Abstract
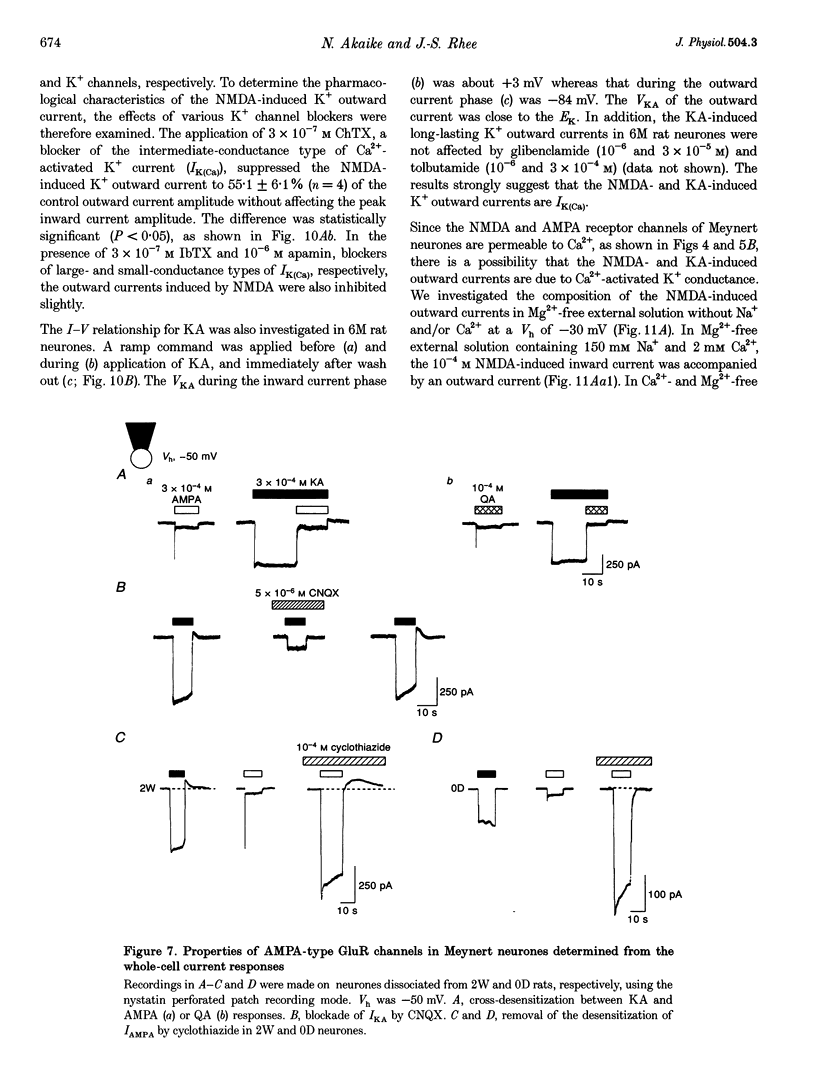
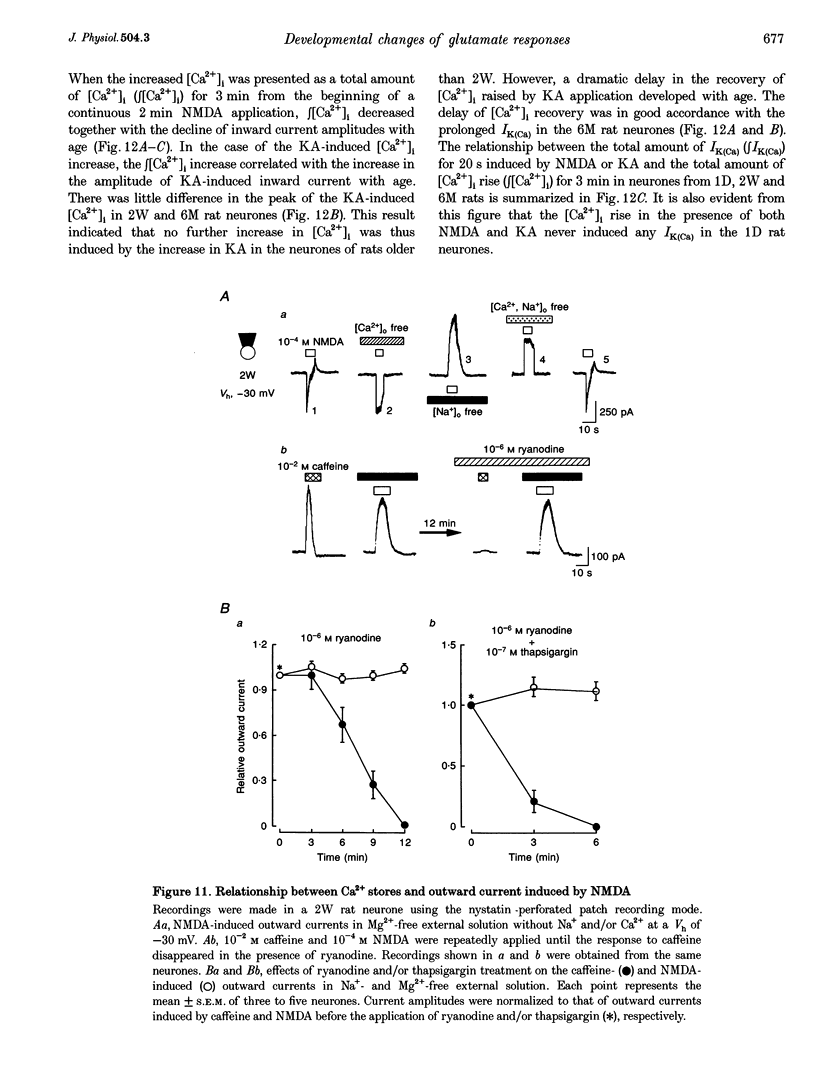
1. The developmental changes of glutamate receptors (GluRs) in acutely dissociated rat Meynert neurones were investigated using the conventional whole cell and nystatin perforated patch recording modes under voltage-clamp conditions. 2. The neurones became less responsive to N-methyl-D-aspartic acid (NMDA) with age, most dramatically between 1 day and 2 weeks, while the responses to kainic acid (KA) and L-alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) gradually increased. The metabotropic GluR response appeared a few days after birth, but thereafter no further change was observed. 3. The decrease in the NMDA response during postnatal development was due to an abrupt reduction in the number of receptors without affecting the affinity, voltage-dependent Mg2+ blockade or high Ca2+ permeability (PCa/PCs approximately 7.0). 4. PCa/PCs in the presence of KA decreased from 2.8 in the 1-day-old (1D) rat neurones to 1.1 and 0.44 in the 2-week-old (2W) and 6-month-old (6M) rat neurones, respectively. The concentration-response relationship for KA shifted to the left with age. The KA response was not affected by NS-102, a KA-selective antagonist, thus indicating that the increased affinity of the receptor for the ligand resulted from the change in the AMPA receptor channel subunits. 5. The AMPA response in the presence of 10(-4) M cyclothiazide showed a change in the inward rectifying current-voltage relationship with age. The KA response was strongly cross-desensitized by the addition of AMPA and was also blocked by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), whereas a rapid desensitization of the AMPA response was removed in a concentration-dependent manner by cyclothiazide. These results indicate that the non-NMDA receptor channels are assembled from the subunits of the AMPA receptor family without the GluR-2 subunit, thus resulting in a high Ca2+ permeability. 6. The L-glutamate (Glu)-induced responses were more sensitive to DL-2-amino-5-phosphonopentanoic acid (APV) in the 1D rat neurones than in the adult rat neurones. 7. Both NMDA and KA raised the intracellular Ca2+ concentration ([Ca2+]i) in all neurones of 1D, 2W and 6M rats, though the charybdotoxin-sensitive Ca(2+)-activated K+ current (IK(Ca)) did not appear in the 1D rat neurones. An age-related prolongation of both IK(Ca) decay and [Ca2+]i clearance was also seen after the removal of KA. 8. It was thus concluded that the age-related changes of ionotropic receptors appear to play a key role in the activities of immature and mature rat Meynert cholinergic neurones. The KA-induced IK(Ca), which developed with ageing, may thus function as one of the negative feedback systems, and thereby prevent excess cell excitation and neural damage, especially in adult rats.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akaike N., Harata N. Nystatin perforated patch recording and its applications to analyses of intracellular mechanisms. Jpn J Physiol. 1994;44(5):433–473. doi: 10.2170/jjphysiol.44.433. [DOI] [PubMed] [Google Scholar]
