Summary
Background and Aims
While immune checkpoint inhibitors (ICIs) are revolutionising cancer therapy, checkpoint inhibitor‐induced liver injury is a significant immune‐related side effect of this immunotherapy. This study focuses on the severity classifications and characteristics of patients with checkpoint inhibitor‐induced hepatitis.
Methods
A retrospective analysis of patients with severe Checkpoint Inhibitor‐induced hepatitis grade 3 and 4 according to the recommended Common Terminology Criteria for Adverse Events (CTCAE) classification was conducted. Data on clinicobiological characteristics, treatment and outcomes were collected from 3 university hospitals, and causality was assessed by using the updated Roussel Uclaf Causality Assessment Method. The severity of hepatitis was assessed using the Model for End‐stage Liver Disease score, the Drug‐Induced Liver Injury Network, and the Drug‐Induced Liver Injury International Expert Working Group classifications.
Results
We retrospectively included 100 patients presenting various hepatitis patterns with a median time to onset of 20 days after checkpoint inhibitors. Severity grading varied significantly among the classifications used. A lower incidence of severe cases was observed when using the Drug‐Induced Liver Injury classifications instead of the recommended CCTCAE classification, and this was correlated with outcomes.
Conclusions
This retrospective study challenges the efficacy of the CTCAE classification in defining the severity of Checkpoint Inhibitor‐induced hepatitis and suggests that the traditional hepatology‐focused scores may be more relevant. The CTCAE classification is inconsistent and gives equal weight to jaundice and elevated transaminases, which leads to steroid overtreatment and limits the rechallenge of ICIs.
The recommended classification Common Terminology Criteria for Adverse Events gives equal weight to jaundice and elevated transaminases and is inconsistent with the actual severity of checkpoint inhibitor‐induced hepatitis.
1. INTRODUCTION
Immune checkpoint inhibitors (ICIs) are blocking monoclonal antibodies targeting immune checkpoint, whose activation inhibits T‐mediated antitumor response. 1 ICIs have revolutionised cancer therapy and have been approved by international drug safety agencies since the early 2010s. 2 The most prescribed ICIs in clinical practice target PD‐1 (programmed cell death‐1), its ligand PDL‐1 (programmed cell death ligand‐1) and CTLA‐4 (cytotoxic T‐lymphocyte‐associated protein‐4). 3 To date, ICIs have increasing indications, and are indicated as first‐line treatments for many advanced solid cancers as adjuvant, neoadjuvant and maintenance therapy. 2 , 4 However, the outstanding efficiency of ICIs is associated with the onset of multisystemic immune‐related adverse events (irAEs), with varying degrees of severity, due to a loss of self‐tolerance when antitumor T‐mediated immunity is restored. 5 Checkpoint inhibitor‐induced liver injury (CHILI) is one of the main side effects of ICI and may occur in up to 30% of patients treated with ICIs. 6 Recently, a retrospective study showed that the real risk of CHILI is greater than described in the literature, with an overall incidence rate of 11.5 per 1000 person‐months. 7 A meta‐analysis published in 2018 showed that CHILI accounted for 17% of fatal adverse events, among patients treated with anti‐PD(L)1. 8 Oncological international guidelines use The Common Terminology Criteria for Adverse Events (CTCAE) v5 to grade the severity of irAEs from grade 1 (mild) to 5 (fatal toxicity), 9 , 10 , 11 and CHILI management is proposed according to these grades. Although the CTCAE classification does not consider liver function, the EASL and AASLD guidelines recommend liver function assessment to evaluate the severity of drug‐induced liver injury (DILI). 12 , 13 Validated grading classifications for DILI are US Drug‐Induced Liver Injury Network (DILI‐N), which ranges from grade 1 (mild) to 5 (fatal toxicity), and International DILI Expert Working Group (DILI‐IEWG), which ranges from grade 1 (mild) to 4 (fatal toxicity). 14 , 15 In addition, the growing use of ICI has led to the emergence of CHILI with acute liver failure (ALF). 16 , 17 , 18 , 19 According to the current guidelines, ALF is defined by markers of liver damage (elevated serum transaminases) associated with impaired liver function (jaundice and INR ≥1.5) and clinical encephalopathy. 20 Acute liver injury (ALI) is defined by impaired liver function without clinical encephalopathy. 20 Drug‐induced liver injury (DILI), especially paracetamol‐related liver injury, is the most frequent cause of ALI and ALF, and may require liver transplantation (LT). 20 Also, the Model for End‐stage Liver Disease (MELD) score, which has been validated and used to predict the prognosis of cirrhotic patients, appears to be suitable in predicting poor outcomes in DILI patients. 21 , 22 Identifying CHILI with liver dysfunction is a growing challenge and it would enable us to recognise patients at risk of poor outcomes. The aim of this study is to compare the CTCAE, MELD score, DILI‐N and DILI‐IEWG classifications to predict the occurrence of ALI in patients with CHILI.
