Abstract
Tidal marshes are threatened coastal ecosystems known for their capacity to store large amounts of carbon in their water-logged soils. Accurate quantification and mapping of global tidal marshes soil organic carbon (SOC) stocks is of considerable value to conservation efforts. Here, we used training data from 3710 unique locations, landscape-level environmental drivers and a global tidal marsh extent map to produce a global, spatially explicit map of SOC storage in tidal marshes at 30 m resolution. Here we show the total global SOC stock to 1 m to be 1.44 Pg C, with a third of this value stored in the United States of America. On average, SOC in tidal marshes’ 0–30 and 30–100 cm soil layers are estimated at 83.1 Mg C ha−1 (average predicted error 44.8 Mg C ha−1) and 185.3 Mg C ha−1 (average predicted error 105.7 Mg C ha−1), respectively.
Subject terms: Carbon cycle, Carbon cycle, Wetlands ecology
A new study shows the total global SOC stock of 1 m in the world’s tidal marshes to be 1.44 Pg C. On average, SOC in tidal marshes’ 0–30 cm and 30–100 cm soil layers are estimated at 83.1 Mg C ha−1 and 185.3 Mg C ha−1, respectively.
Introduction
Tidal marshes, like other blue carbon1,2 ecosystems (BCEs: mangroves, seagrasses and tidal freshwater emergent and forested wetlands), are a global soil organic carbon (SOC) hotspot owing to high rates of autochthonous and allochthonous organic matter deposition and slow decomposition in temporarily or permanently waterlogged soils. In addition to securing this carbon (C) over millennia, tidal marshes are also considered to be one of the most effective ecosystems for C accumulation3. Tidal marsh soils are capable of accreting vertically with sea level rise with inputs from allochthonous and autochthonous sources, thus limitations to C accumulation are far less likely to occur in marshes compared to terrestrial ecosystems, providing potential for continuous climate change mitigation benefits.
Tidal marshes were estimated to cover an area of 52,880 km2 in 2020, distributed across 120 countries and territories4, however, this is only a fraction of the prior extent. It is likely that over 50% of global tidal marsh habitat has been lost since 18005, with modern annual loss rates of between 0.2% and 2%6–8 resulting from global warming, sea-level rise, and anthropogenic activities such as agricultural or urban expansion, and human engineering of coastal floodplains and river systems9. Owing to the many ecosystem services tidal marshes provide, as well as their role in climate change mitigation, there is an increasing need to conserve and restore tidal marsh habitats globally10,11.
As part of effectively managing tidal marshes and quantifying their climate mitigation potential, there is a need to first understand the quantities of C stored within their soils12. Though we have information on SOC in tidal marshes at local to regional scales in areas like the conterminous United States13, Great Britain14 and Australia15, we currently lack a global scale analysis supported by extensive field-based observations and scaled up beyond temperate marshes16. Without this information, the scientific community and practitioners have to rely on global averages that are not ecosystem-specific17 and that are based on data mainly from temperate regions18, or they must collect resource-intensive in-situ field measurements.
Here, we present a global spatial model of SOC stock in tidal marshes and sampling bias-associated uncertainties. We did this by coupling a global tidal marsh extent map4 with a global dataset containing 3710 measurements of tidal marsh soil properties and C content19,20, and using a machine-learning approach including environmental covariates identified by expert elicitation as potential drivers of soil C density. To align with IPCC and other guidance21, we present spatially explicit SOC stocks to 1 m depths. This depth profile is used to represent soil more susceptible to emissions linked to disturbances. We also provide estimates for both the 0–30 cm and 30–100 cm soil layers, to align with both the numerous SOC studies that are confined to the upper layers and to match the conventional 30 cm depth for terrestrial soil C stock estimates.
Results and discussion
Global distribution of tidal marsh SOC
Our spatially explicit model predicts 1.44 Pg C in the top metre of tidal marsh soils globally. This estimate incorporates the spatial variability in tidal marsh SOC more adequately than previous studies, given that the model used training data from 3710 unique locations19,20 and hypothesis-driven landscape-level drivers (Supplementary Table 1), while previous estimates have relied on averaged values from a smaller subset of data22,23. The data used to train the model are representative of most of the environmental conditions found in tidal marshes across the world (Supplementary Fig. 1), although representation is more limited from areas with different rates of Holocene relative sea-level rise (Supplementary Fig. 1h), certain coastal morphologies (Supplementary Fig. 1i), lower minimum temperatures (Supplementary Fig. 1k) and lower potential evapotranspiration (PET) rates (Supplementary Fig. 1n). Whilst our training dataset is extensive, there is also a bias in the geographic coverage of the training data, with over 85% from the USA, UK and Australia (Supplementary Fig. 2). Given the lack of data from the Arctic and the tropics, predictions from those regions are less certain and these are identified as locations for future assessments (see “Locations for priority sampling”). To account for these limitations in the training data, our model used an area of applicability (AOA) approach24 which identifies predictions where the environmental covariates are highly dissimilar to the environmental envelope captured by the training data. Due to the high expected error associated with the predictions outside the AOA, they were removed from our final SOC maps and statistics.
Previous global tidal marsh C stock estimates have taken a wide range of values. With lower values such as the 0.43 ± 0.03 Pg C estimated in the top 0.5 m from a dataset based mostly on North American tidal marshes18, continental SOC averages to 1 m multiplied by extent estimates (1.41–2.44 Pg)25, or ranging between 0.86 and 1.35 Pg C16 estimated to a depth of 1 m from the SoilGrids map, a global machine-learning map from agricultural soils and terrestrial ecosystems data17. Conversely, simple calculations based on an average SOC value, applied to an overestimated tidal marsh extent have indicated that the global stock could be as high as 6.5 Pg C22. Our prediction of total global SOC in tidal marshes is significantly lower than this upper estimate, with our model predicting a range of 0.87–1.62 Pg C. It should be noted that our global tidal marshes SOC stock estimate is conservative because the underlying global map of tidal marshes we used only extends to 60° N and there are considerable unmapped areas of tidal marsh in the Arctic4. In addition, the combination of high expected error of predictions resulted in many areas in the tropics being removed from the statistics (Figs. S3 and S4). These removals, coupled with our currently incomplete understanding of the full distribution of tidal marshes4, suggest that carbon stocks could also be underestimated in the tropics.
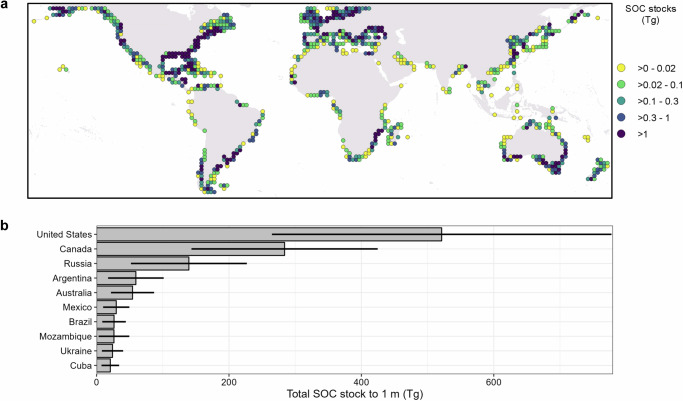
The magnitude of regional and national C stocks is strongly driven by tidal marsh area. Thus, a high proportion of the total global marsh C was located in the Temperate Northern Atlantic (Fig. 1a), which holds almost half (45%) of the global tidal marsh extent4. Countries with the highest predicted total SOC in tidal marshes (the U.S., Canada, and Russia, followed by Argentina, Australia, and Mexico—Fig. 1b and Supplementary Table 2) had both high C per unit area and large marsh extents4. Our country-level tidal marsh SOC stock estimates are consistent with those found in several regional studies. For example, stocks of 720 Tg C to 1 m were projected in the conterminous United States13, which is comparable with our value of 520 Tg C. Similarly, 5.2 Tg C was estimated for the shallow (28 ± 16 cm) tidal marsh soils of Great Britain26, which aligned with our prediction for the top 30 cm being 4.7 Tg C. Our results highlight the importance of geographical context when quantifying tidal marsh soil C stocks. For example, many marshes across Great Britain have relatively shallow soil profiles26, and as such our estimate of 1 m would overestimate the national stock. Conversely, in settings with a longer history of relative sea-level rise or very high tidal ranges, marsh soil can reach depths exceeding 1 m27,28, and thus we may underestimate total SOC stock. Not all of our findings are so well aligned with other studies. For example, we predict 19.3 Tg for China, while 57 Tg C was estimated in an earlier study (although this also included the contribution of mangroves and tidal flats)29. Such differences are likely to be driven by several factors, most strongly of which is the area of tidal marsh estimated for each country26. However, the availability of training data that accurately captures the variability of environmental conditions and the inclusion of finer-scale model predictors (e.g. data on tidal marsh plant communities) of C stocks will impact estimates.
Fig. 1. Total SOC stocks (SOC, Teragrams (Tg)) in the top 1 m of tidal marshes.
a Aggregated per 2° cell, and (b) for the ten countries with the highest total SOC stock. Values refer to predicted SOC stocks after removing pixels outside the AOA, i.e. where we enabled the model to learn about the relationship between SOC stocks and the environmental drivers. Whiskers represent the expected model error.
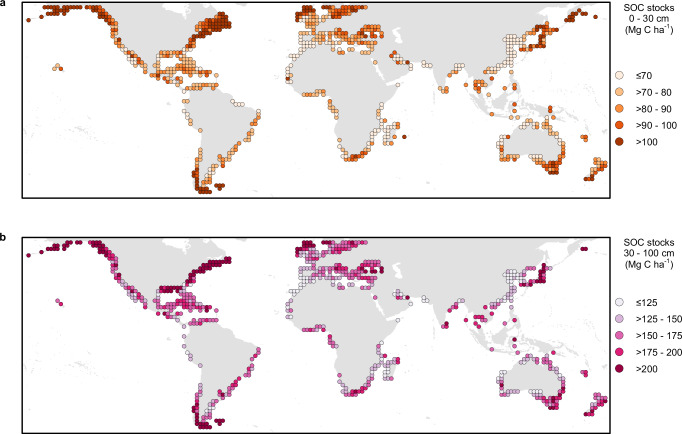
We predicted that the average SOC per hectare in tidal marshes globally is approximately 83.1 Mg C ha−1 in the 0–30 cm layer and 185.3 Mg C ha−1 in the 30–100 cm layer (Fig. 2), with an average predicted error of 44.8 Mg C ha−1 (Supplementary Fig. 3) and 105.7 Mg C ha−1 (Supplementary Fig. 4), respectively. Our value for 1 m of 268 Mg C ha−1 refines previous global estimates, such as 162 Mg C ha−1 derived from local C data of unclear origin22, and 317 Mg C ha−1 averaged from an unspecified number of studies23. Our approach accounts for the spatial variability in C and is an improvement upon averages based on reported values alone. Further, our central estimate for tidal marsh soils is within the range of those predicted for mangrove soils (232–470 Mg C ha−1)30, confirming that these BCEs store significantly more SOC per unit area than many terrestrial ecosystems31. The average expected error associated with our predictions was reasonably consistent at the regional level (Supplementary Table 3, 0–30 cm layer: 43.0–52.5; 30–100 cm layer: 102.6–122.1); however, greater variation was more apparent at finer spatial scales (Supplementary Figs. 3 and 4).
Fig. 2. Global distribution of tidal marsh SOC.
a For the 0–30 cm soil layer and (b) the 30–100 cm soil layer (aggregated per 2° cell). Values refer to predicted SOC per unit area (megagrams carbon per hectare (Mg C ha−1)) after removing pixels outside the AOA, i.e. where we enabled the model to learn about the relationship between SOC and the environmental drivers. Cells with 0% of pixels within the AOA are not displayed. Because fewer training data points were available in the deeper soil layer, more pixels are outside the AOA and thus fewer cells are displayed in the lower panel. Initial predicted values and the proportion of pixels in each cell within the AOA are presented in Supplementary Figs. 5 and 6.
