Abstract
Background
Precision medicine is an evolving predictive, early preventive, and treatment method that takes into account human gene heterogeneity for each individual. Personalized Medicine Research Center (PMRC) of Endocrinology and Metabolism Research Institute (EMRI) has been officially founded by the authority of the Ministry of Health and Medical Education on 18 December 2016 with the primary purpose of research, education, and dissemination of the personalized medicine approach throughout the country. In this review, we aimed to introduce the Personalized Medicine Research Center activities in the field of precision medicine since the establishment.
Methods
We systematically reviewed PubMed, Scopus, and WoS from 2016 to December 2019 to identify our relevant topics with PMRC affiliation.
Results
After screening processes, a total of 18 studies met eligibility criteria to include in the systematic review.
Conclusions
Personalized medicine that interchangeably called precision medicine is a new concept in medicine, the implementation of which needs to organize some infrastructures which are physical and the required education for healthcare professionals in different countries.
Keywords: Personalized Medicine Research Center, EMRI, Precision medicine, Personalized medicine
Introduction
Precision or individualized, stratified, p4 medicine (is derived from the first letter of predictive, preventative, personalized, participatory) or also commonly referred personalized medicine, as the art of medicine, is an approach that uses an individual’s genomic information to predict, prevent and personalized treatment through omics technology [1, 2].
Personalized medicine is a movement from a ‘one size fits all’ treatment and care approach towards one new approach in order to better health management and targeted therapies of patients in achieving the best clinical outcomes in dealing a patient’s disease or disease predisposition.
Precision medicine and personalized medicine have been introduced with some differences by considering their approach and the healthcare delivery model. The term “personalized” suggests that therapies and preventions are provided specifically for each patient, while the emphasis in precision medicine is to determine which interventions are more effective for patients according to the genetic, environmental, and lifestyle factors. However, the personalized and precision medicine terms still used interchangeably [3, 4].
Pharmacogenetics and pharmacogenomics have been widely recognized as the most important component of precision medicine. Pharmacogenetics describes how a single genetic variant influences medication response while pharmacogenomics shows this association with several genes (genome) [5]. Pharmacogenetic information can help clinicians optimize drug selection, dose adjustment, duration of treatment, and prevent adverse drug reactions. Pharmacogenetics can also provide new insights into drug action mechanisms and help to develop new therapeutic agents [6]. Changes in drug pharmacokinetics and pharmacodynamics are two main ways in which pharmacogenomics can influence drug response [1].
Formerly, the clinical applications of the precision medicine approach are known in the diagnosis of cancer and treatment but its impact on non-communicable diseases (NCDs) like type 2 diabetes mellitus (T2DM), required further research [7].
NCDs mainly including cardiovascular diseases, cancers, type 2 diabetes mellitus (T2DM), and chronic respiratory diseases are known as one of the most important reasons for death in the world.
The World Health Organization (WHO) global action plan comprises three main pillars for prevention of common disease until 2025 in order to integrate the preventive strategies through governments and health policy approach to reduce morbidity and mortality [8–10]. Precision medicine would have an important role for achieving the considered plans.
The genomics technology in precision medicine approach can help to classification of type 2 diabetes patients who share particular biological features [1]. Precision medicine is changing the nature of health care system [7, 11, 12].
Personalized medicine is a new era of medicine and it needs new knowledge amongst healthcare professionals and the public. The clinicians require to discuss with their patient’s information about their genomic characteristics and benefits of personalized treatment approach. Therefore, knowledge production is the first step for the implementation of this new subject in any community. Personalized medicine research center (PMRC) affiliated to Endocrinology and Metabolism Research Institute (EMRI) is the only approved research center in the field of personalized medicine by the authority of the Iran Ministry of Health and Medical Education for development of the personalized medicine in the research, education, and dissemination throughout the country. The present study aimed to introduce some research activities of PMRC after establishment since 2016.
Methods
A comprehensive search was performed in PubMed, Scopus, and Web of Science (WoS) databases from December 2016 to December 2019 to select all studies that conducted on the precision medicine approach in PMRC.