2. MATERIALS AND METHODS
2.1. Patients and data collection
We conducted a multi‐center retrospective study of consecutive CHILI patients discussed during “ToxImmun” multidisciplinary meetings between December 2018 and November 2023 at Montpellier University Hospital. The patient files were submitted from Montpellier University Hospital, Montpellier Cancer Institute and Nimes University Hospital. Inclusion criteria were (i) adult patients treated by ICI; (ii) patients with previous normal liver tests, defined as normal transaminases, ALP, GGT and bilirubin levels; (iii) CHILI presentation with abnormal liver tests, after ruling out other causes of hepatitis; and (iv) CHILI grade CTCAE 3 (severe) or 4 (life‐threatening). Patients with underlying liver disease and normal baseline liver tests were included. Patients on anticoagulants with uninterpretable international normalised ratio (INR) value were excluded. Clinical and biological CHILI‐related data were collected at diagnosis; at weeks 1, 2, and 4; and then monthly until recovery from hepatitis. Data regarding cancer, treatment of CHILI, and ICI rechallenge were also collected.
2.2. Definitions and outcomes
All patients were referred to the hepatologist and underwent an extensive evaluation to exclude other potential causes of liver enzyme abnormalities, such as viral hepatitis, autoimmune disease, cancer progression, vascular complications, or other treatments causing DILI. Liver imaging (ultrasound, computed tomography, or magnetic resonance imaging) was systematically performed. CHILI was diagnosed at onset using the updated Roussel Uclaf Causality Assessment Method (RUCAM), which aims to assess DILI causality 23 ; CHILI was defined as possible (RUCAM 3–5), probable (RUCAM 6–8), and highly probable (RUCAM ≥9). CHILI CTCAE G3 and G4 were included, and severity was graded at onset according to (i) MELD score, (ii) DILI‐N classification (the US severity classification) and (iii) DILI‐IEWG classification (the European severity classification, which is the RUCAM severity classification). 14 , 15 The DILI‐N and DILI‐IEWG scores have been measured according to the presence of jaundice, INR value ≥1.5, hospitalisation, liver or other organ failure, and death. Table S1 shows the thresholds used to define CTCAE, DILI‐N and DILI‐IEWG grades of hepatotoxicity. Hyperbilirubinemia was defined as total bilirubin ≥ N, and jaundice was defined as hyperbilirubinemia ≥42.5 μmol/L. 24 CHILI severity was classified as non‐severe (grades 1–2) and severe (grades 3–4) for each severity classification (i.e. DILI‐N, DILI‐IEWG), and we compared G3 to G4 for CTCAE severity. Liver biopsy was performed based on the referring physician's discretion, and liver histology has been blinded‐analysed by an expert pathologist. The hepatitis pattern was analysed by the serum ALT and ALP ratio (R value = (ALT/ULN)/(ALP/ULN), and classified as cholestatic (R ≤ 2), hepatocellular (R ≥ 5), or mixed (2 < R < 5)). ALT and ALP thresholds were indicated by each laboratory, as the blood tests were carried out both in the hospital and in external laboratories. The primary endpoint was the occurrence of liver dysfunction, defined as ALI, i.e. jaundice (hyperbilirubinemia ≥42.5 μmol/L) and INR ≥1.5. Secondary endpoints were hospitalisation, hepatic encephalopathy, use of a second‐line immunosuppressant, plasmapheresis, 3‐month mortality, and overall mortality.
