doi: 10.1523/JNEUROSCI.16-03-01008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. J., Reynolds I. J. Mitochondria and Na+/Ca2+ exchange buffer glutamate-induced calcium loads in cultured cortical neurons. J Neurosci. 1995 Feb;15(2):1318–1328. doi: 10.1523/JNEUROSCI.15-02-01318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]