The following Search strategy ((Personalized[ad] AND (Medicine[ad] OR Med[AD])) AND (Research[ad] OR Res[ad]) AND (Center[ad] OR ctr[ad])) AND ((Endocrino* [ad] AND (Metabolic[ad] OR Metab*[ad]) AND Clin*[ad] AND Inst*[ad]) OR EMRI[ad]) AND (TUMS[ad] OR “Tehran University of Medical Sciences“[ad] OR “Med Sci Univ Tehran“[ad] OR “Med Univ Tehran“[ad] OR “Shariati Hosp“[ad] OR “Tehran Med Sci Univ“[ad] OR “Tehran Med Univ“[ad] OR “Tehran Univ Med“[ad] OR “Tehran Univ Med Sci“[ad] OR “Univ Tehran Med Sci“[ad] OR “Univ Tehran Med Sci Hlth Sci“[ad] OR “Univ Tehran Med Sci Hlth Serv“[ad])) was used in PubMed and modified in other data bases.
In the first step, the titles and abstracts of the articles were screened to choose relevant studies. Next, the full-text of articles were assessed for selection of the included studies according to the eligibility criteria in the final investigation. Opposing views were addressed to achieve consensus through further debate.
The inclusion criteria that were checked in all included studies are followed as follow:
Affiliation of PMRC ”Personalized Medicine Research Center, Endocrinology and Metabolism Clinical Sciences Institute, Tehran University of Medical Sciences, Tehran, Iran”
English article
Review or original or editorial
We considered all of the articles with the umbrella of personalized medicine that use omics approach including genomics
The articles that discuss topics on different aspects of personalized/precision medicine concept
Any article which is working on genetic association studies
Articles that assess epigenetic topic
Studies with precision medicine approach in non-communicable diseases
All published studies in peer reviewed journal
Any article that discussed topics other than personalized/precision medicine and also abstracts was excluded from the present study.
Data extraction
The items such as first name of authors, publication year, study title, and study conclusion were extracted from all eligible studies according to the above mentioned inclusion and exclusion parameters.
Results
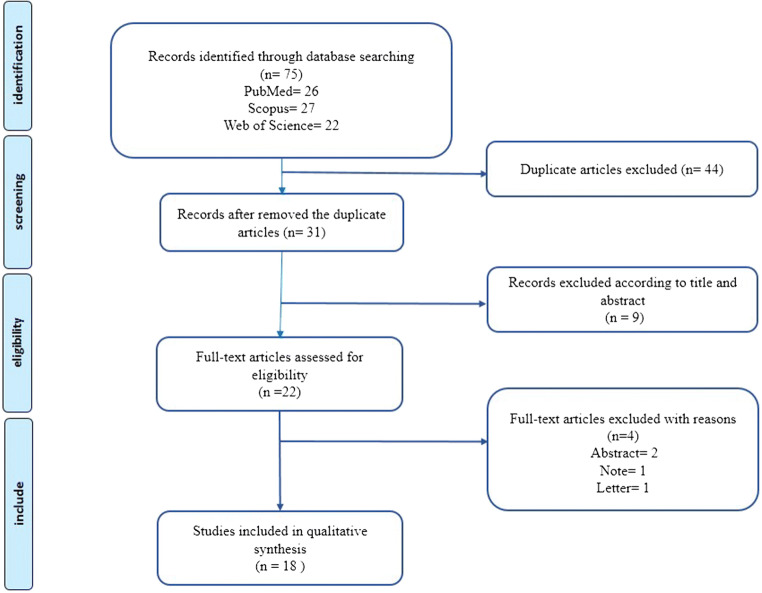
We identified 75 articles after a primary search of PubMed, Scopus, and WoS databases from December 2016 to December 2019 (Fig. 1). Forty-four duplicate articles were removed and 31 articles remained. After reviewing the titles and abstracts, we excluded 9 records because they were topics other than personalized/precision medicine and the other 22 articles were collected for full texts evaluation. Four items were excluded for different reasons (one study was a letter, one study was Note, and two studies were abstract). Finally, a total of 18 studies met the inclusion criteria for assessing the present systematic review. Table 1 provides a description of each included study.
Fig. 1.
Flow diagram presenting the results of the literature search and study selection process
Table 1.