2.3. Statistical analysis
Descriptive statistics are presented as medians (ranges) and mean (SD) for quantitative variables and counts (percentages) for qualitative variables. The Wilcoxon rank sum test was applied to compare the distribution of continuous variables and chi‐squared test (or Fisher's exact test when appropriate) was used to test the association of categorical variables. A p < 0.05 was considered statistically significant and all statistical tests were two‐sided. Survival curves were calculated using the Kaplan–Meier method and compared with the log‐rank test. We used Receiver‐Operating Characteristic curves to assess the ability to predict ALI according to CTCAE, DILI‐N and DILI‐IEWG. The area under the curve and 95% confidence intervals were calculated. DeLong test was performed to make pairwise comparisons of the predictive variables CTCAE, DILI‐N and DILI‐IEWG according to ALI. Youden index was used to select an optimal threshold value or cutoff point, based on the receiver‐operating characteristic curve. Easymedstat software was used for statistical analysis (version 3.30.2; www.easymedstat.com). This study was conducted in accordance with both the Declarations of Helsinki and Istanbul. Institutional review board approval (IRB ID 202100908) and the written informed consent of each patient were obtained.
3. RESULTS
3.1. Patients' characteristics
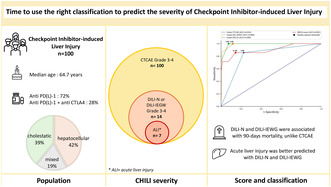
A total of 100 patients with severe CHILI according to CTCAE (G3 and G4) were included in the study (Figure 1). Clinical characteristics of CHILI cases are indicated in Table 1. The median updated RUCAM score was 8 (probable) [6 “possible”; 50 “probable”; 44 “highly probable”], and the median age was 64.7 (23–88) years, with a sex ratio of 1.4 (58 males). ICIs were mostly used to treat lung carcinoma (n = 33), melanoma (n = 32), and renal cell carcinoma (n = 20). Most patients received PD‐1 inhibitors, either alone (n = 64) or with a CTLA‐4 inhibitor (n = 28), and concurrent chemotherapy or TKI (tyrosine kinase inhibitor) were respectively given in 15% and 13%. CHILI pattern was hepatocellular in 42%, cholestatic in 39%, and mixed in 19% of patients. Median delay from last ICI infusion until CHILI was 20 days (1–175). Twenty‐six patients had bilirubinemia ≥ULN (26%), including 19 patients with jaundice (73%). Sixteen patients were admitted to hospital (16%), including four patients requiring intensive care. Liver biopsy has been performed in 37 patients: 18 patients had microscopic biliary injury (48.6%), 5 patients had interface hepatitis (13.5%), and 5 patients had bridge necrosis (13.5%). Regarding hepatitis treatment, 75 patients received steroids (median RUCAM 8; IQR 2), 41 patients received UDCA (median RUCAM 8; IQR 2), 15 patients received UDCA only (median RUCAM 8; IQR 1.5), 10 patients had no treatment (median RUCAM 8; IQR 2.75), and 9 patients received second‐line immunosuppressant (median RUCAM 7; IQR 2), mostly mycophenolate mofetil (MMF) (88.8%). The mean follow‐up duration was 15.5 months.
FIGURE 1.
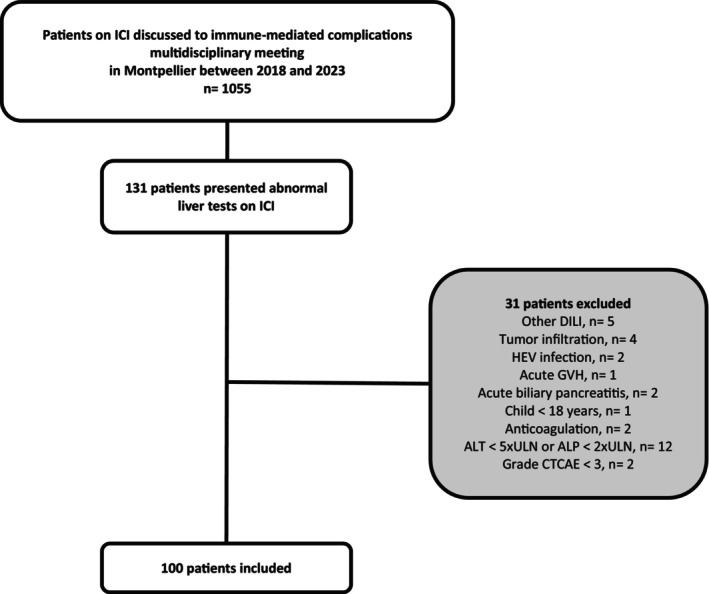
Flow chart.