- Arendt T., Bigl V., Arendt A., Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer's disease, paralysis agitans and Korsakoff's Disease. Acta Neuropathol. 1983;61(2):101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y., Cherubini E., Krnjevic K. Changes in voltage dependence of NMDA currents during development. Neurosci Lett. 1988 Nov 22;94(1-2):88–92. doi: 10.1016/0304-3940(88)90275-3. [DOI] [PubMed] [Google Scholar]
- Bode-Greuel K. M., Singer W. The development of N-methyl-D-aspartate receptors in cat visual cortex. Brain Res Dev Brain Res. 1989 Apr 1;46(2):197–204. doi: 10.1016/0165-3806(89)90283-6. [DOI] [PubMed] [Google Scholar]
- Burnashev N., Khodorova A., Jonas P., Helm P. J., Wisden W., Monyer H., Seeburg P. H., Sakmann B. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992 Jun 12;256(5063):1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Carmignoto G., Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992 Nov 6;258(5084):1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Carnes K. M., Fuller T. A., Price J. L. Sources of presumptive glutamatergic/aspartatergic afferents to the magnocellular basal forebrain in the rat. J Comp Neurol. 1990 Dec 22;302(4):824–852. doi: 10.1002/cne.903020413. [DOI] [PubMed] [Google Scholar]
- Coyle J. T., Price D. L., DeLong M. R. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983 Mar 11;219(4589):1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- D'Angelo E., Rossi P., Taglietti V. Different proportions of N-methyl-D-aspartate and non-N-methyl-D-aspartate receptor currents at the mossy fibre-granule cell synapse of developing rat cerebellum. Neuroscience. 1993 Mar;53(1):121–130. doi: 10.1016/0306-4522(93)90290-v. [DOI] [PubMed] [Google Scholar]
- Dilts S. L. Berry CA: Effect of cholinergic drugs on passive avoidance in the mouse. J Pharmacol Exp Ther. 1967 Nov;158(2):279–285. [PubMed] [Google Scholar]
- Durand G. M., Zukin R. S. Developmental regulation of mRNAs encoding rat brain kainate/AMPA receptors: a northern analysis study. J Neurochem. 1993 Dec;61(6):2239–2246. doi: 10.1111/j.1471-4159.1993.tb07465.x. [DOI] [PubMed] [Google Scholar]
- Ebihara S., Shirato K., Harata N., Akaike N. Gramicidin-perforated patch recording: GABA response in mammalian neurones with intact intracellular chloride. J Physiol. 1995 Apr 1;484(Pt 1):77–86. doi: 10.1113/jphysiol.1995.sp020649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebihara S., Takishima T., Shirasaki T., Akaike N. Regional variation of excitatory and inhibitory amino acid-induced responses in rat dissociated CNS neurons. Neurosci Res. 1992 Jun;14(1):61–71. doi: 10.1016/s0168-0102(05)80006-9. [DOI] [PubMed] [Google Scholar]
