Abstract
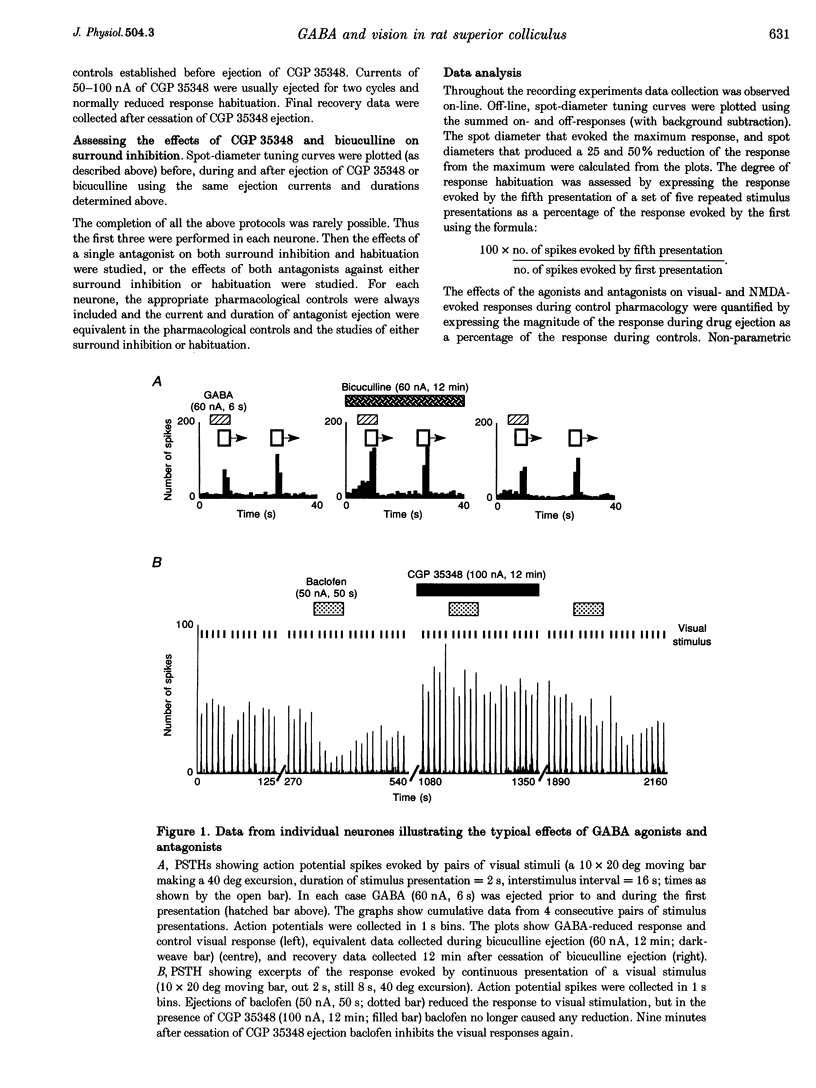
1. The superficial grey layer of the superior colliculus (SGS) contains a high proportion of GABAergic inhibitory neurones. We have investigated the role of GABA receptors in synaptic transmission of aspects of visual activity in the SGS that may be driven by inhibitory mechanisms, such as surround inhibition and response habituation. 2. Multi-barrel glass iontophoretic pipettes were used to record single neuronal activity in the SGS of urethane-anaesthetized rats. Visual stimulation was provided by the display of moving bars and stationary spots of light on a monitor placed in the receptive field. 3. Both ejection of GABA and the GABAB agonist baclofen reduced responses to moving bars (interstimulus intervals > or = 8 s). The effects of GABA were reversed by the GABAA antagonist bicuculline, and the effects of baclofen were antagonized by the GABAB antagonist CGP 35,348. 4. Surround inhibition was estimated by plotting the response to flashed spots of increasing diameter. In controls, expanding the spot diameter beyond the excitatory receptive field caused a decrease in the response. This inhibitory surround was reversibly reduced by bicuculline, but CGP 35,348 had no effect. 5. Response habituation is the progressive reduction in the visual response during repetitive stimulus presentation. In controls, the visual response was reduced to 44 +/- 3% of its initial level when a stimulus (moving bar) was presented 5 times with an interstimulus interval of 0.5 s. During CGP 35,348 ejection, response habituation was reversibly reduced. Bicuculline had no effect on response habituation. 6. The effects of bicuculline on surround inhibition in the superior colliculus are consistent with similar studies in the lateral geniculate nucleus which indicate that GABAA receptors mediate this effect. The function of GABAB receptors in the visual system is less well researched. The reduction of response habituation with CGP 35,348 demonstrates that, at least in the SGS, GABAB receptors have an important role in visual transmission which is distinct from that of GABAA receptors.