TABLE 1.
Characteristics of patients with immune checkpoint‐induced liver injury (CHILI).
| N = 100 | |
|---|---|
| Age at diagnosis (years), median (range) | 64.7 (23–88) |
| Sex, n (%) | |
| Female | 42 (42) |
| Male | 58 (58) |
| Medical history, n (%) | |
| Chronic alcohol consumption | 8 (8) |
| Autoimmune disease | 2 (2) |
| Anti‐HBc IgG | 6 (6) |
| Liver transplant | 1 (1) |
| Pre‐existing liver disease, n (%) | |
| Liver metastasis | 7 (7) |
| Cirrhosis | 4 (4) |
| Cancer, n (%) | |
| Lung | 33 (33) |
| Melanoma | 32 (32) |
| Renal and urothelial | 20 (20) |
| Other cancers a | 15 (15) |
| Cancer stade, n (%) | |
| Stade III | 14 (14) |
| Stade IV | 48 (48) |
| Not evaluable | 38 (38) |
| Checkpoint inhibitor, n (%) | |
| Anti‐PD1 | 64 (64) |
| Anti‐PDL1 | 8 (8) |
| Anti‐PD1 + anti‐CTLA4 | 28 (28) |
| Concomitant oncologic treatment, n (%) | |
| Chemotherapy | 15 (15) |
| Tyrosine kinase inhibitor | 85 (85) |
| RUCAM, median (range) | 8 (4–12) |
| RUCAM, n (%) | |
| Possible (3–5) | 6 (6) |
| Probable (6–8) | 50 (50) |
| Highly probable (≥9) | 44 (44) |
| Laboratory liver tests, median (range) | |
| ALT (IU/L) | 274 (22–3111) |
| AST (IU/L) | 161 (23–4400) |
| GGT (IU/L) | 327 (10–2216) |
| ALP (IU/L) | 228 (38–2459) |
| Total bilirubin (μmol/L) | 11 (3–300) |
| Hyperbilirubinemia (total bilirubin >ULN), n (%) | 26 (26) |
| Jaundice (total bilirubin ≥2 ULN), n (%) | 19 (19) |
| INR | 1 (0.8–2.8) |
| Peak INR ≥1.5, n (%) | 9 (9) |
| Liver biopsy, n (%) | 37 (37) |
| Hospitalisation, n (%) | 16 (16) |
| CTCAE severity score, n (%) | |
| Grade 3 | 73 (73) |
| Grade 4 | 27 (27) |
| MELD score, median (median) | 7 (6–26) |
| DILI‐N severity score, n (%) | |
| Grade 1 | 82 (82) |
| Grade 2 | 4 (4) |
| Grade 3 | 9 (9) |
| Grade 4 | 5 (5) |
| DILI‐IEWG severity score, n (%) | |
| Grade 1 | 82 (82) |
| Grade 2 | 11 (11) |
| Grade 3 | 7 (7) |
Abbreviations: ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; DILI‐IEWG, Drug‐induced liver injury International Working Group; DILI‐N, Drug‐induced liver injury Network; GGT, Gamma‐glutamyl transferase; INR, International normalised ratio; RUCAM, updated Roussel Uclaf Causality Assessment Method 23 ; TBL, total bilirubin; ULN, upper limit of normal.
Head and neck cancer, hepatocellular carcinoma, colorectal cancer, oesophageal cancer, breast cancer, intestinal T‐cell lymphoma.
