Abstract
1. Exocytosis and intracellular [Ca2+] were determined simultaneously in single anterior pituitary gonadotrophs from ovariectomized female rats. Dispersed cells were cultured for 2-4 days with or without 0.2 nM oestradiol-17 beta (E2) before use. Cells were stimulated with either gonadotrophin releasing hormone (GnRH) or by membrane depolarization. Exocytosis was determined from the change in membrane capacitance (Cm) using the perforated-patch whole-cell recording technique. Intracellular [Ca2+] was measured using fura-2 fluorescence. 2. The exocytotic response to 1 nM GnRH was characterized by a wide spectrum of responses, ranging from exocytotic bursts to relatively slow, graded increases that were dependent on the evoked intracellular Ca2+ pattern. A kinetic model is presented that incorporates the observed steep dependence of exocytosis on measured intracellular [Ca2+]; simulated exocytosis reasonably approximated observed exocytotic responses, both kinetically and quantitatively. The model also suggests that the modulatory effects of E2 are brought about either by a change in the Ca2+ sensitivity of exocytosis or by a preferential clustering of docked-secretory granules close to sites of Ca2+ release. The results suggest that in gonadotrophs an oscillatory Ca2+ signal is sensed by the exocytotic apparatus in a modified form of digital encoding. 3. Exocytosis in E2-treated cells was 3-fold greater than in non-treated cells for GnRH-evoked secretion, and 38% greater for depolarization; however, there was no effect of E2 on the intracellular Ca2+ response to either stimulus. The results show that maximum expression of the effect of E2 on exocytosis requires activation of GnRH-dependent pathways.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Elhamdani A., Palfrey H. C. Calmodulin is the divalent cation receptor for rapid endocytosis, but not exocytosis, in adrenal chromaffin cells. Neuron. 1996 Jan;16(1):195–205. doi: 10.1016/s0896-6273(00)80036-7. [DOI] [PubMed] [Google Scholar]