Full text
PDF

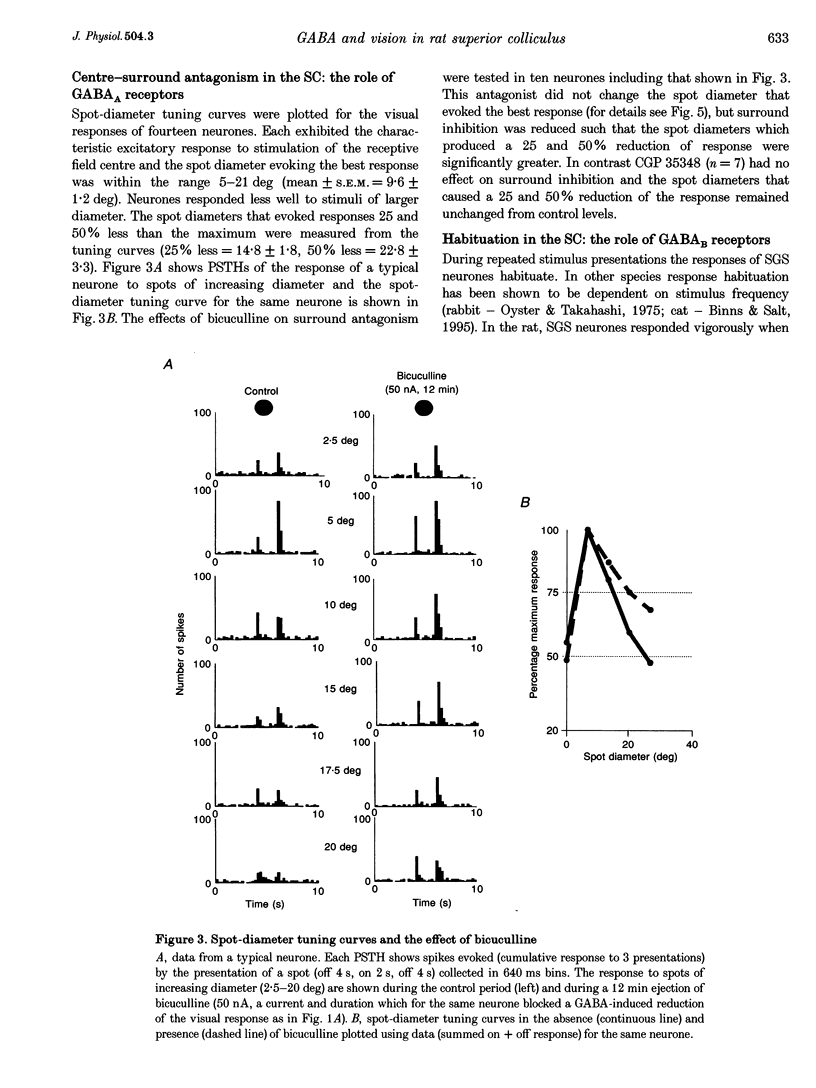
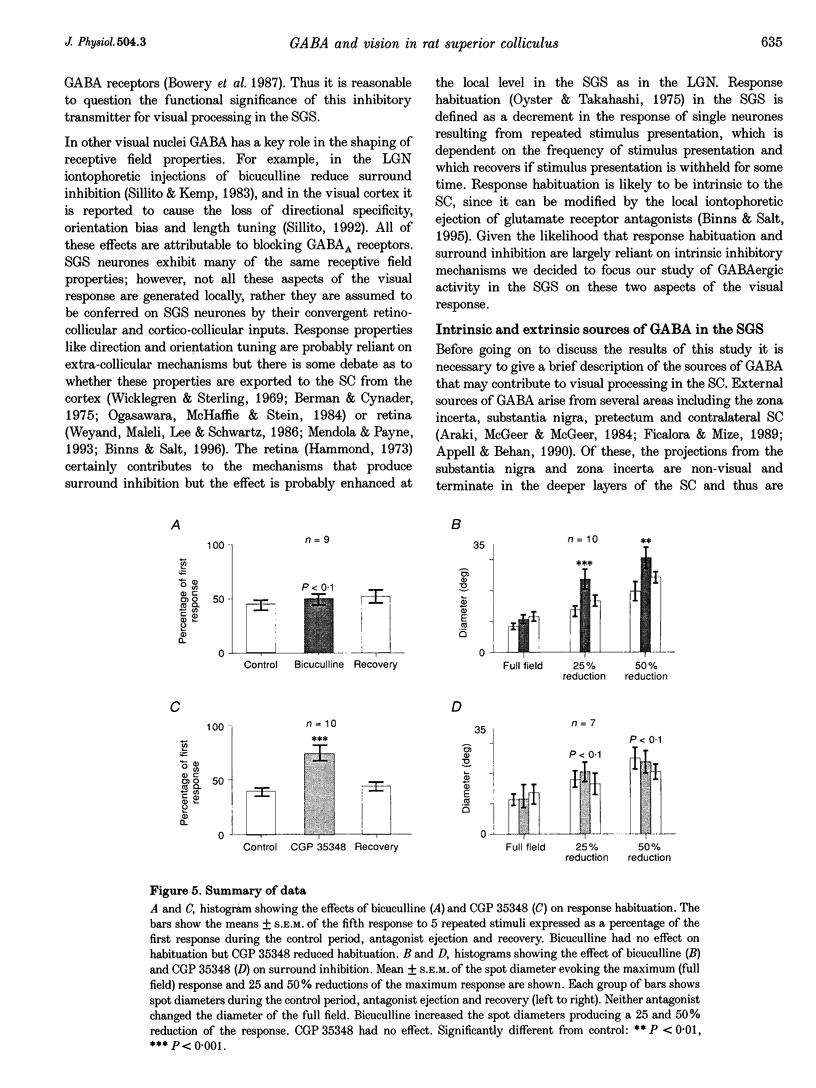



Images in this article
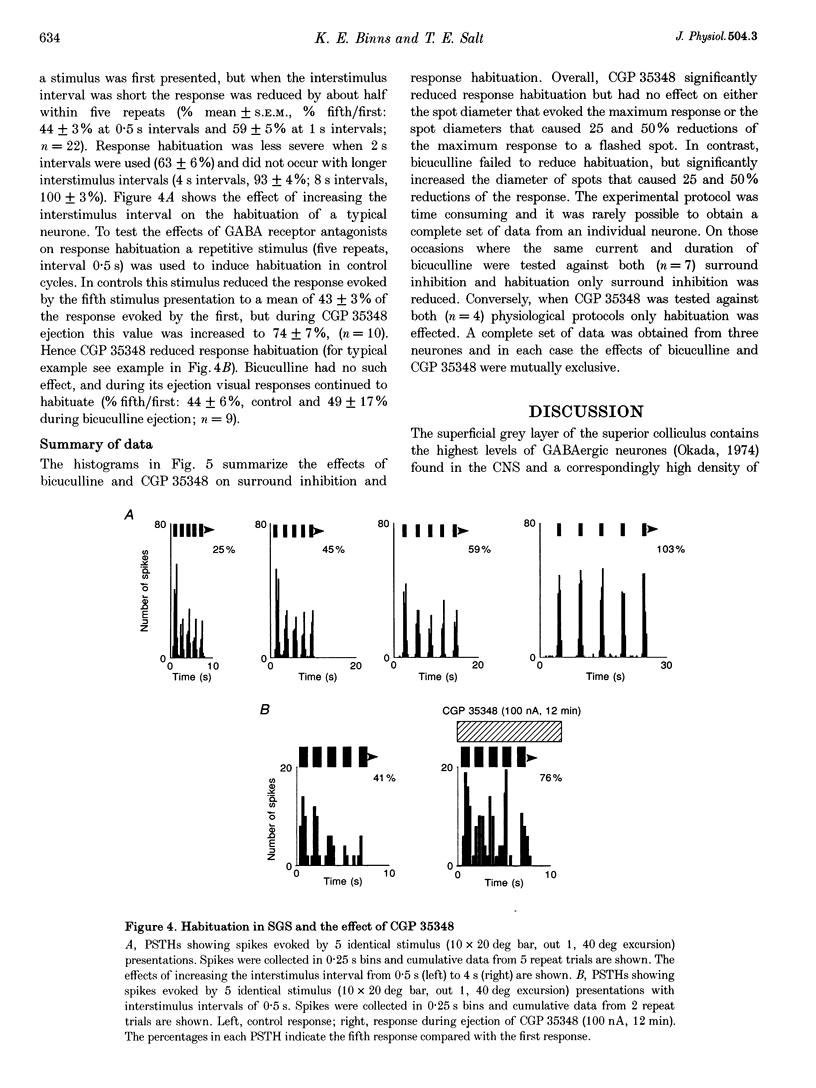
Selected References
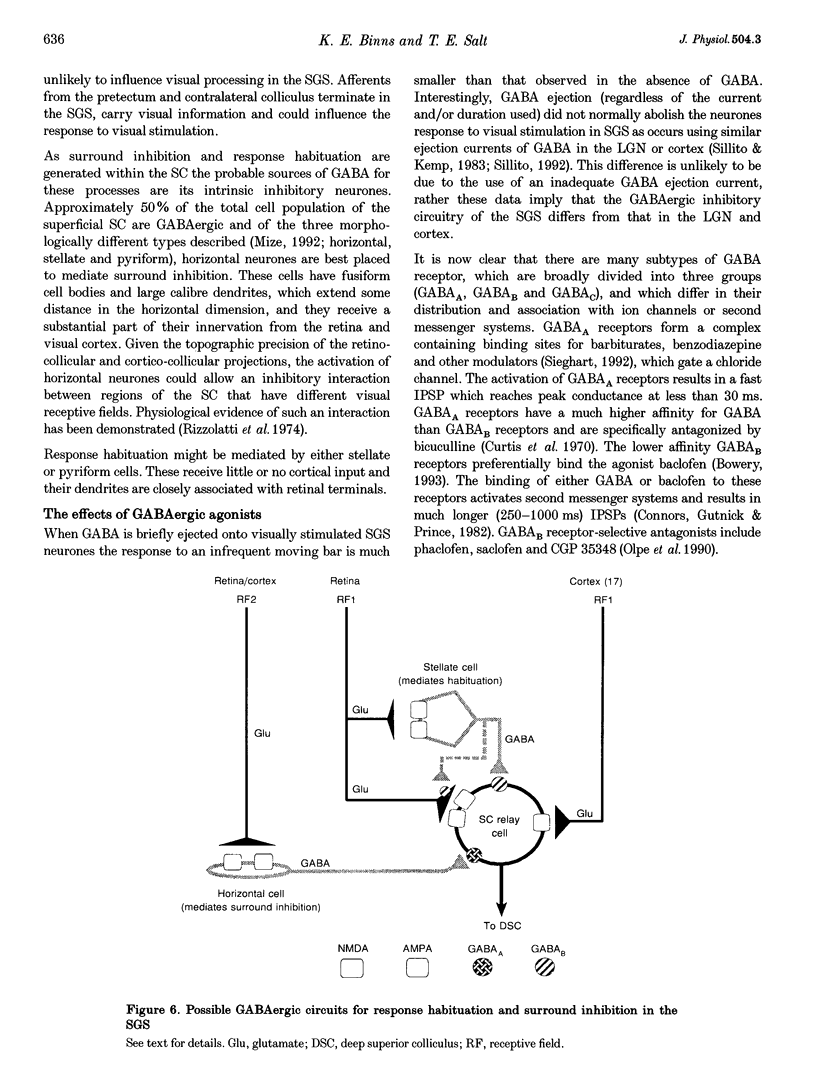
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J. D., Kabara J. F., Snider R. K., Casagrande V. A., Bonds A. B. GABAB-receptor-mediated inhibition reduces the orientation selectivity of the sustained response of striate cortical neurons in cats. Vis Neurosci. 1996 May-Jun;13(3):559–566. doi: 10.1017/s0952523800008233. [DOI] [PubMed] [Google Scholar]
- Appell P. P., Behan M. Sources of subcortical GABAergic projections to the superior colliculus in the cat. J Comp Neurol. 1990 Dec 1;302(1):143–158. doi: 10.1002/cne.903020111. [DOI] [PubMed] [Google Scholar]
- Araki M., McGeer P. L., McGeer E. G. Presumptive gamma-aminobutyric acid pathways from the midbrain to the superior colliculus studied by a combined horseradish peroxidase-gamma-aminobutyric acid transaminase pharmacohistochemical method. Neuroscience. 1984 Oct;13(2):433–439. doi: 10.1016/0306-4522(84)90241-0. [DOI] [PubMed] [Google Scholar]
- Behan M. Identification and distribution of retinocollicular terminals in the cat: an electron microscopic autoradiographic analysis. J Comp Neurol. 1981 Jun 10;199(1):1–15. doi: 10.1002/cne.901990102. [DOI] [PubMed] [Google Scholar]