3.2. Severity classifications
When grading CHILI according to DILI‐N and DILI‐IEWG classifications, severe hepatitis was observed in 14% and 7%, respectively. No patients presented CHILI grade 5 according to DILI‐N, nor grade 4 according to DILI‐IEWG, as severity was assessed at onset. According to CTCAE classification, 73 patients were G3 and 27 patients were G4; Table 2 shows the comparison between these two grades. The median MELD score was 7 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 and was associated with each severity classification according to CTCAE, DILI‐N and DILI‐IEWG (p < 0.001 respectively). Liver biopsies were not significantly more frequent in CTCAE G4 hepatitis compared to G3 (51.9% vs. 31.5%; p = 0.10), but severe hepatitis according to DILI‐N (n = 10, 71.4%, p = 0.006), and DILI‐IEWG (n = 6, 85.7%; p = 0.01) were significantly more biopsied (Tables 3 and 4). Bridging necrosis was statistically associated with severe hepatitis according to DILI‐N (n = 3, 21.4%; p = 0.019), and DILI‐IEWG (n = 3, 50%; p = 0.022). There was no significant difference regarding the presence of multiple adverse events, autoimmune feature, history of liver disease, or response to cancer treatment, when comparing severity. Steroid administration and days until hepatitis resolution were not significantly increased in case of severe hepatitis, whatever the severity classification. ICI was significantly more likely to be resumed after CTCAE grade 3 hepatitis (n = 44, 61.1%; p = 0.02), and after non‐severe hepatitis according to DILI‐N (n = 51, 60%; p < 0.001) and DILI‐IEWG (n = 52, 57.1%; p = 0.004). CHILI recurrence after re‐challenging was not associated with the severity of initial CHILI, regardless of the severity classifications.
TABLE 2.
Comparison of severity according to CTCAE.
| Grade 3, n = 73 (73%) | Grade 4, n = 27 (27%) | p value | |
|---|---|---|---|
| Age (years), mean (±SD) | 64.5 (±14.1) | 66.1 (±12.2) | 0.69 |
| Sex, n (%) | |||
| Female | 31 (42.5) | 11 (40.7) | 0.99 |
| Male | 42 (57.5) | 16 (59.3) | |
| Medical history, n (%) | |||
| Cirrhosis | 3 (4.1) | 1 (3.7) | 0.99 |
| Autoimmune disease | 2 (2.8) | 0 | 0.99 |
| Anti‐HBc IgG | 5 (6.8) | 1 (3.7) | 0.64 |
| Cancer, n (%) | |||
| Lung | 24 (32.9) | 9 (33.3) | 0.70 |
| Melanoma | 22 (30.1) | 10 (37) | |
| Renal and urothelial | 17 (23.3) | 3 (11.2) | |
| Other cancers a | 10 (13.7) | 5 (18.5) | |
| Cancer stade, n (%) | |||
| Stade III | 10 (20.4) | 4 (30.8) | 0.47 |
| Stade IV | 39 (79.6) | 9 (69.2) | 0.47 |
| Liver metastasis | 4 (5.8) | 3 (13) | 0.36 |
| Checkpoint inhibitor, n (%) | |||
| Anti‐PD1 | 46 (63) | 18 (66.7) | 0.63 |
| Anti‐PDL1 | 5 (6.9) | 3 (11.1) | |
| Combotherapy with anti‐CTLA4 | 22 (30.1) | 6 (22.2) | |
| Cycles of ICI infusion, mean (±SD) | 5.9 (±7.8) | 6.3 (±6.0) | 0.24 |
| Time from last infusion until onset (days), mean (±SD) | 26.6 (±31.5) | 32.3 (±25.6) | 0.06 |
| Pattern, n (%) | |||
| Cholestatic | 26 (35.6) | 13 (48.2) | 0.18 |
| Mixed | 17 (23.3) | 2 (7.4) | |
| Hepatocellular | 30 (41.1) | 12 (44.4) | |
| Autoantibodies | |||