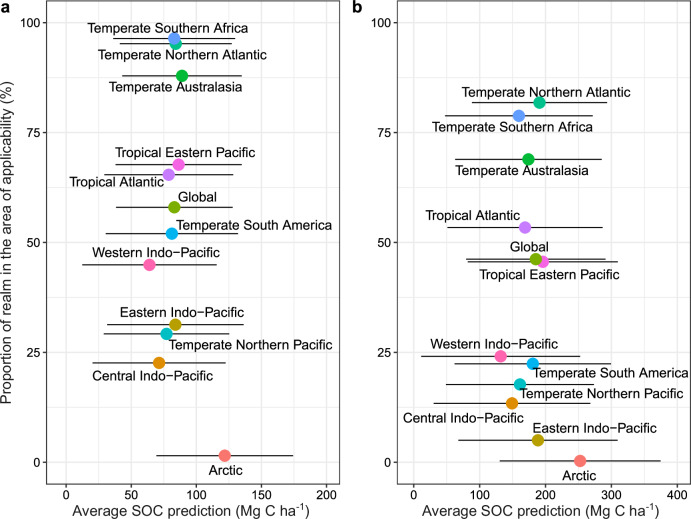
Our analysis indicates larger SOC per hectare in higher latitudes, with northern areas of the Temperate Northern Atlantic and Pacific realms having particularly high values (Fig. 2). At the regional level, within both depth layers, temperate areas generally have slightly higher average SOC values than tropical regions (Supplementary Table 3), although there is high expected model error associated with each prediction (Supplementary Figs. 3 and 4). This finding goes against the hypothesis that higher temperatures are generally associated with higher SOC32, due to the increase in productivity and growth of vegetation33. Instead, the lower soil temperature could limit SOC breakdown enhancing its storage potential33, or temperature could be a weak driver at the global scale34. The large SOC predicted to 1 m in higher latitudes is influenced by limited training data and a low proportion of our predictions in the AOA (Fig. 3). In addition, the limited understanding of processes such as glacial isostatic adjustment and the impacts of relative sea level rise and how they influence C accumulation, decomposition and storage may profoundly alter estimates for the region, and remains an ongoing area of research.
Fig. 3. Realm-level summary statistics of SOC.
In (a) the 0–30 cm soil layer and (b) the 30–100 cm soil layer. For each soil layer (0–30 cm and 30–100 cm), the x-axis shows the average final predicted SOC per unit area (megagrams carbon per hectare (Mg C ha−1)), after masking out areas outside the AOA and the y-axis shows the proportion of the realm within the AOA, i.e. where we enabled the model to learn about the relationship between SOC and the environmental drivers. Whiskers represent the expected model error for each prediction. Colours are mapped to realms, which correspond to the biogeographical realms of the Marine Ecoregions of the World, and the global average.
Drivers of SOC in tidal marshes
Overall, our model performed well in describing variation in our SOC training data, with an R2 of 0.59. We selected hypothesis-driven environmental drivers (Supplementary Table 1) to limit the number of covariates included, as well as a spatial cross-validation strategy to avoid issues of overfitting35. Soil depth was the most important driver of SOC density (Fig. 4). This is consistent with findings quantifying SOC in mangrove ecosystems30,36. Depth has a strong influence on SOC concentration and bulk density, the interaction of which results in relatively stable SOC density measurements across the soil profile13,19. Elevation was also an important variable, with higher SOC values predicted at lower elevations, which are generally characterised by higher sedimentation rates allowing more trapping of organic C37, as well as more frequent inundation providing an opportunity for deposition of tidally-distributed sediments to settle on tidal marsh surfaces38 and for plants to allocate resources to their roots39, adding organic material to the soils. Maximum SOC storage occurs just above mean sea level where (i) tidal marsh vegetation can thrive and serve as a C source, (ii) vertical space (or accommodation space) remains available for accumulation of organic matter enriched sediments from autochthonous and allochthonous sources, and (iii) decomposition of SOC is hampered by the anaerobic conditions created by higher inundation frequencies34. Temperature has been highlighted as being strongly correlated with C stocks in coastal wetlands32,40; however, within our model temperature (both maximum and minimum) had similar relative variable importance as many other covariates (Fig. 4). While our model training data does not sample the full temperature covariate space for minimum temperatures (Supplementary Fig. 1k), other research has suggested that climate may not be a significant predictor of C stocks34, as increased production and decomposition may balance out at higher temperatures. While the variable importance of the model indicates that temperatures partially drive the patterns of SOC, there is uncertainty in this assessment as underlying interactions between variables are not captured.
Fig. 4. Variable importance of the random forest model used to make the predictions of tidal marsh SOC stocks.
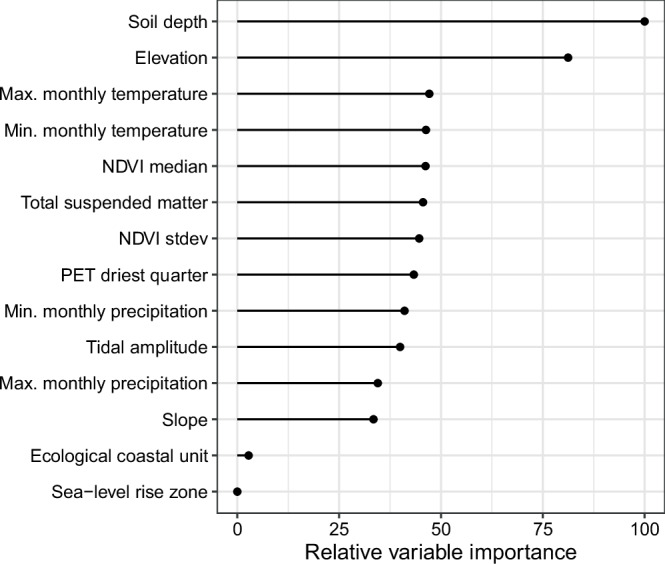
Max maximum, Min minimum, NDVI normalised difference vegetation index, stdev standard deviation, PET potential evapotranspiration. Importance was set to impurity in the model settings.
Our model includes the best globally available environmental covariates used to predict SOC in tidal marshes. However, there are a number of broad-scale drivers identified as potentially important predictors of SOC density which were poorly represented in our model or for which there were no globally available data products. For example, sea-level rise history has been linked to C storage in marshes34,41. This relationship was not apparent using our modelling method, but that is likely driven by the relatively coarse-scale classification of sea-level rise history zones42 used in the analysis. While we included the normalised difference vegetation index (NDVI) as a proxy for tidal marsh vegetation type and productivity (the source of SOC), data sources representing the distribution of dominant plant species, diversity, or species assemblages at a global scale would be an important covariate to develop43, as C stocks can vary with species and plant community14,44. Additionally, a high-resolution map of the coastal typology of tidal marshes would help refine predictions as geomorphic settings (for example deltas, estuaries, lagoons, composite deltas and lagoons, as well as sediment conditions and geology) influence the SOC stock via the type and rate of sediment supply to the coastline, nutrient loading/limitation, and organic matter diagenesis40. A global map of shoreline morphology would also better represent the accommodation space available for C storage34,45, rather than using coastal typology. Finally, our analysis provides a static estimate of tidal marsh SOC stocks driven by environmental covariates. However, it is well established that natural and anthropogenic drivers can impact tidal marsh persistence and condition, and as such their SOC stocks8. Anthropogenic disturbances could both deplete C storage (e.g. from erosion or direct habitat removal, although such impacts would remove them from our map and model) or increase C storage (e.g. from improved productivity due to nutrient additions) beyond what would be predicted by our model.
Locations for priority sampling
While our global tidal marsh SOC model is underpinned by an extensive training dataset19,20 of 42,741 observations from 3710 unique locations (Supplementary Fig. 1), the applicability of the model output is reduced in some regions where data is scarce or inexistent. Over 85% of the training locations are from the USA (n = 2005), the UK (n = 944) or Australia (n = 284). By implementing an AOA method24 we restrict predictions to locations where the relationship between the training data and environmental covariates is meaningful. Due to the sparsity of training data from some locations, areas where our model predictions are robust (the AOA) represent 58% of mapped marshes globally for the 0–30 cm layer and 46.2% for the 30–100 cm layer.
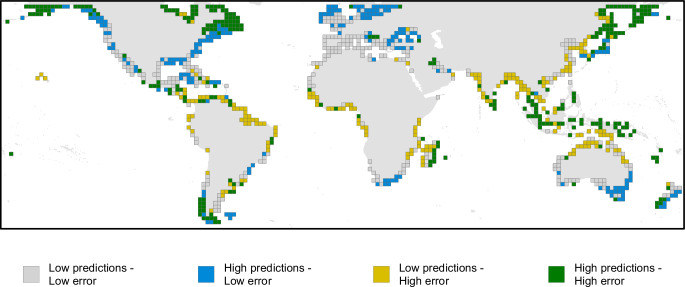
The spatial applicability of our model varies between regions, with high AOAs (>85%) for many temperate regions, but very low applicability for the Arctic and much of the tropics (Fig. 3 and Table S3). By understanding this interplay between the predictions and their uncertainty, our analysis identifies priority areas for sampling to better parameterise future models (Fig. 5). For example, our analysis predicts high SOC across the high Arctic; however, this region is characterised by limited training data and thus high per pixel expected error. In our final analysis, these areas are outside the AOA, and therefore there are significant uncertainties when estimating SOC in this region. Many temperate areas were also predicted to have high SOC, but here predictions were underpinned by extensive field measurements and thus subject to greater certainty. Finally, many tropical and subtropical areas of tidal marsh were predicted to have low SOC. Tidal marshes in these regions, where mangroves are more likely to occur, are poorly characterised46, and our predictions in these regions are limited by our knowledge of their structure and extent. SOC in tropical tidal marshes has been shown to be highly variable47 and as such our model predictions will be sensitive to the limited training data available. Our analysis suggests that the average SOC per unit area is lower (Table S3, 0–30 cm layer: 5–23%; 30–100 cm layer: 9–29%) for three out of the five tropical regions compared to the global average; a result supported by the currently available studies48.
Fig. 5. Bivariate plot showing predicted SOC stocks per unit area and expected error.
Values are for the initial model predictions and expected model error (i.e. not masked by the AOA), aggregated per 2° cell. The plot shows locations with low predictions and low error (grey), high predictions and low error (blue), low predictions and high error (yellow), and high predictions with high error (green).
Our research highlights two key pathways for future work, firstly greater SOC field measurements from the extensive areas of Arctic tidal marsh to better quantify potential stocks in those regions, areas which are significantly threatened by climate change49. Secondly, we require far greater understanding of tidal marshes within the marsh-mangrove ecotone in the tropics to characterise their role as a key BCE. Tidal marshes in tropical regions are an important component of the coastal seascape, yet our understanding of both their extent and SOC stocks is limited by available data. Within our model there was variation in the predicted SOC per unit area across tropical realms (Table S3), but the majority of predictions were associated with high expected error (Fig. 5). The mechanisms behind the variability in SOC are unknown but may relate to variation in the community composition and productivity of tropical tidal marshes.
The uncertainties in our model also have a depth component, with consistently reduced AOAs in the 30–100 cm layer compared to the 0–30 cm layer (Fig. 3). This reduction is driven by the smaller number of data points in this deeper layer (35% of all data). This disparity in data coverage over soil depth could be addressed by more consistent sampling to 1 m across studies; however, not all marshes have an organic matter layer that exceeds 30 cm depth, as is often the case in the northern Pacific coast50 and Great Britain26. These shallow, organic-rich tidal marsh soils formed more recently on top of tidal flats - thus the deeper, minerogenic layers do not hold equivalent levels of SOC found in the deeper organic-rich tidal marsh soils of many other locations. These findings indicate that estimating SOC stocks to 1 m following IPCC and other guidelines may not be appropriate in all cases, and as such we advocate providing SOC estimates across both the 0–30 cm and 30–100 cm layers.
Outlooks and policy implications
Tidal marshes hold a substantial stock of SOC, and our globally consistent map improves knowledge of climate regulation services while practically supporting the inclusion of tidal marsh blue carbon in national inventories that could be used as a baseline for C accounting. Such data could catalyse coastal ecosystem conservation and restoration efforts. Disentangling sources of error from the available training data allows a greater understanding of prediction uncertainty, while our spatially explicit analyses of expected model error clearly highlight locations where future work is urgently needed. These areas include the Arctic and the tropics, and this work should include targeted field data collection of tidal marsh extent, plant community composition and C stocks. Our dataset enables quantification of potential losses in SOC from land use change or conversely benefits from conservation and restoration. International interest in BCEs is high, strongly driven by the calls for climate action under the United Nations Framework Convention on Climate Change (UNFCCC) and the UN Sustainable Development Goals. The very high C content and high sequestration rates of tidal marshes mean they can contribute to growing efforts to mitigate climate change by avoiding future losses, securing current stocks and also through ecosystem restoration11,25. A growing number of countries are including such ecosystems in their national commitments to climate change mitigation as part of their Nationally Determined Contributions, where our study can fill in significant gaps where data is scarce or is not available.