Characteristics of included studies
| First author et al. (Year) | Title | Results | Ref |
|---|---|---|---|
| Erfani T et al. (2020) | KCNQ1 common genetic variant and type 2 diabetes mellitus risk | This study could not find any relation between rs2237892 variant of KCNQ1 gene and type 2 diabetes susceptibility. | [13] |
| Samimi H et al. (2020) | Gut Microbiome and Radioiodine-Refractory Papillary Thyroid Carcinoma Pathophysiology | The gut microbiome could be associated with RAI-resistant PTC through several mechanisms dependent on Na+/I- symporter regulation. | [14] |
| Mahdiannasser, M et al. (2020) |
Investigation of promoter methylation of FSCN1 gene and FSCN1 protein expression in differentiated thyroid carcinomas |
Although the methylation condition is not significantly changed in FTC, the FSCN1 promoter is significantly hypomethylated in PTC subjects which condition does not cause FSCN1 overexpression. So it is possible that other regulatory mechanisms involved in FSCN1 upregulation. | [15] |
| Khooshemehri, P et al. (2020) | Genetic Polymorphism of Mismatch Repair Genes and Susceptibility to Prostate Cancer | The study did not show any significant association between MLH1 and MSH3 polymorphisms with the risk of prostate cancer | [16] |
| Montazeri, V et al. (2020) | A preliminary study of NER and MMR pathways involved in chemotherapy response in bladder transitional cell carcinoma: Impact on progression-free survival | It may that the mRNA expression level of ERCC1, MLH1, MSH2, and CTR1 genes is correlated to PFS time as targets of treatment in order to decrease cisplatin resistance. | [17] |
| Saki, N et al. (2020) | MTNR1B common genetic variant is associated with type 2 diabetes mellitus risk | The results of the study are showed MTNR1B rs10830963 gene variant may influence on T2DM risk in a group of the Iranian population. | [18] |
| Afshardoost, Sh et al. (2019) | The influence of a genetic variant in the KCNQ1 gene on type 2 diabetes mellitus development | Rs2237895 variant in the KCNQ1 gene was not associated with type 2 diabetes risk but could have a protective role against developing renal complications. | [19] |
| Khatami, F et al. (2019) | Personalized treatment options for thyroid cancer: current perspectives |
Genetic modifications in BRAF, VEGF receptors, RET, and RET/PTC, KDR, KIT, PDGFRA, CD274, and JAK2 could be used, as therapeutic targets, in individual treatment to better prognostic consequence. RASSF1, PTEN, SLC, DNMTs, MGMT, TSHR, and E-cadherin promoter methylation have been also considered to select the best treatment option based on genetic history in thyroid cancer types. |
[5] |
| Motamedi, R et al. (2019) | Kallikarein-related peptidase 3 common genetic variant and the risk of prostate cancer | Rs2735839 variant of the KLK3 gene could considerate as a risk factor for the risk of prostate cancer | [20] |
| Rashidi, B et al. (2019) | Association of vascular endothelial growth factor (VEGF) Gene polymorphisms and expression with the risk of endometriosis: a case-control study | The findings of the study did not show any impact of the VEGF rs699947 and rs3025039 variants and also mRNA expression on the endometriosis risk. | [21] |
| Hasanzad, M et al. (2019) | The Pathway from Gene Therapy to Genome Editing: A Nightmare or Dream | Gene therapy might be considered as a type of personalized therapy. | [22] |
| Sarhangi, N et al. (2019) | The Association Analysis of Vascular Endothelial Growth Factor 2549 Insertion/ DeletionVariant and Endometriosis Risk | It was not found an association of VEGF − 2549 I/D variant with susceptibility to endometriosis in a group of Iranian women subjects. | [23] |
| Azizi, SM et al. (2019) | Association Analysis of the HNF4A Common Genetic Variants with Type 2 Diabetes Mellitus Risk | Rs1884613 and rs1884614 variants of the HNF4A were not associated with susceptibility to T2DM or its renal and ophthalmic complications in a group of type 2 diabetes patients. | [24] |
| Asgarbeik, S et al. (2019) | The Role of ERRFI1 + 808T/G Polymorphism in Diabetic Nephropathy | Although, no significant relation was shown between the + 808T/G variant of the ERRFI1 gene and the diabetic nephropathy development in patients with type 2 diabetes, the protective effect of the T allele against the risk of diabetes was found. | [25] |
| Hasanzad, M et al. (2019) | Precision Medicine in Non-Communicable Diseases | Precision medicine is going to become a revolution in the practice of medicine, especially in NCDs. | [2] |
| Mirfeizi, M et al. (2018) | Association of eNOS and ACE gene polymorphisms as a genetic risk factor in gestational diabetes in Iranian women | The eNOS (Glu298Asp) and ACE (I/D) genetic variants were not linked to the risk of gestational diabetes mellitus. | [26] |