| ANA only | 24 (32.9) | 7 (26.9) | 0.63 |
| ASMA | 3 (4.1) | 2 (7.4) | 0.71 |
| Bile duct injury, n (%) | 6 (8.2) | 5 (18.5) | 0.16 |
| Liver biopsy, n (%) | 23 (31.5) | 14 (51.9) | 0.10 |
| Multiple irAEs, n (%) | 27 (37) | 13 (48.1) | 0.47 |
| Hospitalisation, n (%) | 4 (5.5) | 12 (44.4) | <0.001 |
| MELD score, median (range) | 7.8 (6–15) | 11.6 (6–26) | 0.006 |
| DILI‐N severity score, n (%) | |||
| Non severe | 71 (97.3) | 15 (55.6) | <0.001 |
| Severe | 2 (2.7) | 12 (44.4) | |
| DILI‐IEWG severity score, n (%) | |||
| Non severe | 73 (100) | 20 (74.1) | <0.001 |
| Severe | 0 | 7 (25.9) | |
| Hepatitis treatment, n (%) | |||
| Both steroids and UDCA | 17 (23.3) | 9 (33.3) | 0.32 |
| Steroids only | 37 (50.7) | 10 (37) | 0.32 |
| UDCA only | 8 (11) | 7 (25.9) | 0.11 |
| No treatment | 9 (12.3) | 1 (3.7) | 0.28 |
| Second‐line immunosuppressant | 3 (4.1) | 6 (22.2) | 0.01 |
| ICI rechallenge, n (%) | 44 (61.1) | 8 (30.8) | 0.02 |
| Response to cancer treatment (RECIST), n (%) | |||
| Progressive disease | 12 (16.4) | 3 (11.1) | 0.89 |
| Stable disease | 15 (20.5) | 5 (18.5) | |
| Complete or partial response | 38 (56.8) | 14 (60.8) | |
| Days until hepatitis resolution, mean (±SD) | 73.4 (±53.2) | 67.8 (±38.1) | 0.97 |
Abbreviations: ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; CTCAE, common Terminology Criteria for Adverse Events; DILI‐IEWG, drug‐induced liver injury International Working Group; DILI‐N, drug‐induced liver injury Network; ICI, immune checkpoint inhibitor; RECIST, response evaluation criteria in solid tumour; UDCA, ursodeoxycholic acid. Bold values are significant values.
Head and neck cancer, hepatocellular carcinoma, colorectal cancer, oesophageal cancer, breast cancer, intestinal T‐cell lymphoma.
TABLE 3.
Comparison of severity according to DILI‐N.
| Non severe, n = 86 (86%) | Severe, n = 14 (14%) | p value | |
|---|---|---|---|
| Age (years), mean (±SD) | 64.5 (±13.9) | 67.4 (±10.4) | 0.65 |
| Sex, n (%) | |||
| Female | 36 (41.9) | 6 (42.9) | 0.99 |
| Male | 50 (58.1) | 8 (57.1) | |
| Medical history, n (%) | |||
| Cirrhosis | 4 (4.7) | 0 | 0.99 |
| Autoimmune disease | 2 (2.4) | 0 | 0.99 |
| Anti‐HBc IgG | 4 (4.7) | 2 (14.3) | 0.59 |
| Cancer, n (%) | |||
| Lung | 31 (36.1) | 2 (14.3) | 0.31 |
| Melanoma | 26 (30.2) | 6 (42.9) | |
| Renal and urothelial | 18 (20.9) | 2 (14.3) | |
| Other cancers a | 11 (12.8) | 4 (28.5) | |
| Cancer stade, n (%) | |||
| Stade III | 13 (15.1) | 1 (7.1) | 0.99 |
| Stade IV | 43 (50) | 3 (21.4) | 0.99 |
| Liver metastasis | 4 (4.7) | 3 (21.4) | 0.04 |
| Checkpoint inhibitor, n (%) | |||
| Anti‐PD1 | 54 (62.8) | 10 (71.4) | 0.26 |
| Anti‐PDL1 | 6 (7) | 2 (14.3) | |
| Combotherapy with anti‐CTLA4 | 26 (30.2) | 2 (14.3) | |
| Cycles of ICI infusion, mean (±SD) | 6.4 (±7.8) | 3.8 (±2.5) | 0.42 |
| Time from last infusion until onset (days), mean (±SD) | 27.9 (±30.1) | 30.6 (±30.5) | 0.44 |
| Pattern, n (%) | |||
| Cholestatic | 34 (39.5) | 5 (35.7) | 0.79 |
| Mixed | 17 (19.8) | 2 (14.3) | |
| Hepatocellular | 35 (40.7) | 7 (50) | |
| Autoantibodies | |||
| ANA only | 27 (31.8) | 4 (28.6) | 0.99 |
