Abstract
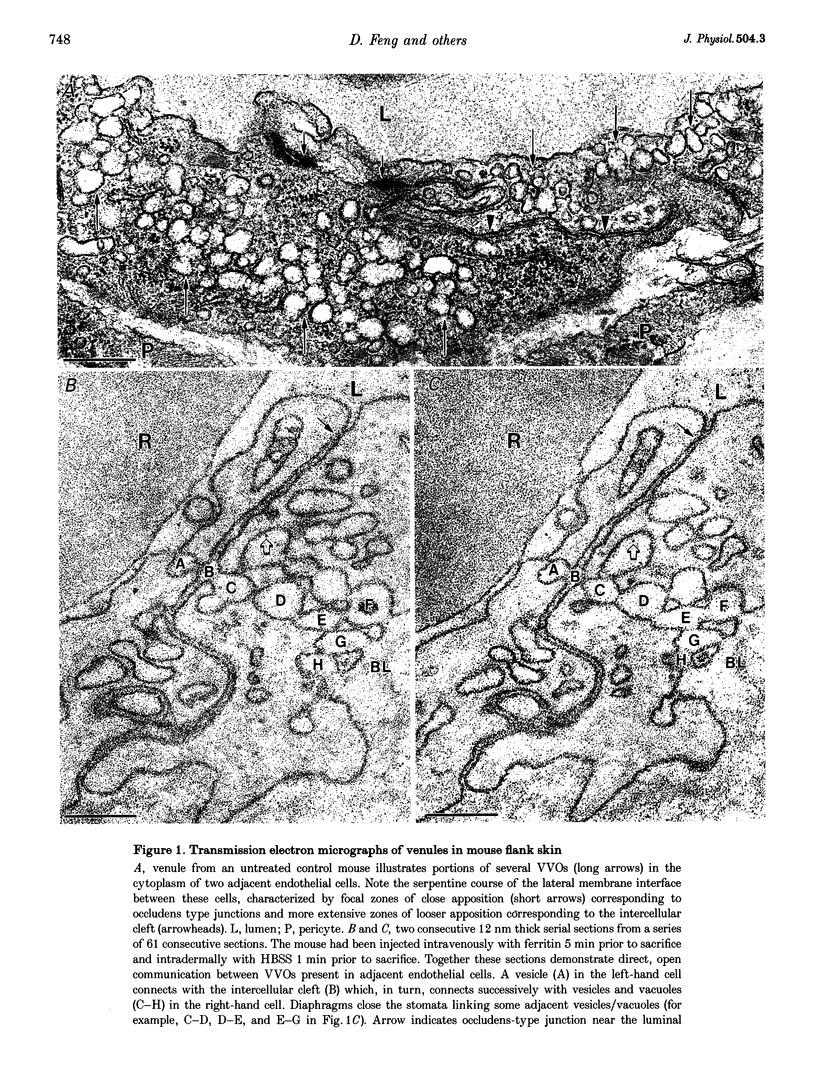
1. In response to vascular permeabilizing agents, particulates circulating in the blood extravasate from venules through endothelial cell openings. These openings have been thought to be intercellular gaps though recently this view has been challenged. 2. To define the precise location of endothelial cell gaps, serial section electron microscopy and three-dimensional reconstructions were performed in skin and cremaster muscle of guinea-pigs, mice and rats injected locally with agents that enhance microvascular permeability: vascular permeability factor, histamine or serotonin. Ferritin and colloidal carbon were injected intravenously as soluble and particulate macromolecular tracers, respectively. 3. Both tracers extravasated from venules in response to all three permeability enhancing agents. The soluble plasma protein ferritin extravasated primarily by way of vesiculo-vacuolar organelles (VVOs), interconnected clusters of vesicles and vacuoles that traverse venular endothelium. In contrast, exogenous particulates (colloidal carbon) and endogenous particulates (erythrocytes, platelets) extravasated from plasma through transendothelial openings. 4. Serial electron microscopic sections and three-dimensional reconstructions demonstrated that eighty-nine of ninety-two openings were transendothelial pores, not intercellular gaps. Pore frequency increased 3- to 33-fold when carbon was used as tracer. 5. The results demonstrate that soluble and particulate tracers extravasate from venules by apparently different transcellular pathways in response to vasoactive mediators. However, some pores may derive from rearrangements of VVOs.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALKSNE J. F. The passage of colloidal particles across the dermal capillary wall under the influence of histamine. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):51–66. doi: 10.1113/expphysiol.1959.sp001376. [DOI] [PubMed] [Google Scholar]
- Baluk P., Hirata A., Thurston G., Fujiwara T., Neal C. R., Michel C. C., McDonald D. M. Endothelial gaps: time course of formation and closure in inflamed venules of rats. Am J Physiol. 1997 Jan;272(1 Pt 1):L155–L170. doi: 10.1152/ajplung.1997.272.1.L155. [DOI] [PubMed] [Google Scholar]
- Braverman I. M., Keh-Yen A. Three-dimensional reconstruction of endothelial cell gaps in psoriatic vessels and their morphologic identity with gaps produced by the intradermal injection of histamine. J Invest Dermatol. 1986 May;86(5):577–581. doi: 10.1111/1523-1747.ep12355222. [DOI] [PubMed] [Google Scholar]
- Clough G. Relationship between microvascular permeability and ultrastructure. Prog Biophys Mol Biol. 1991;55(1):47–69. doi: 10.1016/0079-6107(91)90011-g. [DOI] [PubMed] [Google Scholar]
- Dvorak A. M., Kohn S., Morgan E. S., Fox P., Nagy J. A., Dvorak H. F. The vesiculo-vacuolar organelle (VVO): a distinct endothelial cell structure that provides a transcellular pathway for macromolecular extravasation. J Leukoc Biol. 1996 Jan;59(1):100–115. [PubMed] [Google Scholar]
- Dvorak H. F., Brown L. F., Detmar M., Dvorak A. M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am J Pathol. 1995 May;146(5):1029–1039. [PMC free article] [PubMed] [Google Scholar]
- Dvorak H. F., Orenstein N. S., Carvalho A. C., Churchill W. H., Dvorak A. M., Galli S. J., Feder J., Bitzer A. M., Rypysc J., Giovinco P. Induction of a fibrin-gel investment: an early event in line 10 hepatocarcinoma growth mediated by tumor-secreted products. J Immunol. 1979 Jan;122(1):166–174. [PubMed] [Google Scholar]