| Sattari, M et al. (2017) | Association between matrix metaloproteinases 2 -1306 C/T polymorphism and the risk of coronary artery disease in Iranian population | It was not found enough evidence regarding the association of the MMP2 -1306 variant with coronary artery disease development. | [27] |
| Aghaei Meybodi, HR et al. (2017) | Path to Personalized Medicine for Type 2 Diabetes Mellitus: Reality and Hope | The potential of integrating “Omics” data with the clinical data will lead to the advancement of diabetes care in the personalized medicine approach in the next and lead to better management of diabetes. | [1] |
KCNQ1, Potassium Voltage-Gated Channel Subfamily Q Member 1; FTC, follicular thyroid cancer; PTC, Papillary Thyroid Carcinoma; RAI, Radioiodine-Refractory; FSCN1, Fascin actin-bundling protein 1; MLH1, human mutL homolog 1; MLH3, MutL Homolog 3; NER, Nucleotide excision repair; MMR, DNA mismatch repair; ERCC1, Excision repair cross-complementation group 1; MSH2, mutS homolog 2; CTR1, Copper Transporter 1; PFS, Progression-free survival; MTNR1B, melatonin receptor 1B; VEGF, vascular endothelial growth factor; BRAF, B-Raf Proto-Oncogene, Serine/Threonine Kinase; RET, ret proto-oncogene; KDR, kinase insert domain receptor; KIT, KIT proto-oncogene, receptor tyrosine kinase; PDGFRA, platelet derived growth factor receptor alpha; JAK2, Janus kinase 2; RASSF1, Ras Association Domain Family Member 1; SLC, Solute Carrier Family; DNMTs, DNA methyltransferases; MGMT, O6-methylguanine DNA methyltransferase; TSHR, Thyroid-stimulating hormone receptor; E-cadherin, epithelial cadherin; KLK3, Kallikarein-related peptidase 3; I/D, insertion/deletion; HNF4A, hepatocyte nuclear factor 4 alpha; T2DM, type 2 diabetes mellitus; ERRFI1, ERBB receptor feedback inhibitor 1; NCDs, Non Communicable Diseases; eNOS, Endothelial nitric oxide synthase 3; ACE, angiotensin I converting enzyme; MMP-2, matrix metalloproteinase-2
Discussion
Although “personalized medicine” is an older term than “precision medicine”, the two terms have a lot in common and are used interchangeably. The difference here is that precision medicine seeks to create treatments that are applicable to groups of individuals who meet certain characteristics based on genetic, environmental, and lifestyle factors. This is different from “personalized medicine,” which implies individualized treatments available for every unique patient [4].
PMRC has been established on December 2016 to promote personalized medicine approach in the research, education, and dissemination throughout the country. Education is one of the main goals of the next 10 years in the strategic plan of the personalized medicine research center. Currently, workshops and conferences have been held at the personalized medicine research center. But after adequate training for healthcare professionals, the subject of precision medicine will enter in the field. For example, the use of the personalized medicine concept in diabetes can change the taxonomy of diabetes, and its therapeutic approach.
By promoting innovative research, the EMRI aims to improve better understanding of NCDs in order to find new ways for prevention and treatment strategies and cure possibilities. Accordingly, PMRC also follows this mission, applying precision medicine approach in NCDs.
Over the past year’s clinical practice pattern trends began to change with the rise of evidence-based medicine. However, evidence-based medicine relies on the individualization of care and focuses attention on unique genomic characteristics of a certain patient. The patterns of genetic variation at loci involving disease risk can provide clinically useful personalized information on disease etiology. This genetic architecture shows a wide spread of risk across many hundreds of loci. In the case of precision medicine implementation, we consider several research targets, in this regards we need to perform systematic reviews in order to provide knowledge of precision medicine [1, 2, 5]. Single nucleotide polymorphisms (SNPs) are the most prevalent form of genetic variations which play important roles in the concept of precision medicine [1, 2, 19]. Some pilot studies on the association of well- known T2DM genetic risk variants were performed at PMRC [13, 18, 19, 24]. The main reason for starting some types of projects as have been introduced in this article was to collect biological samples for the future T2DM initiative projects on precision medicine. Because the application of personalized medicine in the clinic is not possible without obtaining the results of initiative projects. Therefore, we can provide valuable sample and data repository of T2DM patients to obtain the genomic data of Iranian patients, so it is necessary to select diabetic patients with well-defined criteria in the first stage.