- Garthwaite G., Yamini B., Jr, Garthwaite J. Selective loss of Purkinje and granule cell responsiveness to N-methyl-D-aspartate in rat cerebellum during development. Brain Res. 1987 Dec 1;433(2):288–292. doi: 10.1016/0165-3806(87)90034-4. [DOI] [PubMed] [Google Scholar]
- Geiger J. R., Melcher T., Koh D. S., Sakmann B., Seeburg P. H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995 Jul;15(1):193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Honore T., Drejeŕ J., Nielsen E. O., Nielsen M. Non-NMDA glutamate receptor antagonist 3H-CNOX binds with equal affinity to two agonist states of quisqualate receptors. Biochem Pharmacol. 1989 Oct 1;38(19):3207–3212. doi: 10.1016/0006-2952(89)90615-1. [DOI] [PubMed] [Google Scholar]
- Iino M., Ozawa S., Tsuzuki K. Permeation of calcium through excitatory amino acid receptor channels in cultured rat hippocampal neurones. J Physiol. 1990 May;424:151–165. doi: 10.1113/jphysiol.1990.sp018060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham C. A., Bolam J. P., Wainer B. H., Smith A. D. A correlated light and electron microscopic study of identified cholinergic basal forebrain neurons that project to the cortex in the rat. J Comp Neurol. 1985 Sep 8;239(2):176–192. doi: 10.1002/cne.902390205. [DOI] [PubMed] [Google Scholar]
- Khateb A., Fort P., Serafin M., Jones B. E., Mühlethaler M. Rhythmical bursts induced by NMDA in guinea-pig cholinergic nucleus basalis neurones in vitro. J Physiol. 1995 Sep 15;487(Pt 3):623–638. doi: 10.1113/jphysiol.1995.sp020905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. S., Geiger J. R., Jonas P., Sakmann B. Ca(2+)-permeable AMPA and NMDA receptor channels in basket cells of rat hippocampal dentate gyrus. J Physiol. 1995 Jun 1;485(Pt 2):383–402. doi: 10.1113/jphysiol.1995.sp020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L., Guthrie P. B. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984 May 17;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. Permeation and block of N-methyl-D-aspartic acid receptor channels by divalent cations in mouse cultured central neurones. J Physiol. 1987 Dec;394:501–527. doi: 10.1113/jphysiol.1987.sp016883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M. M., Mufson E. J., Wainer B. H., Levey A. I. Central cholinergic pathways in the rat: an overview based on an alternative nomenclature (Ch1-Ch6). Neuroscience. 1983 Dec;10(4):1185–1201. doi: 10.1016/0306-4522(83)90108-2. [DOI] [PubMed] [Google Scholar]
- Muir J. L., Page K. J., Sirinathsinghji D. J., Robbins T. W., Everitt B. J. Excitotoxic lesions of basal forebrain cholinergic neurons: effects on learning, memory and attention. Behav Brain Res. 1993 Nov 30;57(2):123–131. doi: 10.1016/0166-4328(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Nabekura J., Kawamoto I., Akaike N. Developmental change in voltage dependency of NMDA receptor-mediated response in nucleus tractus solitarii neurons. Brain Res. 1994 Jun 13;648(1):152–156. doi: 10.1016/0006-8993(94)91915-1. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nakashima Y., Ishibashi H., Harata N., Akaike N. Effects of glucose deprivation on NMDA-induced current and intracellular Ca2+ in rat substantia nigra neurons. J Neurophysiol. 1996 Feb;75(2):740–749. doi: 10.1152/jn.1996.75.2.740. [DOI] [PubMed] [Google Scholar]