- Berman N., Cynader M. Receptive fields in cat superior colliculus after visual cortex lesions. J Physiol. 1975 Feb;245(1):261–270. doi: 10.1113/jphysiol.1975.sp010844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binns K. E., Salt T. E. Corticofugal influences on visual responses in cat superior colliculus: the role of NMDA receptors. Vis Neurosci. 1996 Jul-Aug;13(4):683–694. doi: 10.1017/s0952523800008579. [DOI] [PubMed] [Google Scholar]
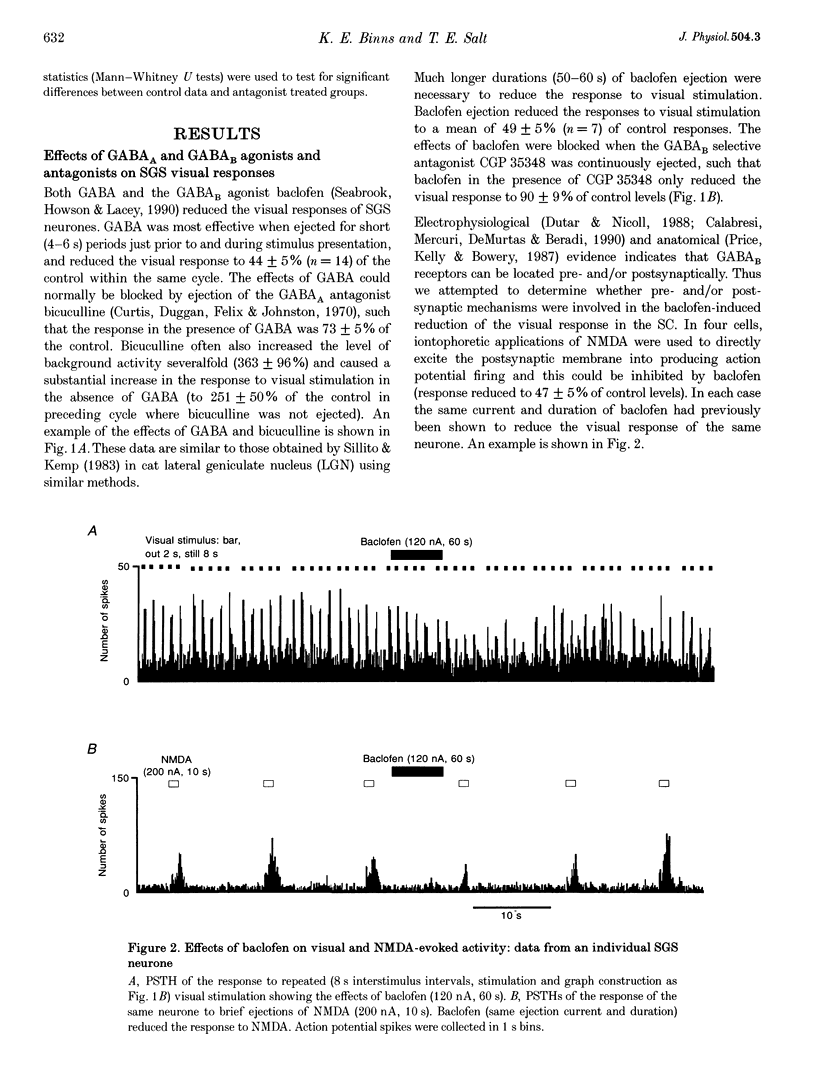
- Binns K. E., Salt T. E. Excitatory amino acid receptors modulate habituation of the response to visual stimulation in the cat superior colliculus. Vis Neurosci. 1995 May-Jun;12(3):563–571. doi: 10.1017/s0952523800008452. [DOI] [PubMed] [Google Scholar]
- Binns K. E., Salt T. E. Excitatory amino acid receptors participate in synaptic transmission of visual responses in the superficial layers of the cat superior colliculus. Eur J Neurosci. 1994 Jan 1;6(1):161–169. doi: 10.1111/j.1460-9568.1994.tb00257.x. [DOI] [PubMed] [Google Scholar]
- Bowery N. G. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hudson A. L., Price G. W. GABAA and GABAB receptor site distribution in the rat central nervous system. Neuroscience. 1987 Feb;20(2):365–383. doi: 10.1016/0306-4522(87)90098-4. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Mercuri N. B., De Murtas M., Bernardi G. Endogenous GABA mediates presynaptic inhibition of spontaneous and evoked excitatory synaptic potentials in the rat neostriatum. Neurosci Lett. 1990 Oct 2;118(1):99–102. doi: 10.1016/0304-3940(90)90258-b. [DOI] [PubMed] [Google Scholar]