- Artalejo C. R., Henley J. R., McNiven M. A., Palfrey H. C. Rapid endocytosis coupled to exocytosis in adrenal chromaffin cells involves Ca2+, GTP, and dynamin but not clathrin. Proc Natl Acad Sci U S A. 1995 Aug 29;92(18):8328–8332. doi: 10.1073/pnas.92.18.8328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine G. J., Neher E. Calcium requirements for secretion in bovine chromaffin cells. J Physiol. 1992 May;450:247–271. doi: 10.1113/jphysiol.1992.sp019126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M. A., Holz R. W. Protein kinase C and clostridial neurotoxins affect discrete and related steps in the secretory pathway. Cell Mol Neurobiol. 1993 Dec;13(6):649–664. doi: 10.1007/BF00711564. [DOI] [PubMed] [Google Scholar]
- Drouva S. V., Gorenne I., Laplante E., Rérat E., Enjalbert A., Kordon C. Estradiol modulates protein kinase C activity in the rat pituitary in vivo and in vitro. Endocrinology. 1990 Jan;126(1):536–544. doi: 10.1210/endo-126-1-536. [DOI] [PubMed] [Google Scholar]
- Emons G., Hoffmann H. G., Brack C., Ortmann O., Sturm R., Ball P., Knuppen R. Modulation of gonadotropin-releasing hormone receptor concentration in cultured female rat pituitary cells by estradiol treatment. J Steroid Biochem. 1988 Nov;31(5):751–756. doi: 10.1016/0022-4731(88)90282-8. [DOI] [PubMed] [Google Scholar]
- Fidler N., Fernandez J. M. Phase tracking: an improved phase detection technique for cell membrane capacitance measurements. Biophys J. 1989 Dec;56(6):1153–1162. doi: 10.1016/S0006-3495(89)82762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis K. D., Mossner R., Neher E. Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron. 1996 Jun;16(6):1209–1220. doi: 10.1016/s0896-6273(00)80147-6. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
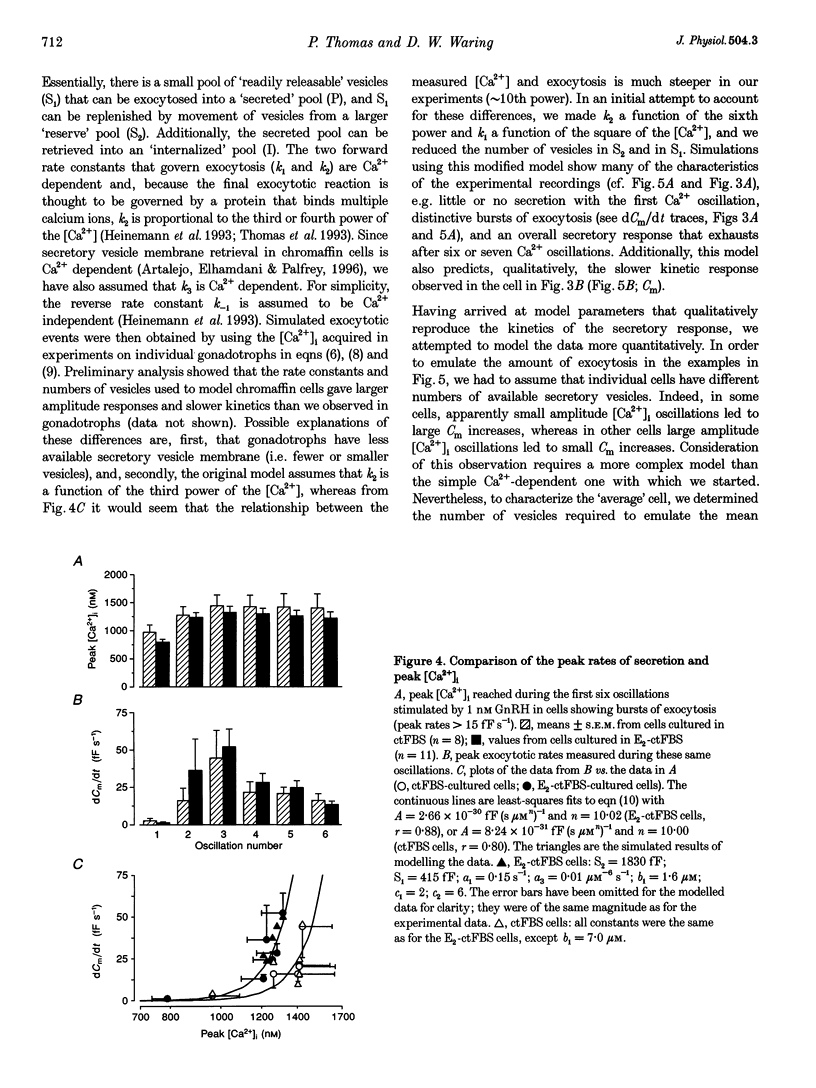
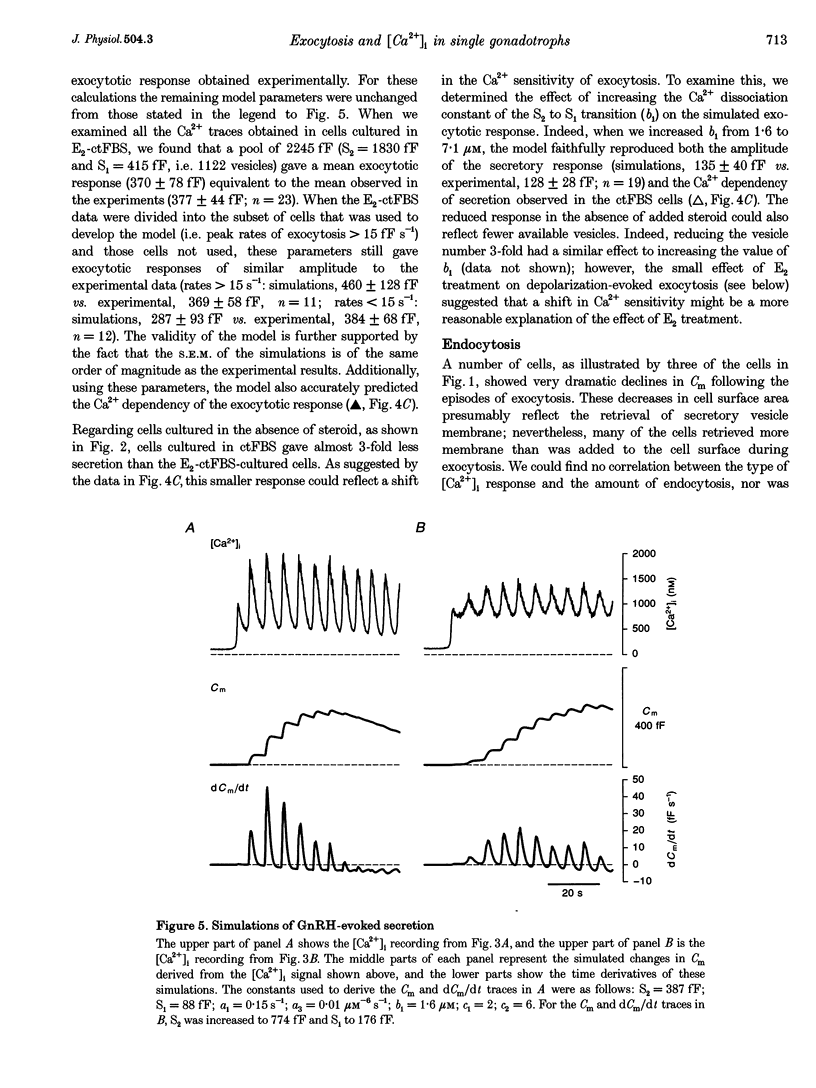
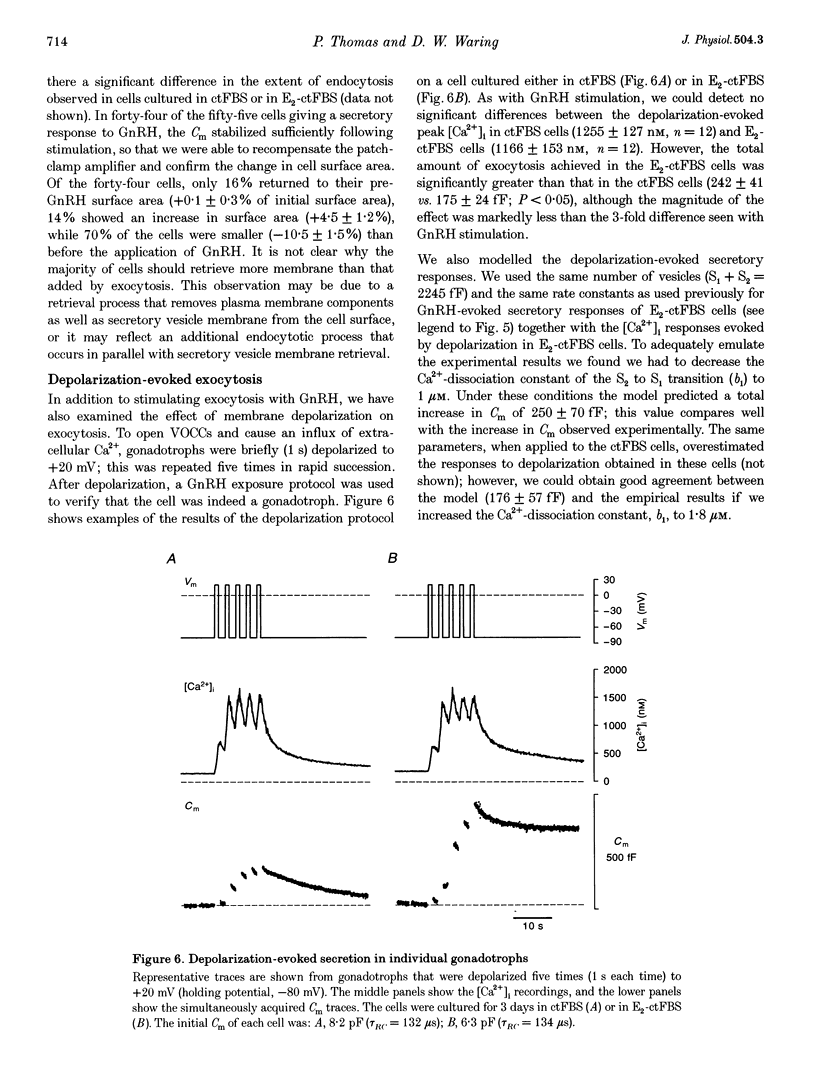