Methods
Tidal marsh soil carbon training data
The global database was compiled from two main sources: the recently produced global tidal Marsh Soil Organic Carbon (MarSOC) Dataset19,51 (https://zenodo.org/records/8414110) and from the Coastal Carbon Research Coordination network20,52 (https://ccrcn.shinyapps.io/CoastalCarbonAtlas/). The data were filtered to those sites located between 60° N to 60° S due to the environmental covariate data being limited to this region, and within a coastal zone data mask53. This dataset contains 42,741 data points from 22 countries and 3710 unique locations27,29,32,37,48,54–247.
Spatial modelling of SOC
We used a 3D approach to model organic carbon density (OCD) to maximise the applicability of the collected data and reduce the need to make assumptions about OCD trends along the soil profile248. We thus modelled OCD as a function of depth (d), and a series of environmental covariate layers (Xn):
| 1 |
where xyd are the 3D coordinates in decimal degrees of latitude and longitude, and soil depth (measured at the centre of a sampled soil layer). The resulting spatial prediction model can then be used to predict OCD at standard depths of 0 cm, 30 cm, and 100 cm, so that the SOC for the 0–30 cm and 30–100 cm soil layer can be calculated using their respective layer thicknesses248:
| 2 |
| 3 |
Thirteen environmental covariates were used based on hypothesised landscape-level drivers of SOC in tidal marshes (Table S1). Drivers were selected by the authors whose expertise includes regional and field-based knowledge as well as considerable prior knowledge of global-scale modelling. This was an iterative process with group discussion and feedback. It was also, however, constrained by data availability. We included covariates representative of potential ecological, environmental, and geomorphological drivers.
We used the NDVI as a proxy for distinguishing vegetation type and the source of SOC. NDVI has been shown to be able to discriminate between broad classes of tidal marsh vegetation249,250. To calculate the NDVI metrics (median and standard deviation), we used Landsat 8 bands from 2014 to 2021 available at 30 m resolution, courtesy of the U.S. Geological Survey, and image processing code from Murray et al.53,251 in Google Earth Engine252.
Elevation and slope data were included, as they can be a proxy of soil age and composition, as well as vegetation structure in marshes253. Additionally, higher SOC stocks may be associated with shallower slopes, due to a lower risk of erosion compared to steeper slopes254. We used the Copernicus Digital Elevation Model GLO-30 dataset, which was developed from the TanDEM-X mission between 2011 and 2015. The product is a global dataset of elevation at 30 m resolution and has an absolute vertical accuracy of less than four metres. The slope was then derived using the ee.Terrain.slope() function in Google Earth Engine252. The dataset can be found here: https://spacedata.copernicus.eu/collections/copernicus-digital-elevation-model.
Tidal amplitude can influence the stability and resilience of marshes255, as well as the accommodation space45. We used the FES2014 Tide Model M2, which has a resolution of ~7 km, available here at https://datastore.cls.fr/catalogues/fes2014-tide-model/.
Higher SOC stocks further from estuaries can be explained by the signature of past sea level rise34. We used the five broad classes originally presented in Clark et al.42.
The total suspended matter (TSM) was retrieved from the GlobColour project, which processed the TSM data from MERIS imagery collected by the Envisat European Space Agency satellite. Monthly values at 4 km resolution were retrieved from the period 2003–2011, as these were the full years available, which were averaged to generate one TSM layer. The data can be found here: https://hermes.acri.fr/.
Each coastal setting (deltas, estuaries, lagoons, composite deltas and lagoons, bedrock, and carbonate) has an environmental signature that controls the SOC stock via the type and rate of sediment supply to the coastline, nutrient loading/limitation, and organic matter diagenesis40. We used the groups of coastlines from the Ecological Coastal Units256, which were generated by clustering the 4 million coastal line segments based on ten variables (two land, five ocean, and three coastline variables). We rasterized the 1 km shoreline segments, which were available from: https://www.arcgis.com/home/item.html?id=54df078334954c5ea6d5e1c34eda2c87.
Higher temperatures generally increase the productivity and growth of vegetation33, and are associated with higher SOC stocks32. Higher rainfall is generally associated with higher SOC by increasing the freshwater runoff and thus potentially higher deposition of allochthonous organic matter254. Both minimum and maximum monthly average values of temperature and precipitation were chosen rather than mean annual values40, as they portray environmental thresholds that may have a stronger effect on SOC stocks by constraining ecosystem functionality257, which regulates both production and decomposition rates. We used data from WorldClim BIO variables258 at 927.67 m resolution, available from: https://www.worldclim.org/data/bioclim.html.
PET is the amount of plant evaporation that would occur if there was a sufficient water source in the surrounding soil. This measure has been found to explain different ecophysiological processes in mangroves259, and may show a tradeoff in extreme climates. We used the average ENVIREM mean monthly PET of the driest quarter260, as this can represent the more extreme cases, when a prolonged dry period can have a negative effect on plant productivity. The dataset can be found here: https://envirem.github.io/#downloads.
Most covariate layers (tidal amplitude, TSM, ECUs, temperature, precipitation, PET) were land or ocean products that needed to be extrapolated to each pixel containing tidal marsh. This was done by calculating the average of neighbouring pixels using a circle-shaped boolean kernel in Google Earth Engine252, with the functions ee.Image.reduceNeighborhood(), either ee.Reducer.mean() or ee.Reducer.mode() for categorical variables, and ee.Kernel.circle(1, ‘pixels’). To get all covariate map layers to the same 30 m resolution as the DEM and the NDVI layers, we re-sampled and re-projected coarser resolution data to a unified pixel grid (World Geodetic System 1984, EPSG:4326) using bilinear interpolation. Collinearity between the continuous variables was visualised using the corrplot package (version 0.92)261, and identified generally low correlations between the variables (Supplementary Fig. 7), and below the threshold of |r| > 0.7262.
We visualised how well our training data captured the variability of the environmental covariates across the global tidal marsh extent, and as such, how potentially biased the environmental covariate data that was used to train the model was. To do this we sampled the covariate values for 10,000 points drawn randomly from across the global tidal marsh extent and compared these to the covariate values for the training locations (Supplementary Fig. 1).
Model training
We used the random forest model implemented in the ranger package (0.15.1)263 and the caret framework (6.0-94)264 within R265. Due to the limited data in many regions of the world, we used the entire dataset for model training. To test model accuracy, R2 and Root Mean Square Error (RMSE) values were calculated from all pairs of observed and predicted response values when held back from model training using the cross-validation method.
We used resampling-based cross-validation to provide an estimate of the predictive performance of the random forest model266. The choice of a cross-validation strategy is key because it determines the estimation of the model performance as well as the variable importance. Our training data is clustered due to the nature of the available measurements (i.e. large amounts of data in the USA, Australia, and the U.K.), which means that random cross-validation (CV) would only indicate the ability of the model to predict within these clusters35,267,268. A spatial cross-validation strategy, in which spatial units are held back for validation268,269, assesses the ability of the model to predict beyond the clusters, which is in line with the purpose of the model to predict into spaces that lack training data269–271. We used the k-fold nearest neighbour distance matching (k-NNDM) cross-validation presented by Linnenbrink et al.272, implemented within the CAST package (0.8.1)273, which is a variant of the leave-one-out NNDM cross-validation with reduced computation time compared to the method developed by Milà et al.270.
This k-NNDM method creates folds such that the geographic distance between sample points of different folds approximates the distance between the training samples and the prediction locations. To create the prediction locations, 5000 points were randomly selected from the global tidal marsh extent map4. We used k = 5 folds (Supplementary Fig. 8), so that the training data were clustered geographically. When the model separates the training data into training and testing at the cross-validation phase, each fold serves as a testing set while the others are used for training. By comparing the geographic distance between folds of our k-NNDM CV to those between random folds, we can see that our method better resembles the distance from prediction locations to training samples (Supplementary Fig. 9).
We implemented a model tuning step, testing 100 possible models by varying the number of variables to consider at each split (1, 2, 3, 4, or 5), the minimum node size (1, 2, 3, 5, or 10), and the number of trees (100, 200, 300, 400, or 500). Between these, the RMSE varied very little, as expected for random forest models274. We thus set mtry to 3, the minimum node size to 5, and the number of trees to 300.
Within the final random forest, variable importance was set to “impurity” within the ranger package, corresponding to the Gini index for classification. This was used to identify the relative importance of the environmental covariates to the SOC predictions.
Predictions
To align with the highest resolution covariate variable, the 10 m tidal marsh data was exported at 30 m resolution using Google Earth Engine252. The predictions were made for every 30 m pixel identified as a tidal marsh in 20204. This recent extent map was derived from earth observation data and estimates 52,880 km2 of tidal marshes between 60° N and 60° S. As described in the spatial modelling of the “SOC” section, the model predicted SOC density at 0 cm and 30 cm, which were then averaged and multiplied by 30 cm (the layer thickness) and by 100 to get a SOC stock in Mg per hectare. This was also undertaken for the 30–100 cm soil layer, using the SOC density values at 30 cm and 100 cm. Thus, each 30 m pixel has a predicted SOC stock (Mg ha−1) for the 0–30 cm and 30–100 cm soil layers.
Pixel-wise accuracy estimation
To estimate how different the prediction areas were from areas on which the model was trained, we first calculated a dissimilarity index (DI). In brief, this is calculated by dividing the minimum distance to the nearest training data point in a multidimensional predictor space that has been scaled and weighted by variable importance, by the average of the distances in the training data24. The DI is calculated based on data points that do not occur in the same cross-validation fold, thus keeping in mind the cross-validation nature of the model.
Then, we used the relationship between the DI and the prediction performance (i.e. the final model RMSE) to produce a spatially continuous estimation of the expected error associated with each SOC prediction (DItoErrormetric() in the developer’s CAST commit version from August 2023)273. This uses shape constrained additive models275 to model the relationship between the DI and the RMSE. This model can then be applied to the DI of every tidal marsh pixel to produce a spatially continuous map of the estimated accuracy of the predictions. We calculated the expected model error similarly as we did for the predictions using the spatial modelling approach, and to get an expected error in the same units as the predictions.
The analysis workflow (model training, predictions, error, and AOA) was completed using Snakemake276. We calculated average prediction and expected error values for eleven out of the twelve biogeographical realms of the Marine Ecoregions of the World277 (Fig. 3 and Table S3). There was no tidal marsh extent predicted in the twelfth realm, Southern Ocean. We also calculated averages per country using the union of the ESRI Country shapefile and the Exclusive Economic Zones from the Flanders Marine Institute278.
AOA
Although we can apply the Random Forest model to all tidal marshes globally because of the availability and preparation of covariate data for all marshes, these predictions can often be extrapolated and rendered meaningless when predictor values are too different compared to the training data271. To ensure our predictions were bounded with the environmental envelope of our training data, we implemented the AOA methodology, introduced by Meyer and Pebesma 24, to mask out areas where the model was not able to learn about the relationship between the predictors and the response (here, SOC density). We specifically excluded areas with a different covariate space where predictions of carbon stocks would be uncertain because of a lack of training and validation data. The threshold for determining the AOA was based on the outlier-removed maximum DI of the training data, i.e. data larger than the 75th percentile plus 1.5 times the interquartile range of the DI values of the cross-validated training data. The calculation of the pixel-level DI and the AOA were generated using the aoa() function, both available in the CAST package (0.8.1)273. Then, the predictor space which is greater than the AOA threshold is considered outside the AOA, and thus is masked from our predictions. For each predicted soil layer (i.e. 0–30 cm and 30–100 cm), the AOA was calculated at the upper and lower depths and then averaged. Locations considered inside the AOA (Supplementary Figs. 5b and 6b) are those where the averaged AOA is equal to 1 (i.e. AOA values of 0 or 0.5 are considered outside the AOA).
Supplementary information
Acknowledgements
We thank Daniele Baisero, Thomas Ball, and Alison Eyres for methodological help. This project benefited from funding from the Bezos Earth Fund and other donors supporting the Nature Conservancy (T.A.W., E.L., and M.D.S.). LH Pérez-Bernal provided assistance in the geochemical analysis of sediment cores from Mexico. This work was performed using resources provided by the Cambridge Service for Data-Driven Discovery (CSD3) operated by the University of Cambridge Research Computing Service (www.csd3.cam.ac.uk), provided by Dell EMC and Intel using Tier-2 funding from the Engineering and Physical Sciences Research Council (capital grant EP/T022159/1), and DiRAC funding from the Science and Technology Facilities Council (www.dirac.ac.uk). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Author contributions
T.A.W., M.D.S., E.L., and T.L.M. designed the research. T.L.M. and T.A.W. performed the analyses. M.F.A., J.A., W.E.N.A, M.S.C., M.D.d.P.C., G.C., D.F., J.H., C.J.T.L., C.E.L., M.M.M., J.R., K.R., A.C.R.-F., O.S., C.S., M.V.d.B., and L.W.-M. provided expert knowledge on tidal marsh soil carbon dynamics. C.J.T.L. M.L., N.M., A.N., A.R., and L.W. helped with the methodology. E.L. provided funding. M.D.S. L.S.S. and T.A.W. provided supervision. The manuscript was draughted by T.L.M. and T.A.W. with contributions from all co-authors.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The training data, tidal marsh extent and environmental covariate data used in this study are publicly available (linked in the Methods). The soil organic carbon predictions, estimated model errors, and area of applicability layer for both the 0–30 cm and 30–100 cm layers are available on Zenodo (10.5281/zenodo.10940066).