- Chalupa L. M., Rhoades R. W. Responses of visual, somatosensory, and auditory neurones in the golden hamster's superior colliculus. J Physiol. 1977 Sep;270(3):595–626. doi: 10.1113/jphysiol.1977.sp011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors B. W., Gutnick M. J., Prince D. A. Electrophysiological properties of neocortical neurons in vitro. J Neurophysiol. 1982 Dec;48(6):1302–1320. doi: 10.1152/jn.1982.48.6.1302. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Dräger U. C., Hubel D. H. Responses to visual stimulation and relationship between visual, auditory, and somatosensory inputs in mouse superior colliculus. J Neurophysiol. 1975 May;38(3):690–713. doi: 10.1152/jn.1975.38.3.690. [DOI] [PubMed] [Google Scholar]
- Dutar P., Nicoll R. A. Pre- and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988 Sep;1(7):585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Ficalora A. S., Mize R. R. The neurons of the substantia nigra and zona incerta which project to the cat superior colliculus are GABA immunoreactive: a double-label study using GABA immunocytochemistry and lectin retrograde transport. Neuroscience. 1989;29(3):567–581. doi: 10.1016/0306-4522(89)90131-0. [DOI] [PubMed] [Google Scholar]
- Hammond P. Contrasts in spatial organization of receptive fields at geniculate and retinal levels: centre, surround and outer surround. J Physiol. 1973 Jan;228(1):115–137. doi: 10.1113/jphysiol.1973.sp010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harutiunian-Kozak B., Dec K., Dreher B. Habituation of unitary responses in the superior colliculus of the cat. Acta Neurobiol Exp (Wars) 1971;31(2):213–217. [PubMed] [Google Scholar]
- Kaneko T., Hicks T. P. GABA(B)-related activity involved in synaptic processing of somatosensory information in S1 cortex of the anaesthetized cat. Br J Pharmacol. 1990 Aug;100(4):689–698. doi: 10.1111/j.1476-5381.1990.tb14077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. H., Li B., Sun J. S., Diao Y. C. [Effects of GABA and bicuculline on the properties of visual superior colliculus neurons in golden hamsters]. Sheng Li Xue Bao. 1991 Dec;43(6):573–579. [PubMed] [Google Scholar]
- Mendola J. D., Payne B. R. Direction selectivity and physiological compensation in the superior colliculus following removal of areas 17 and 18. Vis Neurosci. 1993 Nov-Dec;10(6):1019–1026. doi: 10.1017/s0952523800010129. [DOI] [PubMed] [Google Scholar]
- Mize R. R. The organization of GABAergic neurons in the mammalian superior colliculus. Prog Brain Res. 1992;90:219–248. doi: 10.1016/s0079-6123(08)63616-x. [DOI] [PubMed] [Google Scholar]
- Ogasawara K., McHaffie J. G., Stein B. E. Two visual corticotectal systems in cat. J Neurophysiol. 1984 Dec;52(6):1226–1245. doi: 10.1152/jn.1984.52.6.1226. [DOI] [PubMed] [Google Scholar]