- Heinemann C., von Rüden L., Chow R. H., Neher E. A two-step model of secretion control in neuroendocrine cells. Pflugers Arch. 1993 Jul;424(2):105–112. doi: 10.1007/BF00374600. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Nuclear mechanisms of estrogen action. Effects of estradiol and anti-estrogens on estrogen receptors and nuclear receptor processing. J Biol Chem. 1978 Nov 25;253(22):8185–8191. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. The phorbol ester TPA increases the affinity of exocytosis for calcium in 'leaky' adrenal medullary cells. FEBS Lett. 1983 Aug 22;160(1-2):98–100. doi: 10.1016/0014-5793(83)80944-2. [DOI] [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Dannies P. S. Ca2+ transients induced by thyrotropin-releasing hormone rapidly lose their ability to cause release of prolactin. Mol Endocrinol. 1989 Mar;3(3):539–546. doi: 10.1210/mend-3-3-539. [DOI] [PubMed] [Google Scholar]
- Leong D. A., Thorner M. O. A potential code of luteinizing hormone-releasing hormone-induced calcium ion responses in the regulation of luteinizing hormone secretion among individual gonadotropes. J Biol Chem. 1991 May 15;266(14):9016–9022. [PubMed] [Google Scholar]
- Meyer T., Stryer L. Calcium spiking. Annu Rev Biophys Biophys Chem. 1991;20:153–174. doi: 10.1146/annurev.bb.20.060191.001101. [DOI] [PubMed] [Google Scholar]
- Ortmann O., Stojilković S. S., Cesnjaj M., Emons G., Catt K. J. Modulation of cytoplasmic calcium signaling in rat pituitary gonadotrophs by estradiol and progesterone. Endocrinology. 1992 Sep;131(3):1565–1567. doi: 10.1210/endo.131.3.1505483. [DOI] [PubMed] [Google Scholar]
- Ortmann O., Tilse B., Emons G. Modulatory actions of estradiol and progesterone on phorbol ester-stimulated LH secretion from cultured rat pituitary cells. J Steroid Biochem Mol Biol. 1992 Dec;43(7):619–627. doi: 10.1016/0960-0760(92)90286-r. [DOI] [PubMed] [Google Scholar]