Code availability
All code used in this study is available on Github279: https://github.com/Tania-Maxwell/global-marshC-map/.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tania L. Maxwell, Email: taniamaxwell7@gmail.com
Thomas A. Worthington, Email: taw52@cam.ac.uk
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-54572-9.
References
- 1.Adame, M. F. et al. All tidal wetlands are blue carbon ecosystems. Bioscience74, 253–268 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macreadie, P. I. et al. The future of blue carbon science. Nat. Commun.10, 1–13 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ouyang, X. & Lee, S. Y. Updated estimates of carbon accumulation rates in coastal marsh sediments. Biogeosciences11, 5057–5071 (2014). [Google Scholar]
- 4.Worthington, T. A. et al. The distribution of global tidal marshes from earth observation data. Glob. Ecol. Biogeogr.33, e13852 (2024). [Google Scholar]
- 5.Davidson, N. C. How much wetland has the world lost? Long-term and recent trends in global wetland area. Mar. Freshw. Res.65, 934–941 (2014). [Google Scholar]
- 6.McLeod, E. et al. A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ.9, 552–560 (2011). [Google Scholar]
- 7.Macreadie, P. I., Allen, K., Kelaher, B. P., Ralph, P. J. & Skilbeck, C. G. Paleoreconstruction of estuarine sediments reveal human-induced weakening of coastal carbon sinks. Glob. Chang. Biol.18, 891–901 (2012). [Google Scholar]
- 8.Campbell, A. D., Fatoyinbo, L., Goldberg, L. & Lagomasino, D. Global hotspots of salt marsh change and carbon emissions. Nature612, 701–706 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkinson, C. S., Cai, W. J. & Hu, X. Carbon sequestration in wetland dominated coastal systems—a global sink of rapidly diminishing magnitude. Curr. Opin. Environ. Sustain.4, 186–194 (2012). [Google Scholar]
- 10.Lotze, H. K. et al. Depletion degradation, and recovery potential of estuaries and coastal seas. Science312, 1806–1809 (2006). [DOI] [PubMed] [Google Scholar]
- 11.McMahon, L. et al. Maximizing blue carbon stocks through saltmarsh restoration. Front. Mar. Sci.10, 1106607 (2023). [Google Scholar]
- 12.Granek, E. F. et al. Ecosystem services as a common language for coastal ecosystem-based management. Conserv. Biol.24, 207–216 (2010). [DOI] [PubMed] [Google Scholar]
- 13.Holmquist, J. R. et al. Accuracy and precision of tidal wetland soil carbon mapping in the conterminous United States. Sci. Rep.8, 9478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeaton, C. et al. Using citizen science to estimate surficial soil Blue Carbon stocks in Great British saltmarshes. Front. Mar. Sci.9, 959459 (2022). [Google Scholar]
- 15.Young, M. A. et al. National scale predictions of contemporary and future blue carbon storage. Sci. Total Environ.800, 149573 (2021). [DOI] [PubMed] [Google Scholar]
- 16.Macreadie, P. I. et al. Blue carbon as a natural climate solution. Nat. Rev. Earth Environ.2, 826–839 (2021). [Google Scholar]
- 17.Hengl, T. et al. SoilGrids250m: global gridded soil information based on machine learning. PLoS One12, e0169748 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chmura, G. L., Anisfeld, S. C., Cahoon, D. R. & Lynch, J. C. Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles17, 1111 (2003). [Google Scholar]
- 19.Maxwell, T. L. et al. Global dataset of soil organic carbon in tidal marshes. Sci. Data10, 797 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmquist, J. R. et al. The Coastal Carbon Library and Atlas: open source soil data and tools supporting blue carbon research and policy. Glob. Chang. Biol.30, e17098 (2024). [DOI] [PubMed] [Google Scholar]
- 21.Howard, J. et al. Coastal Blue Carbon: Methods for Assessing Carbon Stocks and Emissions Factors in Mangroves, Tidal Salt Marshes, and Seagrass Meadows (Conservation International, Intergovernmental Oceanographic Commission of UNESCO, International Union for Conservation of Nature, Arlington, 2014).
- 22.Duarte, C. M., Losada, I. J., Hendriks, I. E., Mazarrasa, I. & Marba, N. The role of coastal plant communities for climate change mitigation and adaptation. Nat. Clim. Chang.3, 961–968 (2013). [Google Scholar]
- 23.Alongi, D. M. Carbon balance in salt marsh and mangrove ecosystems: a global synthesis. J. Mar. Sci. Eng.8, 767 (2020). [Google Scholar]
- 24.Meyer, H. & Pebesma, E. Predicting into unknown space? Estimating the area of applicability of spatial prediction models. Methods Ecol. Evol.12, 1620–1633 (2021). [Google Scholar]
- 25.Mason, V. G. et al. Blue carbon benefits from global saltmarsh restoration. Glob. Chang. Biol.29, 6517–6545 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Smeaton, C. et al. Organic carbon stocks of Great British saltmarshes. Front. Mar. Sci.10, 1229486 (2023). [Google Scholar]
- 27.Hansen, K. et al. Factors influencing the organic carbon pools in tidal marsh soils of the Elbe estuary (Germany). J. Soils Sediment.17, 47–60 (2017). [Google Scholar]
- 28.Artigas, F. et al. Long term carbon storage potential and CO2 sink strength of a restored salt marsh in New Jersey. Agric. Meteorol.200, 313–321 (2015). [Google Scholar]
- 29.Xia, S. et al. Storage, patterns and influencing factors for soil organic carbon in coastal wetlands of China. Glob. Chang. Biol.28, 6065–6085 (2022). [DOI] [PubMed] [Google Scholar]
- 30.Maxwell, T. L. et al. Global mangrove soil organic carbon stocks dataset at 30 m resolution for the year 2020 based on spatiotemporal predictive machine learning. Data Br.50, 109621 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein, A. et al. Protecting irrecoverable carbon in Earth’s ecosystems. Nat. Clim. Chang.10, 287–295 (2020). [Google Scholar]
- 32.Serrano, O. et al. Australian vegetated coastal ecosystems as global hotspots for climate change mitigation. Nat. Commun.10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith, A. J., Noyce, G. L., Megonigal, J. P., Guntenspergen, G. R. & Kirwan, M. L. Temperature optimum for marsh resilience and carbon accumulation revealed in a whole-ecosystem warming experiment. Glob. Chang. Biol.28, 3236–3245 (2022). [DOI] [PubMed] [Google Scholar]
- 34.Rogers, K. et al. Wetland carbon storage controlled by millennial-scale variation in relative sea-level rise. Nature567, 91–95 (2019). [DOI] [PubMed] [Google Scholar]
- 35.Meyer, H., Reudenbach, C., Wöllauer, S. & Nauss, T. Importance of spatial predictor variable selection in machine learning applications—moving from data reproduction to spatial prediction. Ecol. Modell.411, 108815 (2019). [Google Scholar]
- 36.Sanderman, J. et al. A global map of mangrove forest soil carbon at 30 m spatial resolution. Environ. Res. Lett.13, 055002 (2018). [Google Scholar]
- 37.Connor, R. F., Chmura, G. L. & Beecher, C. B. Carbon accumulation in Bay of Fundy salt marshes: Implications for restoration of reclaimed marshes. Glob. Biogeochem. Cycles15, 943–954 (2001). [Google Scholar]
- 38.Rogers, K., Macreadie, P. I., Kelleway, J. J. & Saintilan, N. Blue carbon in coastal landscapes: a spatial framework for assessment of stocks and additionality. Sustain. Sci.14, 453–467 (2019). [Google Scholar]
- 39.Kirwan, M. L. & Guntenspergen, G. R. Feedbacks between inundation, root production, and shoot growth in a rapidly submerging brackish marsh. J. Ecol.100, 764–770 (2012). [Google Scholar]
- 40.Rovai, A. S. et al. Global controls on carbon storage in mangrove soils. Nat. Clim. Chang.8, 534–538 (2018). [Google Scholar]
- 41.Gore, C. et al. Saltmarsh blue carbon accumulation rates and their relationship with sea-level rise on a multi-decadal timescale in northern England. Estuar. Coast. Shelf Sci.299, 108665 (2024). [Google Scholar]
- 42.Clark, J. A., Farrell, W. E. & Peltier, W. R. Global changes in postglacial sea level: a numerical calculation. Quat. Res.9, 265–287 (1978). [Google Scholar]
- 43.Sharma, R., Mishra, D. R., Levi, M. R. & Sutter, L. A. Remote sensing of surface and subsurface soil organic carbon in tidal wetlands: a review and ideas for future research. Remote Sens14, 2940 (2022). [Google Scholar]
- 44.Martinetto, P. et al. The blue carbon of southern southwest Atlantic salt marshes and their biotic and abiotic drivers. Nat. Commun.14, 1–10 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers, K. Accommodation space as a framework for assessing the response of mangroves to relative sea-level rise. Singap. J. Trop. Geogr.42, 163–183 (2021). [Google Scholar]
- 46.Viswanathan, C. et al. Salt marsh vegetation in India: Species composition, distribution, zonation pattern and conservation implications. Estuar. Coast. Shelf Sci.242, 106792 (2020). [Google Scholar]
- 47.Ruiz-Fernández, A. C. et al. Carbon burial and storage in tropical salt marshes under the influence of sea level rise. Sci. Total Environ.630, 1628–1640 (2018). [DOI] [PubMed] [Google Scholar]
- 48.Perera, N., Lokupitiya, E., Halwatura, D. & Udagedara, S. Quantification of blue carbon in tropical salt marshes and their role in climate change mitigation. Sci. Total Environ.820, 153313 (2022). [DOI] [PubMed] [Google Scholar]
- 49.Mcguire, A. D. et al. Sensitivity of the carbon cycle in the Arctic to climate change. Ecol. Monogr.79, 523–555 (2009). [Google Scholar]
- 50.Chastain, S. G., Kohfeld, K. E., Pellatt, M. G., Olid, C. & Gailis, M. Quantification of blue carbon in salt marshes of the Pacific coast of Canada. Biogeosciences19, 5751–5777 (2022). [Google Scholar]
- 51.Maxwell, T. L. et al. Database: tidal marsh soil organic carbon (MarSOC) dataset. 10.5281/zenodo.8414110 (2023).
- 52.Coastal Carbon Network. Database: coastal carbon library (version 1.3.0). 10.25573/serc.21565671.v5 (2023).
- 53.Murray, N. J. et al. High-resolution mapping of losses and gains of Earth’s tidal wetlands. Science376, 744–749 (2022). [DOI] [PubMed] [Google Scholar]
- 54.Abbott, K. M., Quirk, T. & DeLaune, R. D. Dataset: factors influencing blue carbon accumulation across a 32‐year chronosequence of created coastal marshes. 10.25573/DATA.10005215 (2019).
- 55.Abbott, K. M., Elsey-Quirk, T. & DeLaune, R. D. Factors influencing blue carbon accumulation across a 32-year chronosequence of created coastal marshes. Ecosphere10, e02828 (2019). [Google Scholar]
- 56.Adame, M. F. et al. Mangroves in arid regions: ecology, threats, and opportunities. Estuar. Coast. Shelf Sci.248, 106796 (2021). [Google Scholar]
- 57.Adame, M. F. et al. Carbon and nitrogen sequestration of Melaleuca floodplain wetlands in tropical Australia. Ecosystems23, 454–466 (2020). [Google Scholar]
- 58.Adame, M. F. et al. Carbon stocks and soil sequestration rates of tropical riverine wetlands. Biogeosciences12, 3805–3818 (2015). [Google Scholar]
- 59.Adame, M. F. et al. Carbon stocks of tropical coastal wetlands within the Karstic landscape of the Mexican Caribbean. PLoS One8, e56569 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen, J. R., Cornwell, J. C. & Baldwin, A. H. Contributions of organic and mineral matter to vertical accretion in tidal wetlands across a Chesapeake Bay subestuary. J. Mar. Sci. Eng. 9, 751 (2022).