- Nowak L., Bregestovski P., Ascher P., Herbet A., Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984 Feb 2;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Omura T., Munakata M., Akaike N. Nystatin-perforated patch recordings disclose KA-operated outward currents in rat cortical neurons. Brain Res. 1993 Nov 12;627(2):345–348. doi: 10.1016/0006-8993(93)90340-s. [DOI] [PubMed] [Google Scholar]
- Partin K. M., Patneau D. K., Winters C. A., Mayer M. L., Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993 Dec;11(6):1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Ramoa A. S., McCormick D. A. Enhanced activation of NMDA receptor responses at the immature retinogeniculate synapse. J Neurosci. 1994 Apr;14(4):2098–2105. doi: 10.1523/JNEUROSCI.14-04-02098.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirasaki T., Nakagawa T., Wakamori M., Tateishi N., Fukuda A., Murase K., Akaike N. Glycine-insensitive desensitization of N-methyl-D-aspartate receptors in acutely isolated mammalian central neurons. Neurosci Lett. 1990 Jan 1;108(1-2):93–98. doi: 10.1016/0304-3940(90)90712-i. [DOI] [PubMed] [Google Scholar]
- Studer L., Spenger C., Luthman J., Seiler R. W. NGF increases neuritic complexity of cholinergic interneurons in organotypic cultures of neonatal rat striatum. J Comp Neurol. 1994 Feb 8;340(2):281–296. doi: 10.1002/cne.903400212. [DOI] [PubMed] [Google Scholar]
- Tateishi N., Takano Y., Honda K., Yamada K., Kamiya Y., Kamiya H. Effects of intrahippocampal injections of the cholinergic neurotoxin AF64A on presynaptic cholinergic markers and on passive avoidance response in the rat. Clin Exp Pharmacol Physiol. 1987 Jul;14(7):611–618. doi: 10.1111/j.1440-1681.1987.tb01881.x. [DOI] [PubMed] [Google Scholar]
- Tremblay E., Roisin M. P., Represa A., Charriaut-Marlangue C., Ben-Ari Y. Transient increased density of NMDA binding sites in the developing rat hippocampus. Brain Res. 1988 Oct 4;461(2):393–396. doi: 10.1016/0006-8993(88)90275-2. [DOI] [PubMed] [Google Scholar]
- Verdoorn T. A., Johansen T. H., Drejer J., Nielsen E. O. Selective block of recombinant glur6 receptors by NS-102, a novel non-NMDA receptor antagonist. Eur J Pharmacol. 1994 Sep 15;269(1):43–49. doi: 10.1016/0922-4106(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Wang L. Y., MacDonald J. F. Modulation by magnesium of the affinity of NMDA receptors for glycine in murine hippocampal neurones. J Physiol. 1995 Jul 1;486(Pt 1):83–95. doi: 10.1113/jphysiol.1995.sp020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk H., Bigl V., Meyer U. Cholinergic projections from magnocellular nuclei of the basal forebrain to cortical areas in rats. Brain Res. 1980 Dec;2(3):295–316. doi: 10.1016/0165-0173(80)90011-9. [DOI] [PubMed] [Google Scholar]