- Parsons T. D., Coorssen J. R., Horstmann H., Almers W. Docked granules, the exocytic burst, and the need for ATP hydrolysis in endocrine cells. Neuron. 1995 Nov;15(5):1085–1096. doi: 10.1016/0896-6273(95)90097-7. [DOI] [PubMed] [Google Scholar]
- Samli M. H., Geschwind I. I. Some effects of energy-transfer inhibitors and of Ca++-free or K+-enhanced media on the release of luteinizing hormone (LH) from the rat pituitary gland in vitro. Endocrinology. 1968 Feb;82(2):225–231. doi: 10.1210/endo-82-2-225. [DOI] [PubMed] [Google Scholar]
- Smith G. D. Analytical steady-state solution to the rapid buffering approximation near an open Ca2+ channel. Biophys J. 1996 Dec;71(6):3064–3072. doi: 10.1016/S0006-3495(96)79500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. D., Wagner J., Keizer J. Validity of the rapid buffering approximation near a point source of calcium ions. Biophys J. 1996 Jun;70(6):2527–2539. doi: 10.1016/S0006-3495(96)79824-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojilkovic S. S., Catt K. J. Expression and signal transduction pathways of gonadotropin-releasing hormone receptors. Recent Prog Horm Res. 1995;50:161–205. doi: 10.1016/b978-0-12-571150-0.50012-3. [DOI] [PubMed] [Google Scholar]
- Stojilković S. S., Iida T., Merelli F., Torsello A., Krsmanović L. Z., Catt K. J. Interactions between calcium and protein kinase C in the control of signaling and secretion in pituitary gonadotrophs. J Biol Chem. 1991 Jun 5;266(16):10377–10384. [PubMed] [Google Scholar]
- Stutzin A., Stojilković S. S., Catt K. J., Rojas E. Characteristics of two types of calcium channels in rat pituitary gonadotrophs. Am J Physiol. 1989 Nov;257(5 Pt 1):C865–C874. doi: 10.1152/ajpcell.1989.257.5.C865. [DOI] [PubMed] [Google Scholar]
- Thomas P., Mellon P. L., Turgeon J., Waring D. W. The L beta T2 clonal gonadotrope: a model for single cell studies of endocrine cell secretion. Endocrinology. 1996 Jul;137(7):2979–2989. doi: 10.1210/endo.137.7.8770922. [DOI] [PubMed] [Google Scholar]