- 61.Allen, J. R., Cornwell, J. C. & Baldwin, A. H. Dataset: contributions of organic and mineral matter to vertical accretion in tidal wetlands across a Chesapeake Bay subestuary. at 10.25573/serc.18130892.v1 (2022).
- 62.Anisfeld, S. C., Tobin, M. J. & Benoit, G. Sedimentation rates in flow-restricted and restored salt marshes in Long Island Sound. Estuaries22, 231 (1999). [Google Scholar]
- 63.Arias-Ortiz, A., Masqué, P., Paytan, A. & Baldocch, D. D. Dataset: tidal and nontidal marsh restoration: a trade-off between carbon sequestration, methane emissions, and soil accretion. J.Geophys. Res. Biogeosci.126, e2021JG006573. 10.25573/serc.15127743.v2 (2021).
- 64.Arias-Ortiz, A. et al. Tidal and nontidal marsh restoration: atrade-off between carbon sequestration, methane emissions, and soil accretion. J. Geophys. Res. Biogeosci.126, e2021JG006573 (2021). [Google Scholar]
- 65.Arriola, J. M. & Cable, J. E. Variations in carbon burial and sediment accretion along a tidal creek in a Florida salt marsh. Limnol. Oceanogr.62, S15–S28 (2017). [Google Scholar]
- 66.Baustian, M. M., Stagg, C. L., Perry, C. L., Moss, L. C. & Carruthers, T. J. B. Long-term carbon sinks in marsh soils of coastal Louisiana are at risk to wetland loss. J. Geophys. Res. Biogeosci.126, e2020JG005832 (2021). [Google Scholar]
- 67.Baustian, M. M. et al. Long-term soil carbon data and accretion from four marsh types in Mississippi River Delta in 2015. at 10.5066/P93U3B3E (Wetland and Aquatic Research Center, Gainesville, 2021).
- 68.Beasy, K. M. & Ellison, J. C. Comparison of three methods for the quantification of sediment organic carbon in salt marshes of the Rubicon Estuary, Tasmania, Australia. Int. J. Biol.5, p1 (2013). [Google Scholar]
- 69.Bernhardt, C. E. et al. Carbon budget assessment of tidal freshwater forested wetland and oligohaline marsh ecosystems along the Waccamaw and Savannah rivers, U.S.A. (2005–2016) (USGS, Gainesville, 018); 10.5066/F7TM7930.
- 70.Boyd, B. Comparison of sediment accumulation and accretion in impounded and unimpounded marshes of the Delaware Estuary (University of Delaware, 2012).
- 71.Boyd, B. M. & Sommerfield, C. K. Marsh accretion and sediment accumulation in a managed tidal wetland complex of Delaware Bay. Ecol. Eng.92, 37–46 (2016). [Google Scholar]
- 72.Boyd, B. M., Sommerfield, C. K. & Elsey-Quirk, T. Hydrogeomorphic influences on salt marsh sediment accumulation and accretion in two estuaries of the U.S. mid-Atlantic coast. Mar. Geol.383, 132–145 (2017). [Google Scholar]
- 73.Boyd, B., Sommerfield, C. K., Quirk, T. & Unger, V. Dataset: accretion and sediment accumulation in impounded and unimpounded marshes in the Delaware Estuary and Barnegat Bay. figshre 10.25573/DATA.9747065 (2019).
- 74.Breithaupt, J. L. et al. Dataset: increasing rates of carbon burial in southwest Florida coastal wetlands (Marine Science Faculty Publications, 2020); 10.25573/data.9894266.
- 75.Bryant, J. C. & Chabreck, R. H. Effects of impoundment on vertical accretion of coastal marsh. Estuaries21, 416 (1998). [Google Scholar]
- 76.Bulmer, R. H. et al. Blue carbon stocks and cross-habitat subsidies. Front. Mar. Sci.7, 380 (2020). [Google Scholar]
- 77.Bunzel, D. et al. (Table A1) Organic carbon measurements for sediment sequences TB13-1, GeoHH-GIE, GeoHH-FK and GeoHH-KWK (PANGAEA, 2019); 10.1594/PANGAEA.905218.
- 78.Burden, A., Garbutt, A. & Evans, C. D. Effect of restoration on saltmarsh carbon accumulation in Eastern England. Biol. Lett.15, 20180773 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Burden, A., Garbutt, A., Hughes, S., Oakley, S. & Tempest, J. A. Soil biochemical measurements from salt marshes of different ages on the Essex Coast, UK (UKCEH, 2011); 10.5285/0b1faab4-3539-457f-9169-b0b1fbd59bc2.
- 80.Burke, S. A., Manahan, J., Eichelmann, E. & Cott, G. M. Dublin’s saltmarshes contain climate-relevant carbon pools. Front. Mar. Sci.9, 976457 (2022). [Google Scholar]
- 81.Cahoon, D. R., Lynch, J. C. & Powell, A. N. Marsh vertical accretion in a southern California Estuary. USA Coast. Shelf Sci.43, 19–32 (1996). [Google Scholar]
- 82.Callaway, J. C., Borgnis, E. L., Turner, R. E. & Milan, C. S. Carbon sequestration and sediment accretion in San Francisco Bay tidal wetlands. Estuar. Coasts35, 1163–1181 (2012). [Google Scholar]
- 83.Callaway, J. C., Evyan L. Borgnis, R., Turner, E. & Milan, C. S. Dataset: carbon sequestration and sediment accretion in San Francisco Bay tidal wetlands. Estuar. Coasts.10.25573/DATA.9693251 (2019).
- 84.Camacho, S., Moura, D., Connor, S., Boski, T. & Gomes, A. Geochemical characteristics of sediments along the margins of an atlantic-mediterranean estuary (the Guadiana, Southeast Portugal): spatial and seasonal variations. Rev. Gest.ão Costeira Integr.14, 129–148 (2014). [Google Scholar]
- 85.Carlin, J. et al. Dataset: sedimentary organic carbon measurements in a restored coastal wetland in San Francisco Bay, CA, USA. figshare 10.25573/SERC.16416684 (2021).
- 86.Chambers, L. et al. Barataria Bay carbon mineralization and biogeochemical properties from nine soil cores. figshare 10.1575/1912/bco-dmo.775547.1 (2019).
- 87.Chmura, G. L. & Hung, G. A. Controls on salt marsh accretion: a test in salt marshes of Eastern Canada. Estuaries27, 70–81 (2004). [Google Scholar]
- 88.Cochran, J. K., Hirschberg, D. J., Wang, J. & Dere, C. Atmospheric deposition of metals to coastal waters (Long Island Sound, New York U.S.A.): evidence from saltmarsh deposits. Estuar. Coast. Shelf Sci.46, 503–522 (1998). [Google Scholar]
- 89.Conrad, S. et al. Does regional development influence sedimentary blue carbon stocks? A case study from three Australian estuaries. Front. Mar. Sci.5, 518 (2019). [Google Scholar]
- 90.Cott, G. M., Chapman, D. V. & Jansen, M. A. K. Salt marshes on substrate enriched in organic matter: the case of ombrogenic Atlantic salt marshes. Estuar. Coasts36, 595–609 (2013). [Google Scholar]
- 91.Craft, C. B., Seneca, E. D. & Broome, S. W. Vertical accretion in microtidal regularly and irregularly flooded estuarine marshes. Estuar. Coast. Shelf Sci.37, 371–386 (1993). [Google Scholar]
- 92.Craft, C. Freshwater input structures soil properties, vertical accretion, and nutrient accumulation of Georgia and U.S tidal marshes. Limnol. Oceanogr.52, 1220–1230 (2007). [Google Scholar]
- 93.Crooks, S. et al. Coastal blue carbon opportunity assessment for the snohomish estuary: the climate benefits of estuary restoration. Tec. Rep.10.13140/RG.2.1.1371.6568 (2014).
- 94.Cuellar-Martinez, T., Ruiz-Fernández, A. C., Sanchez-Cabeza, J. A., Pérez-Bernal, L. H. & Sandoval-Gil, J. Relevance of carbon burial and storage in two contrasting blue carbon ecosystems of a north-east Pacific coastal lagoon. Sci. Total Environ.675, 581–593 (2019). [DOI] [PubMed] [Google Scholar]
- 95.Cuellar-Martinez, T. et al. Temporal records of organic carbon stocks and burial rates in Mexican blue carbon coastal ecosystems throughout the Anthropocene. Glob. Planet. Change192, 103215 (2020). [Google Scholar]
- 96.Cusack, M. et al. Organic carbon sequestration and storage in vegetated coastal habitats along the western coast of the Arabian Gulf. Environ. Res. Lett.13, 074007 (2018). [Google Scholar]
- 97.Day, J. W. et al. Vegetation death and rapid loss of surface elevation in two contrasting Mississippi delta salt marshes: The role of sedimentation, autocompaction and sea-level rise. Ecol. Eng.37, 229–240 (2011). [Google Scholar]
- 98.de los Santos, C. B. et al. Sedimentary organic carbon and nitrogen sequestration across a vertical gradient on a temperate wetland seascape including salt marshes, seagrass meadows and rhizophytic macroalgae beds. Ecosystems26, 826–842 (2023). [Google Scholar]
- 99.de los Santos, C. B. et al. Vertical intertidal variation of organic matter stocks and patterns of sediment deposition in a mesotidal coastal wetland. Estuar. Coast. Shelf Sci.272, 107896 (2022). [Google Scholar]
- 100.Doughty, C. et al. Mangroves marching northward: The impacts of rising seas and temperatures on ecosystems at Kennedy Space Center. figshrae 10.25573/DATA.9695918.V1 (2019).
- 101.Doughty, C. L. et al. Mangrove range expansion rapidly increases coastal wetland carbon storage. Estua. Coasts39, 385–396 (2016). [Google Scholar]
- 102.Drake, K., Halifax, H., Adamowicz, S. C. & Craft, C. Carbon sequestration in tidal salt marshes of the northeast United States. Environ. Manag.56, 998–1008 (2015). [DOI] [PubMed] [Google Scholar]
- 103.Drexler, J. Z. et al. A long-term comparison of carbon sequestration rates in impounded and naturally tidal freshwater marshes along the Lower Waccamaw River, South Carolina. Wetlands33, 965–974 (2013). [Google Scholar]
- 104.Elsey-Quirk, T., Seliskar, D. M., Sommerfield, C. K. & Gallagher, J. L. Salt marsh carbon pool distribution in a mid-Atlantic Lagoon, USA: sea level rise implications. Wetlands31, 87–99 (2011). [Google Scholar]
- 105.Ensign, S. H., Noe, G. B., Hupp, C. R. & Skalak, K. J. Head-of-tide bottleneck of particulate material transport from watersheds to estuaries. Geophys. Res. Lett.42, 10,671–10,679 (2015). [Google Scholar]
- 106.Ensign, S. H., Noe, G. B., Hupp, C. R. & Skalak, K. J. Dataset: head of tide bottleneck of particulate material transport from watersheds to estuaries (USGS, 2021); 10.25573/SERC.13483332.
- 107.Ewers Lewis, C. J., Carnell, P. E., Sanderman, J., Baldock, J. A. & Macreadie, P. I. Variability and vulnerability of coastal ‘blue carbon’ stocks: a case study from southeast Australia. Ecosystems21, 263–279 (2018). [Google Scholar]
- 108.Drexler, J. Z., Woo, I., Fuller, C. C. & Nakai, G. Carbon accumulation and vertical accretion in a restored versus historic salt marsh in southern Puget Sound, Washington, United States. Restor. Ecol.27, 1117–1127 (2019). [Google Scholar]
- 109.Fell, C., Adgie, T. & Chapman, S. Dataset: carbon sequestration across northeastern Florida coastal wetlands. figshare 10.25573/serc.15043920.v1 (2021).
- 110.Ferronato, C. et al. Effect of waterlogging on soil biochemical properties and organic matter quality in different salt marsh systems. Geoderma338, 302–312 (2019). [Google Scholar]
- 111.Ford, H., Garbutt, A. & Skov, M. Coastal biodiversity and ecosystem service sustainability (CBESS) soil organic matter content from three soil depths on saltmarsh sites at Morecambe Bay and Essex (UKCEH, 2016); 10.5285/90457ba1-f291-4158-82dc-425d7cbb1ac5.