As the practice of precision medicine has achieved fruitful results in the oncology, thus precision cancer medicine was another target of personalized medicine research center [2, 5]. Today, cancer patients can be stratified according to what will be most effective for their condition. The genetic association studies have also performed in order to the analyzing the association between genetic variant and susceptibility to prostate cancer at PMRC [16, 20]. These findings could help in the development of precision medicine approach to targeted treatment of patients.
It was reported in a study by Khatami et al. [5] that the association of genetic mutation and personalized treatment could improve the prognosis outcome through more optimum precision treatment strategies. Several genetic changes including mutation and polymorphism, copy number variation (CNV) could be used for precision thyroid cancer treatment in order to improve prognostic consequences. In order to select better therapeutic option, it is necessary that each subject with thyroid cancer considered according to their genetic background.
Altogether, precision medicine is a medical model that strengthens medical practice in the immediate future.
Conclusions
Personalized medicine is a new approach in medicine, the realization of which in different populations requires the establishment of physical infrastructure and the necessary education for healthcare professionals. Large genomics population studies are needed in each country in order to implement precision medicine approach in clinical practice. These types of studies can eliminate trial and errors in clinical practice that ultimately help to choose the best treatments against disease. Accordingly, understanding the genetic variations underlying different common diseases and integration of these data into clinical practice will require efforts from all health components. The use of big data extracted from “omics,” could make the dream of precision medicine in NCDs care to a reality in the near future. Precision medicine has provided the infrastructure for efficient utilization of several drugs in each patient population with specific genetic variations.
Abbreviations
- NCDs
Non-communicable disease
- T2DM
Type 2 diabetes mellitus
- WHO
World Health Organization
- PMRC
Personalized medicine research center
- EMRI
Endocrinology and Metabolism Research Institute
- WoS
Web of Science
- SNPs
Single nucleotide polymorphisms
- CNV
copy number variation
Author contributions
MH has developed the project and written the manuscript with a collaboration by NS; SHN provided some guidance to the research; and BL designed the project and revised the manuscript. All authors read and approved the final version of manuscript before submission.
Data availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mandana Hasanzad, Email: mandanahasanzad@yahoo.com.
Bagher Larijani, Email: larijanib1340@gmail.com.
References
- 1.Aghaei Meybodi HR, Hasanzad M, Larijani B. Path to personalized medicine for Type 2 Diabetes Mellitus: reality and hope. Acta Med Iran. 2017;55(3):166–74. [PubMed] [Google Scholar]
- 2.Hasanzad M, Sarhangi N, Aghaei Meybodi HR, Nikfar S, Khatami F, Larijani B. Precision medicine in non communicable diseases. Int J Mol Cell Med. 2019;8(2):0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ginsburg GS, Phillips KA. Precision medicine: from science to value. Health Aff. 2018;37(5):694–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jameson JL, Longo DL. Precision medicine—personalized, problematic, and promising. N Engl J Med. 2015;372(23):2229–34. [DOI] [PubMed] [Google Scholar]
- 5.Khatami F, Larijani B, Nikfar S, Hasanzad M, Fendereski K, Tavangar SM. Personalized treatment options for thyroid cancer: current perspectives. Pharmgenomics Pers Med. 2019;12:235–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arjmand B, Abdollahi M, Larijani B. Precision medicine: A new revolution in healthcare system. Iran Biomed J. 2017;21(5):282–3. [PubMed] [Google Scholar]
- 8.Peykari N, Larijani B. A multi-sectoral approach to combatting non-communicable diseases: Iran’s experience. J Diabetes Metab Disord. 2019;18(2):719–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roth GA, Abate D, Abate KH, Abay SM, Abbafati C, Abbasi N, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett JE, Stevens GA, Mathers CD, Bonita R, Rehm J, Kruk ME, et al. NCD Countdown 2030: worldwide trends in non-communicable disease mortality and progress towards Sustainable Development Goal target 3.4. Lancet. 2018;392(10152):1072–88. [DOI] [PubMed] [Google Scholar]