- Feng D., Nagy J. A., Hipp J., Dvorak H. F., Dvorak A. M. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996 May 1;183(5):1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Galey F., Wayland H. Action of histamine on the mesenteric microvasculature. Microvasc Res. 1980 Jan;19(1):108–126. doi: 10.1016/0026-2862(80)90087-4. [DOI] [PubMed] [Google Scholar]
- Hashimoto P. H., Takaesu S., Chazono M., Amano T. Vascular leakage through intraendothelial channels induced by cholera toxin in the skin of guinea pigs. Am J Pathol. 1974 Apr;75(1):171–180. [PMC free article] [PubMed] [Google Scholar]
- Hirata A., Baluk P., Fujiwara T., McDonald D. M. Location of focal silver staining at endothelial gaps in inflamed venules examined by scanning electron microscopy. Am J Physiol. 1995 Sep;269(3 Pt 1):L403–L418. doi: 10.1152/ajplung.1995.269.3.L403. [DOI] [PubMed] [Google Scholar]
- Joris I., Majno G., Corey E. J., Lewis R. A. The mechanism of vascular leakage induced by leukotriene E4. Endothelial contraction. Am J Pathol. 1987 Jan;126(1):19–24. [PMC free article] [PubMed] [Google Scholar]
- Kohn S., Nagy J. A., Dvorak H. F., Dvorak A. M. Pathways of macromolecular tracer transport across venules and small veins. Structural basis for the hyperpermeability of tumor blood vessels. Lab Invest. 1992 Nov;67(5):596–607. [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E., SCHOEFL G. I. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961 Dec;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJNO G., PALADE G. E. Studies on inflammation. 1. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961 Dec;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G., Gilmore V., Leventhal M. On the mechanism of vascular leakage caused by histaminetype mediators. A microscopic study in vivo. Circ Res. 1967 Dec;21(6):833–847. doi: 10.1161/01.res.21.6.833. [DOI] [PubMed] [Google Scholar]
- Majno G., Shea S. M., Leventhal M. Endothelial contraction induced by histamine-type mediators: an electron microscopic study. J Cell Biol. 1969 Sep;42(3):647–672. doi: 10.1083/jcb.42.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C. C. Capillary permeability and how it may change. J Physiol. 1988 Oct;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal C. R., Michel C. C. Openings in frog microvascular endothelium induced by high intravascular pressures. J Physiol. 1996 Apr 1;492(Pt 1):39–52. doi: 10.1113/jphysiol.1996.sp021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal C. R., Michel C. C. Transcellular gaps in microvascular walls of frog and rat when permeability is increased by perfusion with the ionophore A23187. J Physiol. 1995 Oct 15;488(Pt 2):427–437. doi: 10.1113/jphysiol.1995.sp020977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippe B., Haraldsson B. Transport of macromolecules across microvascular walls: the two-pore theory. Physiol Rev. 1994 Jan;74(1):163–219. doi: 10.1152/physrev.1994.74.1.163. [DOI] [PubMed] [Google Scholar]
- Roberts W. G., Palade G. E. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995 Jun;108(Pt 6):2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Schnittler H. J., Wilke A., Gress T., Suttorp N., Drenckhahn D. Role of actin and myosin in the control of paracellular permeability in pig, rat and human vascular endothelium. J Physiol. 1990 Dec;431:379–401. doi: 10.1113/jphysiol.1990.sp018335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Van de Water L., Brown L. F., Nagy J. A., Yeo K. T., Yeo T. K., Berse B., Jackman R. W., Dvorak A. M., Dvorak H. F. Vascular permeability factor (VPF, VEGF) in tumor biology. Cancer Metastasis Rev. 1993 Sep;12(3-4):303–324. doi: 10.1007/BF00665960. [DOI] [PubMed] [Google Scholar]