- 112.Fu, C. et al. Stocks and losses of soil organic carbon from Chinese vegetated coastal habitats. Glob. Chang. Biol.27, 202–214 (2021). [DOI] [PubMed] [Google Scholar]
- 113.Fuchs, M. et al. Soil carbon and nitrogen stocks in Arctic river deltas: new data for three Northwest Alaskan deltas. Epic. Eur. Conf. Permafr. Chamonix Mont-Blanc (2018).
- 114.Gailis, M., Kohfeld, K. E., Pellatt, M. G. & Carlson, D. Quantifying blue carbon for the largest salt marsh in southern British Columbia: implications for regional coastal management. Coast. Eng. J.63, 275–309 (2021). [Google Scholar]
- 115.Gallagher, J. B., Prahalad, V. & Aalders, J. Inorganic and black carbon hotspots constrain blue carbon mitigation services across tropical seagrass and temperate tidal marshes. Wetlands41, 65 (2021). [Google Scholar]
- 116.Gerlach, M. J. et al. Reconstructing common era relative sea-level change on the Gulf Coast of Florida. Mar. Geol.390, 254–269 (2017). [Google Scholar]
- 117.Giblin, A., Forbrich, I. & Plum Island Ecosystems LTER. PIE LTER High Marsh Sediment Chemistry and Activity Measurements, Nelson Island Creek Marsh, Rowley, MA. 10.6073/PASTA/D1D5CBF87602CCF51DE30B87B8E46D01 (2018).
- 118.Gispert, M. et al. Appraising soil carbon storage potential under perennial and annual Chenopodiaceae in salt marsh of NE Spain. Estuar. Coast. Shelf Sci.252, 107240 (2021). [Google Scholar]
- 119.Gispert, M., Phang, C. & Carrasco-Barea, L. The role of soil as a carbon sink in coastal salt-marsh and agropastoral systems at La Pletera, NE Spain. CATENA185, 104331 (2020). [Google Scholar]
- 120.Gonneea, M. E., O’Keefe Suttles, J. A. & Kroeger, K. D. Collection, analysis, and age-dating of sediment cores from salt marshes on the south shore of Cape Cod, Massachusetts, from 2013 through 2014 (U.S. Geological Survey data release, 2018); 10.5066/F7H41QPP.
- 121.González-Alcaraz, M. N., Aránega, B., Conesa, H. M., Delgado, M. J. & Álvarez-Rogel, J. Contribution of soil properties to the assessment of a seawater irrigation programme as a management strategy for abandoned solar saltworks. CATENA126, 189–200 (2015). [Google Scholar]
- 122.González-Alcaraz, M. N. et al. Storage of organic carbon, nitrogen and phosphorus in the soil–plant system of Phragmites australis stands from a eutrophicated Mediterranean salt marsh. Geoderma185–186, 61–72 (2012). [Google Scholar]
- 123.Gorham, C., Lavery, P., Kelleway, J. J., Salinas, C. & Serrano, O. Soil carbon stocks vary across geomorphic settings in Australian temperate tidal marsh ecosystems. Ecosystems24, 319–334 (2021). [Google Scholar]
- 124.Graversen, A. E. L., Banta, G. T., Masque, P. & Krause-Jensen, D. Carbon sequestration is not inhibited by livestock grazing in Danish salt marshes. Limnol. Oceanogr.67, S19–S35 (2022). [Google Scholar]
- 125.Grey, A. et al. Geochemical mapping of a blue carbon zone: Investigation of the influence of riverine input on tidal affected zones in Bull Island. Reg. Stud. Mar. Sci.45, 101834 (2021). [Google Scholar]
- 126.Gu, J., VanArdenne, L. & Chmura, G. Data for: invasive phragmites increases blue carbon stock and soil volume in a St. Lawrence estuary marsh. J.Geophys.Res.Biogeosci.10.17632/2DG3SPXSBH.2 (2020).
- 127.Guerra, R., Simoncelli, S. & Pasteris, A. Carbon accumulation and storage in a temperate coastal lagoon under the influence of recent climate change (Northwestern Adriatic Sea). Reg. Stud. Mar. Sci.53, 102439 (2022). [Google Scholar]
- 128.Hatje, V. et al. Vegetated coastal ecosystems in the Southwestern Atlantic Ocean are an unexploited opportunity for climate change mitigation. Commun. Earth Environ.4, 160 (2023). [Google Scholar]
- 129.Hatton, R. S., DeLaune, R. D. & Patrick, W. H. Sedimentation, accretion, and subsidence in marshes of Barataria Basin, Louisiana. Limnol. Oceanogr.28, 494–502 (1983). [Google Scholar]
- 130.Hayes, M. A. et al. Dynamics of sediment carbon stocks across intertidal wetland habitats of Moreton Bay, Australia. Glob. Chang. Biol.23, 4222–4234 (2017). [DOI] [PubMed] [Google Scholar]
- 131.He, Q. et al. Consumer regulation of the carbon cycle in coastal wetland ecosystems. Philos. Trans. R. Soc. B375, 20190451 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hill, T. D. & Anisfeld, S. C. Coastal wetland response to sea level rise in Connecticut and New York. Estuar. Coast. Shelf Sci.163, 185–193 (2015). [Google Scholar]
- 133.Holmquist, J. R. et al. [Dataset:] Accuracy and Precision of Tidal Wetland Soil Carbon Mapping in the Conterminous United States: Public Soil Carbon Data Release Version 1. 10.25572/ccrcn/10088/35684 (2018).
- 134.Hu, M., Sardans, J., Yang, X., Peñuelas, J. & Tong, C. Patterns and environmental drivers of greenhouse gas fluxes in the coastal wetlands of China: a systematic review and synthesis. Environ. Res.186, 109576 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Human, L. R. D., Els, J., Wasserman, J. & Adams, J. B. Blue carbon and nutrient stocks in salt marsh and seagrass from an urban African estuary. Sci. Total Environ.842, 156955 (2022). [DOI] [PubMed] [Google Scholar]
- 136.Kauffman, J. B. et al. Dataset: Carbon stocks in seagrass meadows, emergent marshes, and forested tidal swamps of the Pacific Northwest. figshare 10.25573/serc.12640172.v2 (2020).
- 137.Johnson, B. J., Moore, K. A., Lehmann, C., Bohlen, C. & Brown, T. A. Middle to late Holocene fluctuations of C3 and C4 vegetation in a Northern New England Salt Marsh, Sprague Marsh, Phippsburg Maine. Org. Geochem.38, 394–403 (2007). [Google Scholar]
- 138.Jones, M. C., Bernhardt, C. E., Krauss, K. W. & Noe, G. B. The impact of late holocene land use change, climate variability, and sea level rise on carbon storage in tidal freshwater wetlands on the southeastern United States coastal plain. J. Geophys. Res. Biogeosci.122, 3126–3141 (2017). [Google Scholar]
- 139.Thorne, K. M. USGS Pacific tidal marsh soil core surveys. figshare 10.5066/F7SJ1HNC (2015).
- 140.Kauffman, J. B. et al. Total ecosystem carbon stocks at the marine-terrestrial interface: blue carbon of the Pacific Northwest Coast, United States. Glob. Chang. Biol.26, 5679–5692 (2020). [DOI] [PubMed] [Google Scholar]
- 141.Kauffman, J. B. et al. SWAMP Dataset-Mangrove Soil Carbon-marisma-2017. 10.17528/CIFOR/DATA.00244.
- 142.Kemp, A. C. et al. Use of lead isotopes for developing chronologies in recent salt-marsh sediments. Quat. Geochronol.12, 40–49 (2012). [Google Scholar]
- 143.Kemp, A. C. et al. Dataset: use of lead isotopes for developing chronologies in recent salt-marsh sediments. figshare 10.25573/serc.11569419 (2020).
- 144.Keshta, A. E. Hydrology, Soil Redox, and Pore-water Iron Regulate Carbon Cycling in Natural and Restored Tidal Freshwater Wetlands in the Chesapeake Bay, Maryland, USA (University of Maryland, 2017).
- 145.Keshta, A. E., Yarwood, S. A. & Baldwin, A. H. Dataset: Soil redox and hydropattern control soil carbon stocks across different habitats in tidal freshwater wetlands in a sub-estuary of the Chesapeake Bay (Wiley, 2020); 10.25573/serc.13187549.
- 146.Keshta, A. E., Yarwood, S. A. & Baldwin, A. H. A new in situ method showed greater persistence of added soil organic matter in natural than restored wetlands. Restor. Ecol.29, e13437 (2021). [Google Scholar]
- 147.Kohfeld, K. E., Chastain, S., Pellatt, M. G. & Olid, C. Salt marsh soil carbon content, loss on ignition, dry bulk density, carbon stocks and carbon accumulation rates for Clayoquot Sound, British Columbia, Canada [dataset] (PANGAEA, 2022); 10.1594/PANGAEA.947824.
- 148.Krauss, K. W. et al. The role of the upper tidal estuary in wetland blue carbon storage and flux. Glob. Biogeochem. Cycles32, 817–839 (2018). [Google Scholar]
- 149.Kulawardhana, R. W. et al. The role of elevation, relative sea-level history and vegetation transition in determining carbon distribution in Spartina alterniflora dominated salt marshes. Estuar. Coast. Shelf Sci.154, 48–57 (2015). [Google Scholar]
- 150.Kumar, M., Boski, T., González-Vila, F. J., Jiménez-Morillo, N. T. & González-Pérez, J. A. Characteristics of organic matter sources from Guadiana Estuary salt marsh sediments (SW Iberian Peninsula). Cont. Shelf Res.197, 104076 (2020). [Google Scholar]
- 151.Lagomasino, D., Reide Corbett, D. & Walsh, J. P. Dataset: influence of wind-driven inundation and coastal geomorphology on sedimentation in two microtidal marshes, pamlico river estuary, NC. Estuar. Coasts. 10.25573/SERC.12043335 (2020).
- 152.Lagomasino, D., Reide Corbett, D. & Walsh, J. P. Influence of wind-driven inundation and coastal geomorphology on sedimentation in two microtidal marshes, Pamlico River Estuary, NC. Estuar. Coasts36, 1165–1180 (2013). [Google Scholar]
- 153.St. Laurent, K. A., Hribar, D. J., Carlson, A. J., Crawford, C. M. & Siok, D. Assessing coastal carbon variability in two Delaware tidal marshes. J. Coast. Conserv.24, 65 (2020). [Google Scholar]
- 154.St. Laurent, K. A., Hribar, D. J., Carlson, A. J., Crawford, C. M. & Siok, D. Dataset: assessing coastal carbon variability in two Delaware tidal marshes. figshare 10.25573/SERC.13315472 (2020).
- 155.Li, Y. et al. Plant biomass and soil organic carbon are main factors influencing dry-season ecosystem carbon rates in the coastal zone of the Yellow River Delta. PLoS One14, e0210768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Loomis, M. J. & Craft, C. B. Carbon sequestration and nutrient (nitrogen, phosphorus) accumulation in river-dominated tidal marshes, Georgia, USA. Soil Sci. Soc. Am. J.74, 1028–1036 (2010). [Google Scholar]
- 157.Luk, S., Spivak, A. C., Eagle, M. J. & O’keefe Suttles, J. A. Collection, analysis, and age-dating of sediment cores from a salt marsh platform and ponds, Rowley, Massachusetts, 2014–15 (USGS, 2020).
- 158.Luk, S. Y. et al. Soil organic carbon development and turnover in natural and disturbed salt marsh environments. Geophys. Res. Lett.48, e2020GL090287 (2021). [Google Scholar]
- 159.Macreadie, P. I. et al. Carbon sequestration by Australian tidal marshes. Sci. Rep.7, 44071 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Markewich, H. W. et al. Detailed descriptions for sampling, sample preparation and analyses of cores from St. Bernard Parish, Louisiana (USGS, 1998); 10.3133/ofr98429.
- 161.Martins, M. et al. Carbon and nitrogen stocks and burial rates in intertidal vegetated habitats of a mesotidal coastal lagoon. Ecosystems25, 372–386 (2022). [Google Scholar]
- 162.Mazarrasa, I. et al. Drivers of variability in blue carbon stocks and burial rates across European estuarine habitats. Sci. Total Environ.886, 163957 (2023). [DOI] [PubMed] [Google Scholar]
- 163.McClellan, S. A., Elsey-Quirk, T., Laws, E. & DeLaune, R. Data for: root-zone carbon and nitrogen pools across two chronosequences of coastal marshes formed using different restoration techniques: dredge sediment versus river sediment diversion. Ecol. Eng. 10.17632/5zbv2mb5zp.1 (2021).