- 11.Arjmand B, Larijani B. Personalized medicine: A new era in endocrinology. Acta Med Iran. 2017;55(3):142–3. [PubMed] [Google Scholar]
- 12.Arjmand B, Abdollahi M, Larijani B. Study break: precision medicine: a new revolution in healthcare system. Iran Biomed J. 2017;21(5):282–3. [PubMed] [Google Scholar]
- 13.Erfani T, Sarhangi N, Afshari M, Abbasi D, Meybodi HRA, Hasanzad M. KCNQ1 common genetic variant and type 2 diabetes mellitus risk. J Diabetes Metab Disord. 2020;19(1):47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samimi H, Haghpanah V. Gut microbiome and radioiodine-refractory papillary thyroid carcinoma pathophysiology. Trends Endocrinol Metab. 2020;27(1–2):14–22. [DOI] [PubMed] [Google Scholar]
- 15.Mahdiannasser M, Haghpanah V, Damavandi E, Kabuli M, Tavangar SM, Larijani B, et al. Investigation of promoter methylation of FSCN1 gene and FSCN1 protein expression in differentiated thyroid carcinomas. Mol Biol Rep. 2020;47(3):2161–9. [DOI] [PubMed] [Google Scholar]
- 16.Khooshemehri P, Jamaldini SH, Ziaee SAM, Afshari M, Sattari M, Narouie B, et al. Genetic polymorphism of mismatch repair genes and susceptibility to prostate cancer. Urol J. 2020;17(3):271–5. [DOI] [PubMed] [Google Scholar]
- 17.Montazeri V, Ghahremani MH, Montazeri H, Hasanzad M, Safavi M, Ayati M, et al. A preliminary study of NER and MMR pathways involved in chemotherapy response in bladder transitional cell carcinoma: Impact on progression-free survival. Iran J Pharm Res. 2020;19(1):355–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saki N, Sarhangi N, Afshari M, Bandarian F, Aghaei Meybodi HR, Hasanzad M. MTNR1B common genetic variant is associated with type 2 diabetes mellitus risk. Gene Rep. 2020;20:100695. [Google Scholar]
- 19.Afshardoost S, Sarhangi N, Afshari M, Aghaei Meybodi HR, Hasanzad M. The influence of a genetic variant in the KCNQ1 gene on type 2 diabetes mellitus development. Gene Rep. 2019;17:100529. [Google Scholar]
- 20.Motamedi RK, Sarhangi N, Afshari M, Sattari M, Jamaldini SH, Samzadeh M, et al. Kallikarein-related peptidase 3 common genetic variant and the risk of prostate cancer. J Cell Biochem. 2019;120(9):14822–30. [DOI] [PubMed] [Google Scholar]
- 21.Rashidi BH, Sarhangi N, Aminimoghaddam S, Haghollahi F, Naji T, Amoli MM, et al. Association of vascular endothelial growth factor (VEGF) Gene polymorphisms and expression with the risk of endometriosis: a case-control study. Mol Biol Rep. 2019;46(3):3445–50. [DOI] [PubMed] [Google Scholar]
- 22.Hasanzad M, Larijani B. The pathway from gene therapy to genome editing: A nightmare or dream. Int J Mol Cell Med. 2019;8(2):69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarhangi N, Mohseni S, Aminimoghaddam S, Hossein Rashidi B, Haghollahi F, Qorbani M, et al. The association analysis of vascular endothelial growth factor – 2549 insertion/ deletion variant and endometriosis risk. Int J Mol Cell Med. 2019;8(Suppl1):63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azizi SM, Sarhangi N, Afshari M, Abbasi D, Aghaei Meybodi HR, Hasanzad M. Association analysis of the HNF4A common genetic variants with Type 2 diabetes mellitus risk. Int J Mol Cell Med. 2019;8(Suppl1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asgarbeik S, Mohammad Amoli M, Enayati S, Bandarian F, Nasli-Esfahani E, Forouzanfar K, et al. The role of ERRFI1 + 808T/G polymorphism in diabetic nephropathy. Int J Mol Cell Med. 2019;8(Suppl1):49–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mirfeizi M, Hasanzad M, Sattari M, Afshari M, Abbasi D, Ajoodani Z, et al. Association of eNOS and ACE gene polymorphisms as a genetic risk factor in gestational diabetes in Iranian women. J Diabetes Metab Disord. 2018;17(2):123–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sattari M, Hassanzad M, Jamaldini SH, Najafi A, Imani M, Mohammadhassani M, et al. Association between matrix metaloproteinases 2-1306C/T polymorphism and the risk of coronary artery disease in Iranian population. Pathophysiology. 2017;24(3):185–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.