- Thomas P., Surprenant A., Almers W. Cytosolic Ca2+, exocytosis, and endocytosis in single melanotrophs of the rat pituitary. Neuron. 1990 Nov;5(5):723–733. doi: 10.1016/0896-6273(90)90226-6. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong J. G., Lee A. K., Almers W. A low affinity Ca2+ receptor controls the final steps in peptide secretion from pituitary melanotrophs. Neuron. 1993 Jul;11(1):93–104. doi: 10.1016/0896-6273(93)90274-u. [DOI] [PubMed] [Google Scholar]
- Tomić M., Cesnajaj M., Catt K. J., Stojilkovic S. S. Developmental and physiological aspects of Ca2+ signaling in agonist-stimulated pituitary gonadotrophs. Endocrinology. 1994 Nov;135(5):1762–1771. doi: 10.1210/endo.135.5.7956899. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Almers W., Hille B. Rhythmic exocytosis stimulated by GnRH-induced calcium oscillations in rat gonadotropes. Science. 1993 Apr 2;260(5104):82–84. doi: 10.1126/science.8385366. [DOI] [PubMed] [Google Scholar]
- Tse A., Tse F. W., Hille B. Calcium homeostasis in identified rat gonadotrophs. J Physiol. 1994 Jun 15;477(Pt 3):511–525. doi: 10.1113/jphysiol.1994.sp020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse F. W., Tse A., Hille B., Horstmann H., Almers W. Local Ca2+ release from internal stores controls exocytosis in pituitary gonadotrophs. Neuron. 1997 Jan;18(1):121–132. doi: 10.1016/s0896-6273(01)80051-9. [DOI] [PubMed] [Google Scholar]
- Tsien R. W., Tsien R. Y. Calcium channels, stores, and oscillations. Annu Rev Cell Biol. 1990;6:715–760. doi: 10.1146/annurev.cb.06.110190.003435. [DOI] [PubMed] [Google Scholar]
- Turgeon J. L., Waring D. W. Rapid augmentation by progesterone of agonist-stimulated luteinizing hormone secretion by cultured pituitary cells. Endocrinology. 1990 Aug;127(2):773–780. doi: 10.1210/endo-127-2-773. [DOI] [PubMed] [Google Scholar]
- von Rüden L., Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993 Nov 12;262(5136):1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]