- 164.McClellan, S. A., Elsey-Quirk, T., Laws, E. A. & DeLaune, R. D. Root-zone carbon and nitrogen pools across two chronosequences of coastal marshes formed using different restoration techniques: dredge sediment versus river sediment diversion. Ecol. Eng.169, 106326 (2021). [Google Scholar]
- 165.McTigue, N. et al. Dataset: carbon accumulation rates in a salt marsh over the past two millennia. figshare 10.25573/serc.11421063 (2020).
- 166.McTigue, N. et al. Sea-level rise explains changing carbon accumulation rates in a salt marsh over the past two millennia. J. Geophys. Res. Biogeosci.124, 2945–2957 (2019). [Google Scholar]
- 167.Merrill, J. Z. Tidal freshwater marshes as nutrient sinks: particulate nutrient burial and denitrification. (University of Maryland, 1999).
- 168.Messerschmidt, T. C. & Kirwan, M. L. Dataset: Soil properties and accretion rates of C3 and C4 marshes at the global change research wetland, Edgewater, Maryland. figshare 10.25573/SERC.11914140 (2020).
- 169.Miller, C. B., Rodriguez, A. B., Bost, M. C., McKee, B. A. & McTigue, N. D. Carbon accumulation rates are highest at young and expanding salt marsh edges. Commun. Earth Environ.3, 173 (2022). [Google Scholar]
- 170.Miller, L. C., Smeaton, C., Yang, H. & Austin, W. E. N. Physical and geochemical properties of Scottish saltmarsh soils (Monitoring, 2022); 10.7489/12422-1.
- 171.Morris, J. T. & Jensen, A. The carbon balance of grazed and non-grazed Spartina anglica saltmarshes at Skallingen, Denmark. J. Ecol.86, 229–242 (1998). [Google Scholar]
- 172.Nahlik, A. M. & Fennessy, M. S. Carbon storage in US wetlands. Nat. Commun.7, 13835 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Neubauer, S. C., Anderson, I. C., Constantine, J. A. & Kuehl, S. A. Sediment deposition and accretion in a mid-Atlantic (U.S.A.) tidal freshwater marsh. Estuar. Coast. Shelf Sci.54, 713–727 (2002). [Google Scholar]
- 174.Noe, G. B., Hupp, C. R., Bernhardt, C. E. & Krauss, K. W. Contemporary deposition and long-term accumulation of sediment and nutrients by tidal freshwater forested wetlands impacted by sea level rise. Estuar. Coasts39, 1006–1019 (2016). [Google Scholar]
- 175.Nogueira, J. et al. Geochemistry of coastal wetland in the Northern Saharan environment through lacustrine sediment core THI [dataset] (PANGAEA, 2020); 10.1594/PANGAEA.925024.
- 176.Nolte, S. Dataset: Does livestock grazing affect sediment deposition and accretion rates in salt marshes? Estuar. Coast. Shelf Sci.10.25573/SERC.11958996 (2020).
- 177.Nolte, S. et al. Does livestock grazing affect sediment deposition and accretion rates in salt marshes? Estuar. Coast. Shelf Sci.135, 296–305 (2013). [Google Scholar]
- 178.Nuttle, W. Tidal Freshwater Marshes as Nutrient Sinks: Particulate Nutrient Burial and Denitrification. (Environmental Data Initiative, 1996).
- 179.Nyman, J. A., DeLaune, R. D., Roberts, H. H. & Patrick, W. H. Relationship between vegetation and soil formation in a rapidly submerging coastal marsh. Mar. Ecol. Prog. Ser.96, 269–279 (1993). [Google Scholar]
- 180.O’Keefe Suttles, J. A. et al. Collection, analysis, and age-dating of sediment cores from Herring River wetlands and other nearby wetlands in Wellfleet, Massachusetts, 2015–17 (USGS, 2021); 10.5066/P95RXPHB.
- 181.O’keefe Suttles, J. A., Eagle, M. J., Mann, A. C. & Kroeger, K. D. Collection, analysis, and age-dating of sediment cores from mangrove and salt marsh ecosystems in Tampa Bay, Florida, 2015 (USGS, 2021); 10.5066/P9QB17H2.
- 182.O’Keefe Suttles, J. A. et al. Collection, analysis, and age-dating of sediment cores from natural and restored salt marshes on Cape Cod, Massachusetts, 2015–2016 (USGS, 2021); 10.5066/P9R154DY.
- 183.O’keefe Suttles, J. A. et al. Collection, analysis, and age-dating of sediment cores from salt marshes, Rhode Island, 2016 (USGS, 2021); 10.5066/P94HIDVU.
- 184.Orson, R. A., Warren, R. S. & Niering, W. A. Interpreting sea level rise and rates of vertical marsh accretion in a southern New England tidal salt marsh. Estuar. Coast. Shelf Sci.47, 419–429 (1998). [Google Scholar]
- 185.Osland, M. J. et al. U.S. Gulf of Mexico coast (TX, MS, AL, and FL) vegetation, soil, and landscape data (2013–2014) (USGS, 2016); 10.5066/F7J1017G.
- 186.Pastore, M. A., Megonigal, J. P. & Langley, J. A. Elevated CO2 and nitrogen addition accelerate net carbon gain in a brackish marsh. Biogeochemistry133, 73–87 (2017). [Google Scholar]
- 187.Patrick, W. H. & De, D. L. Subsidence. accretion. and sea level rise in south San Francisco Bay marshes. Limnol. Oceanogr.35, 1389–1395 (1990). [Google Scholar]
- 188.Peck, E. K., Wheatcroft, R. A. & Brophy, L. S. Controls on sediment accretion and blue carbon burial in tidal saline wetlands: insights from the Oregon coast, USA. J. Geophys. Res. Biogeosci.125, e2019JG005464 (2020). [Google Scholar]
- 189.Peck, E., Wheatcroft, R. & Brophy, L. Dataset: Controls on sediment accretion and blue carbon burial in tidal saline wetlands: Insights from the Oregon coast, U.S.A. J. Geophys. Res. Biogeosci. 10.25573/SERC.11317820.V2 (2020).
- 190.Piazza, S. C. et al. Geomorphic and ecological effects of Hurricanes Katrina and Rita on coastal Louisiana marsh communities (USGS, 2011); 10.3133/ofr20111094.
- 191.Pollmann, T., Böttcher, M. E. & Giani, L. Young soils of a temperate barrier island under the impact of formation and resetting by tides and wind. CATENA202, 105275 (2021). [Google Scholar]
- 192.Poppe, K. L. & Rybczyk, J. M. Dataset: Sediment carbon stocks and sequestration rates in the Pacific Northwest region of Washington, USA. figshare 10.25573/DATA.10005248 (2019).
- 193.Poppe, K. L. & Rybczyk, J. M. Tidal marsh restoration enhances sediment accretion and carbon accumulation in the Stillaguamish River estuary, Washington. PLoS One16, e0257244 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Radabaugh, K. R. et al. Coastal blue carbon assessment of mangroves, salt marshes, and salt barrens in Tampa Bay, Florida. Estuar. Coasts41, 1496–1510 (2018). [Google Scholar]
- 195.Rathore, A. P., Chaudhary, D. R. & Jha, B. Biomass production, nutrient cycling, and carbon fixation by Salicornia brachiata Roxb.: a promising halophyte for coastal saline soil rehabilitation. Int. J. Phytoremediat.18, 801–811 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Raw, J. L. et al. Salt marsh elevation and responses to future sea-level rise in the Knysna Estuary, South Africa. Afr. J. Aquat. Sci.45, 49–64 (2020). [Google Scholar]
- 197.Orson, R. A., Simpson, R. L. & Good, R. E. Rates of sediment accumulation in a tidal freshwater marsh. J. Sediment. Res.60, 859–869 (1990). [Google Scholar]
- 198.Rodriguez, A., Miller, C. & Bost, M. Salt marsh radiocarbon and loss on ignition data. figshare 10.6084/m9.figshare.20137649.v2 (2022).
- 199.Roman, C. T., Peck, J. A., Allen, J. R., King, J. W. & Appleby, P. G. Accretion of a New England (U.S.A.) salt marsh in response to inlet migration, storms, and sea-level rise. Estuar. Coast. Shelf Sci.45, 717–727 (1997). [Google Scholar]
- 200.Ruranska, P., Ladd, C. J. T., Smeaton, C., Skov, M. W. & Austin, W. E. N. Dry bulk density, loss on ignition and organic carbon content of surficial soils from English and Welsh salt marshes 2019 (UKCEH, 2022); 10.5285/e5554b83-910f-4030-8f4e-81967dc7047c.
- 201.Ruranska, P. et al. Dry bulk density, loss on ignition and organic carbon content of surficial soils from Scottish salt marshes, 2018–2019 (UKCEH, 2020); 10.5285/81a1301f-e5e2-44f9-afe0-0ea5bb08010f.
- 202.Russell, S. K., Gillanders, B. M., Detmar, S., Fotheringham, D. & Jones, A. R. Determining environmental drivers of fine-scale variability in blue carbon soil stocks. Estuar. Coasts47, 48–59 (2024). [Google Scholar]
- 203.Rybczyk, J. M. & Cahoon, D. R. Estimating the potential for submergence for two wetlands in the Mississippi River delta. Estuaries25, 985–998 (2002). [Google Scholar]
- 204.Sammul, M., Kauer, K. & Köster, T. Biomass accumulation during reed encroachment reduces efficiency of restoration of Baltic coastal grasslands. Appl. Veg. Sci.15, 219–230 (2012). [Google Scholar]
- 205.Sanborn, P. & Coxson, D. S. Dataset: Carbon data for intertidal soils and sediments, Skeena River estuary, British Columbia. figshare 10.25573/serc.12252005 (2020).
- 206.Santos, R. et al. Superficial sedimentary stocks and sources of carbon and nitrogen in coastal vegetated assemblages along a flow gradient. Sci. Rep.9, 610 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schile, L., Kauffman, J. B., Megonigal, J. P., Fourqurean, J. & Crooks, S. Abu Dhabi blue carbon project [Dataset] (Dryad, 2016); 10.15146/R3K59Z.
- 208.Schile, L. & Megonigal, J. P. Abu Dhabi Blue Carbon Demonstration Project. 10.5479/data_serc/10088/31949 (2017).
- 209.Shamrikova, E. V., Deneva, S. V. & Kubik, O. S. Spatial patterns of carbon and nitrogen in soils of the Barents Sea coastal area (Khaypudyrskaya Bay). Eurasia. Soil Sci.52, 507–517 (2019). [Google Scholar]
- 210.Siewert, M. B., Hugelius, G., Heim, B. & Faucherre, S. Landscape controls and vertical variability of soil organic carbon storage in permafrost-affected soils of the Lena River Delta. CATENA147, 725–741 (2016). [Google Scholar]
- 211.Smeaton, C. et al. Physical and Geochemical Properties of Saltmarsh Soils From Narrow Diameter Gouge Cores in Uk Saltmarshes Collected Between 2018 and 202110.5285/d301c5f5-77f5-41ba-934e-a80e1293d4cd (2022).
- 212.Smeaton, C. et al. Physical and Geochemical Properties of Saltmarsh Soils From Wide Diameter Gouge Cores in Uk Saltmarshes Collected Between 2018 And 202110.5285/279558cd-20fb-4f19-8077-4400817a4482 (2022).
- 213.Smeaton, C. et al. Physical and Geochemical Properties of Saltmarsh Soils From Wide Diameter Gouge Cores In Essex, Uk, Collected In 201910.5285/fa3f4087-528e-4c5d-90d8-6bb4675d6317 (2023).
- 214.Smeaton, C., Rees-Hughs, L., Barlow, N. L. M. & Austin, W. E. N. Sedimentological and Organic Carbon Data From the Kyle of Tongue Saltmarsh, Scotland, 201810.5285/b57ef444-54d4-47f9-8cbf-3cfef1182b55 (2021).
- 215.Smith, K. E. L., Flocks, J. G., Steyer, G. D. & Piazza, S. C. Wetland Paleoecological Study of Southwest Coastal Louisiana: Sediment Cores and Diatom Calibration Dataset10.3133/ds877 (2015).
- 216.Smith, K. E. L. Paleoecological Study of Coastal Marsh in the Chenier Plain, Louisiana: Investigating the Diatom Composition of Hurricane-deposited Sediments and a Diatom-Based Quantitative Reconstruction of Sea-Level Characteristics (University of Florida, Gainesville, 2012).