- Okada Y. Distribution of gamma-aminobutyric acid (GABA) in the layers of the superior colliculus of the rabbit. Brain Res. 1974 Jul 26;75(2):362–366. doi: 10.1016/0006-8993(74)90762-8. [DOI] [PubMed] [Google Scholar]
- Olpe H. R., Karlsson G., Pozza M. F., Brugger F., Steinmann M., Van Riezen H., Fagg G., Hall R. G., Froestl W., Bittiger H. CGP 35348: a centrally active blocker of GABAB receptors. Eur J Pharmacol. 1990 Oct 2;187(1):27–38. doi: 10.1016/0014-2999(90)90337-6. [DOI] [PubMed] [Google Scholar]
- Oyster C. W., Takahashi E. S. Responses of rabbit superior colliculus neurons to repeated visual stimuli. J Neurophysiol. 1975 Mar;38(2):301–312. doi: 10.1152/jn.1975.38.2.301. [DOI] [PubMed] [Google Scholar]
- Price G. W., Kelly J. S., Bowery N. G. The location of GABAB receptor binding sites in mammalian spinal cord. Synapse. 1987;1(6):530–538. doi: 10.1002/syn.890010605. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G., Camarda R., Grupp L. A., Pisa M. Inhibitory effect of remote visual stimuli on visual responses of cat superior colliculus: spatial and temporal factors. J Neurophysiol. 1974 Nov;37(6):1262–1275. doi: 10.1152/jn.1974.37.6.1262. [DOI] [PubMed] [Google Scholar]
- Seabrook G. R., Howson W., Lacey M. G. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990 Dec;101(4):949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. GABAA receptors: ligand-gated Cl- ion channels modulated by multiple drug-binding sites. Trends Pharmacol Sci. 1992 Dec;13(12):446–450. doi: 10.1016/0165-6147(92)90142-s. [DOI] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A. The influence of GABAergic inhibitory processes on the receptive field structure of X and Y cells in cat dorsal lateral geniculate nucleus (dLGN). Brain Res. 1983 Oct 24;277(1):63–77. doi: 10.1016/0006-8993(83)90908-3. [DOI] [PubMed] [Google Scholar]
- Siminoff R., Schwassmann H. O., Kruger L. An electrophysiological study of the visual projection to the superior colliculus of the rat. J Comp Neurol. 1966 Aug;127(4):435–444. doi: 10.1002/cne.901270402. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Crunelli V. On the properties and origin of the GABAB inhibitory postsynaptic potential recorded in morphologically identified projection cells of the cat dorsal lateral geniculate nucleus. Neuroscience. 1989;33(1):23–33. doi: 10.1016/0306-4522(89)90307-2. [DOI] [PubMed] [Google Scholar]
- Weyand T. G., Malpeli J. G., Lee C., Schwark H. D. Cat area 17. III. Response properties and orientation anisotropies of corticotectal cells. J Neurophysiol. 1986 Oct;56(4):1088–1101. doi: 10.1152/jn.1986.56.4.1088. [DOI] [PubMed] [Google Scholar]
- Wickelgren B. G., Sterling P. Influence of visual cortex on receptive fields in the superior colliculus of the cat. J Neurophysiol. 1969 Jan;32(1):16–23. doi: 10.1152/jn.1969.32.1.16. [DOI] [PubMed] [Google Scholar]