- 217.Sousa, A. I., Lillebø, A. I., Pardal, M. A. & Caçador, I. The influence of Spartina maritima on carbon retention capacity in salt marshes from warm-temperate estuaries. Mar. Pollut. Bull.61, 215–223 (2010). [DOI] [PubMed] [Google Scholar]
- 218.Spivak, A. Bulk Soil and Elemental Properties of Marsh and Infilled Pond Soils Collected in 2014–2015 within Plum Island Ecosystems LTER10.26008/1912/BCO-DMO.827298.1 (2020).
- 219.Thom, R. M. Accretion rates of low intertidal salt marshes in the Pacific Northwest. Wetlands12, 147–156 (1992). [Google Scholar]
- 220.Sun, H. et al. Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems. Sci. Total Environ.673, 502–510 (2019). [DOI] [PubMed] [Google Scholar]
- 221.Thom, R. M. Dataset: Accretion Rates of Low Intertidal Salt Marshes in the Pacific Northwest10.25573/DATA.10046189 (2019).
- 222.Thorne, K. et al. U.S. Pacific coastal wetland resilience and vulnerability to sea-level rise. Sci. Adv.4, eaao3270 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Unger, V., Elsey-Quirk, T., Sommerfield, C. & Velinsky, D. Stability of organic carbon accumulating in Spartina alterniflora-dominated salt marshes of the Mid-Atlantic U.S. Estuar. Coast. Shelf Sci.182, 179–189 (2016). [Google Scholar]
- 224.Van de Broek, M., et al. GCB, Supplementary Data. 10.17632/2nnv9bw3hh.2 (2018).
- 225.Vaughn, D., Bianchi, T., Shields, M., Kenney, W. & Osborne, T. Dataset: Increased Organic Carbon Burial in Northern Florida Mangrove-Salt Marsh Transition Zones10.25573/serc.10552004.v2 (2021).
- 226.Vaughn, D. R., Bianchi, T. S., Shields, M. R., Kenney, W. F. & Osborne, T. Z. Increased organic carbon burial in northern Florida mangrove-salt marsh transition zones. Glob. Biogeochem. Cycles34, e2019GB006334 (2020). [Google Scholar]
- 227.Vitti, S., Pellegrini, E., Casolo, V., Trotta, G. & Boscutti, F. Contrasting responses of native and alien plant species to soil properties shed new light on the invasion of dune systems. J. Plant Ecol.13, 667–675 (2020). [Google Scholar]
- 228.Voltz, B. et al. A multiproxy study of intertidal surface sediments from two macrotidal estuarine systems (Canche, Authie) in northern France: insights into environmental processes. Cont. Shelf Res.230, 104554 (2021). [Google Scholar]
- 229.Wails, C. N. et al. Assessing changes to ecosystem structure and function following invasion by Spartina alterniflora and Phragmites australis: a meta-analysis. Biol. Invasions23, 2695–2709 (2021). [Google Scholar]
- 230.Ward, M. A. et al. Blue carbon stocks and exchanges along the California coast. Biogeosciences18, 4717–4732 (2021). [Google Scholar]
- 231.Ward, M. Data From: Organic Carbon, Grain Size, Elemental/isotopic Composition [Dataset]10.5061/dryad.m0cfxpp31 (2021).
- 232.Ward, R. D. Carbon sequestration and storage in Norwegian Arctic coastal wetlands: impacts of climate change. Sci. Total Environ.748, 141343 (2020). [DOI] [PubMed] [Google Scholar]
- 233.Watson, E. B. & Byrne, R. Late holocene marsh expansion in southern San Francisco Bay, California. Estuar. Coasts36, 643–653 (2013). [Google Scholar]
- 234.Weis, A. D. Vertical Accretion Rates and Heavy Metal Chronologies in Wetland Sediments of Tijuana Estuary (San Diego State University, 1999).
- 235.Weis, D. A., Callaway, J. C. & Gersberg, R. M. Vertical accretion rates and heavy metal chronologies in wetland sediments of the Tijuana Estuary. Estuaries24, 840–850 (2001). [Google Scholar]
- 236.Weston, N. B. et al. Dataset: Recent Acceleration of Coastal Wetland Accretion Along the U.S. East Coast10.25573/serc.13043054.v2 (2022).
- 237.White, J. R., Sapkota, Y., Chambers, L. G., Cook, R. L. & Xue, Z. Biogeochemical Properties of Sediment Cores from Barataria Basin, Louisiana, 2018 and 2019. Biological and Chemical Oceanography Data Management Office (BCO-DMO)10.26008/1912/bco-dmo.833824.1 (2020).
- 238.Windham-Myers, L. et al. Biogeochemical Processes in an Urban, Restored Wetland of San Francisco Bay, California, 2007–2009; Methods and Data for Plant, Sediment and Water Parameters (USGS, 2010).
- 239.Wollenberg, J. T., Ollerhead, J. & Chmura, G. L. Rapid carbon accumulation following managed realignment on the Bay of Fundy. PLoS One13, e0193930 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yando, E. S. et al. Salt marsh-mangrove ecotones: using structural gradients to investigate the effects of woody plant encroachment on plant–soil interactions and ecosystem carbon pools. J. Ecol.104, 1020–1031 (2016). [Google Scholar]
- 241.Yang, R.-M. & Chen, L.-M. Spartina alterniflora invasion alters soil bulk density in coastal wetlands of China. L. Degrad. Dev.32, 1993–1999 (2021). [Google Scholar]
- 242.Ye, S. et al. Carbon sequestration and soil accretion in coastal wetland communities of the Yellow River Delta and Liaohe Delta, China. Estuar. Coasts38, 1885–1897 (2015). [Google Scholar]
- 243.Yu, O. T. & Chmura, G. L. Soil carbon may be maintained under grazing in a St Lawrence Estuary tidal marsh. Environ. Conserv.36, 312–320 (2009). [Google Scholar]
- 244.Yuan, H. W. et al. Sources and distribution of sedimentary organic matter along the Andong salt marsh, Hangzhou Bay. J. Mar. Syst.174, 78–88 (2017). [Google Scholar]
- 245.Yuan, J. et al. Data From: Spartina alterniflora Invasion Drastically Increases Methane Production Potential by Shifting Methanogenesis From Hydrogenotrophic to Methylotrophic Pathway in a Coastal Marsh [Dataset]10.5061/dryad.6f60v3q (2019).
- 246.Zubrzycki, S., Kutzbach, L., Grosse, G., Desyatkin, A. & Pfeiffer, E.-M. Organic carbon and total nitrogen stocks in soils of the Lena River Delta. Biogeosciences10, 3507–3524 (2013). [Google Scholar]
- 247.U.S. Environmental Protection Agency. National Wetland Cndition Assessment 2011: A Collaborative Survey of the Nation’s Wetlands (U.S. Environmental Protection Agency, Washington, 2016).
- 248.Hengl, T. & MacMilan, R. A. Predictive Soil Mapping with R. (OpenGeoHub Foundation, Wageningen, 2019).
- 249.Sun, C., Fagherazzi, S. & Liu, Y. Classification mapping of salt marsh vegetation by flexible monthly NDVI time-series using Landsat imagery. Estuar. Coast. Shelf Sci.213, 61–80 (2018). [Google Scholar]
- 250.Yeo, S. et al. Classification and mapping of saltmarsh vegetation combining multispectral images with field data. Estuar. Coast. Shelf Sci.236, 106643 (2020). [Google Scholar]
- 251.Murray, N. nick-murray/gee-global-intertidal-change-ARCHIVE: v1.0 for Manuscript Publication10.5281/zenodo.6503069 (2022).
- 252.Gorelick, N. et al. Google earth engine: planetary-scale geospatial analysis for everyone. Remote Sens. Environ.202, 18–27 (2017). [Google Scholar]
- 253.Allen, J. R. L. Morphodynamics of Holocene salt marshes: a review sketch from the Atlantic and Southern North Sea coasts of Europe. Quat. Sci. Rev.19, 1155–1231 (2000). [Google Scholar]
- 254.Spivak, A. C., Sanderman, J., Bowen, J. L., Canuel, E. A. & Hopkinson, C. S. Global-change controls on soil-carbon accumulation and loss in coastal vegetated ecosystems. Nat. Geosci.12, 685–692 (2019). [Google Scholar]
- 255.Kirwan, M. L. & Guntenspergen, G. R. Influence of tidal range on the stability of coastal marshland. J. Geophys. Res. Earth Surf.115, 2009 (2010). [Google Scholar]
- 256.Sayre, R., et al. Ecological coastal units—standardized global shoreline characteristics. In OCEANS 2022, Hampton Roads 1–4 (Hampton Roads, VA, 2022)
- 257.Easterling, D. R. et al. Climate extremes: observations, modeling, and impacts. Science289, 2068–2074 (2000). [DOI] [PubMed] [Google Scholar]
- 258.Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G. & Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol.25, 1965–1978 (2005). [Google Scholar]
- 259.Krauss, K. W. et al. Mangroves provide blue carbon ecological value at a low freshwater cost. Sci. Rep.12, 17636 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Title, P. O. & Bemmels, J. B. ENVIREM: an expanded set of bioclimatic and topographic variables increases flexibility and improves performance of ecological niche modeling. Ecography41, 291–307 (2018). [Google Scholar]
- 261.Wei, T. & Simko, V. R Package ‘corrplot’: Visualization of a Correlation Matrix (version 0.92)https://github.com/taiyun/corrplot (2021).
- 262.Dormann, C. F. et al. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography36, 27–46 (2013). [Google Scholar]
- 263.Wright, M. N. & Ziegler, A. ranger: a fast implementation of random forests for high dimensional data in C++ and R. J. Stat. Softw.77, 1–17 (2017). [Google Scholar]
- 264.Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw.28, 1–26 (2008).27774042 [Google Scholar]
- 265.R Core Team. R: A Language and Environment for Statistical Computinghttps://www.r-project.org/ (2023).
- 266.James, G., Witten, D., Hastie, T. & Tibshirani, R. An Introduction to Statistical Learning (Springer, New York, 2013).
- 267.Wenger, S. J. & Olden, J. D. Assessing transferability of ecological models: an underappreciated aspect of statistical validation. Methods Ecol. Evol.3, 260–267 (2012). [Google Scholar]
- 268.Roberts, D. R. et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography40, 913–929 (2017). [Google Scholar]
- 269.Ludwig, M., Moreno-Martinez, A., Hölzel, N., Pebesma, E. & Meyer, H. Assessing and improving the transferability of current global spatial prediction models. Glob. Ecol. Biogeogr.32, 356–368 (2023). [Google Scholar]
- 270.Milà, C., Mateu, J., Pebesma, E. & Meyer, H. Nearest neighbour distance matching leave-one-out cross-validation for map validation. Methods Ecol. Evol.13, 1304–1316 (2022). [Google Scholar]
- 271.Meyer, H. & Pebesma, E. Machine learning-based global maps of ecological variables and the challenge of assessing them. Nat. Commun.13, 2208 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Linnenbrink, J., Milà, C., Ludwig, M. & Meyer, H. kNNDM: k-fold Nearest Neighbour Distance Matching Cross-Validation for map accuracy estimation. EGUsphere10.5194/egusphere-2023-1308 (2023).
- 273.Meyer, H., Milà, C., Ludwig, M., Linnenbrink, J. & Schumacher, F. CAST: ‘caret’ Applications for Spatial-Temporal Models. R package version 1.0.2https://hannameyer.github.io/CAST/ (2024).
- 274.Schratz, P., Muenchow, J., Iturritxa, E., Richter, J. & Brenning, A. Hyperparameter tuning and performance assessment of statistical and machine-learning algorithms using spatial data. Ecol. Modell.406, 109–120 (2019). [Google Scholar]
- 275.Pya, N. & Wood, S. N. Shape constrained additive models. Stat. Comput.25, 543–559 (2015). [Google Scholar]
- 276.Mölder, F. et al. Sustainable data analysis with Snakemake. F1000Research10, 33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Spalding, M. D. et al. Marine ecoregions of the world: a bioregionalization of coastal and shelf areas. Bioscience57, 573–583 (2007). [Google Scholar]
- 278.Flanders Marine Institute. Union of the ESRI Country Shapefile and the Exclusive Economic Zones (version 3)https://www.marineregions.org/ (2020).
- 279.Maxwell, T. L. Tania-Maxwell/global-marshC-map: Release for Zenodo10.5281/zenodo.14031247 (2024).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The training data, tidal marsh extent and environmental covariate data used in this study are publicly available (linked in the Methods). The soil organic carbon predictions, estimated model errors, and area of applicability layer for both the 0–30 cm and 30–100 cm layers are available on Zenodo (10.5281/zenodo.10940066).
All code used in this study is available on Github279: https://github.com/Tania-Maxwell/global-marshC-map/.