| ASMA | 4 (4.7) | 1 (7.1) | 0.60 |
| Bile duct injury, n (%) | 10 (11.6) | 1 (7.1) | 0.99 |
| Liver biopsy, n (%) | 27 (31.4) | 10 (71.4) | 0.006 |
| Multiple irAEs, n (%) | 42 (42.4) | 6 (40.0) | 0.99 |
| Hospitalisation, n (%) | 2 (2.3) | 14 (100) | <0.001 |
| MELD score, median (range) | 7.7 (6–15) | 14.7 (7–26) | <0.001 |
| CTCAE severity score, n (%) | |||
| Grade 3 | 71 (82.6) | 2 (14.3) | <0.001 |
| Grade 4 | 15 (17.4) | 12 (85.7) | |
| DILI‐IEWG severity score, n (%) | |||
| Non severe | 86 (100) | 7 (50) | <0.001 |
| Severe | 0 | 7 (50) | |
| Hepatitis treatment, n (%) | |||
| Both steroids and UDCA | 51 (59.3) | 7 (46.7) | 0.79 |
| Steroids only | 42 (48.8) | 5 (35.7) | 0.40 |
| UDCA only | 13 (15.1) | 2 (14.3) | 0.99 |
| No treatment | 15 (17.4) | 0 | 0.21 |
| Second‐line immunosuppressant | 4 (4.7) | 4 (26.7) | 0.01 |
| ICI rechallenge, n (%) | 51 (60) | 1 (7.1) | <0.001 |
| Response to cancer treatment (RECIST), n (%) | |||
| Progressive disease | 11 (13.3) | 2 (18.2) | 0.19 |
| Stable disease | 20 (24.1) | 2 (18.2) | |
| Complete or partial response | 61 (62.6) | 7 (63.7) | |
| Days until hepatitis resolution, mean (±SD) | 73.9 (±51.2) | 51.5 (±32.7) | 0.37 |
Abbreviations: ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; CTCAE, Common Terminology Criteria for Adverse Events; DILI‐IEWG, drug‐induced liver injury international working group; DILI‐N, drug‐induced liver injury network; ICI, immune checkpoint inhibitor; RECIST, response evaluation criteria in solid tumour; UDCA, ursodeoxycholic acid. Bold values are significant values.
Head and neck cancer, hepatocellular carcinoma, colorectal cancer, oesophageal cancer, breast cancer, intestinal T‐cell lymphoma.
TABLE 4.
Comparison of severity according to DILI‐IEWG.
| Non severe, n = 93 (93%) | Severe, n = 7 (7%) | p value | |
|---|---|---|---|
| Age (years), mean (±SD) | 64.5 (±13.6) | 69.7 (±12.6) | 0.37 |
| Sex, n (%) | |||
| Female | 31 (42.5) | 11 (40.7) | 0.99 |
| Male | 42 (57.5) | 16 (59.3) | |
| Cancer, n (%) | |||
| Lung | 32 (34.4) | 1 (14.3) | 0.50 |
| Melanoma | 29 (31.2) | 3 (42.9) | |
| Renal and urothelial | 19 (20.4) | 1 (14.3) | |
| Other cancers a | 13 (14) | 2 (28.5) | |
| Cancer stade, n (%) | |||
| Stade III | 13 (14) | 1 (14.3) | 0.54 |
| Stade IV | 46 (49.5) | 2 (28.6) | 0.54 |
| Liver metastasis | 6 (6.5) | 1 (14.3) | 0.33 |
| Checkpoint inhibitor, n (%) | |||
| Anti‐PD1 | 59 (63.4) | 5 (71.4) | 0.44 |
| Anti‐PDL1 | 7 (7.5) | 1 (14.3) | |
| Combotherapy with anti‐CTLA4 | 27 (29.1) | 1 (14.3) | |
| Cycles of ICI infusion, mean (±SD) | 6.2 (±7.5) | 3.3 (±1.5) | 0.69 |
| Time until onset (days), mean (±SD) | 29.2 (±31.1) | 18.7 (±8.2) | 0.62 |
| Pattern, n (%) | |||
| Cholestatic | 38 (40.9) | 1 (14.3) | 0.08 |
| Mixed | 19 (20.4) | 0 | |
| Hepatocellular | 36 (38.7) | 6 (85.7) | |
| Autoantibodies | |||
| ANA only | 29 (31.5) | 2 (28.6) | 0.99 |
| ASMA | 5 (5.4) | 0 | 0.99 |
| Bile duct injury, n (%) | 10 (10.8) | 1 (14.3) | 0.57 |
| Liver biopsy, n (%) | 31 (33.3) | 6 (85.7) | 0.01 |
| Multiple irAEs, n (%) | 37 (39.8) | 3 (42.9) | 0.99 |
| Hospitalisation, n (%) | 9 (9.7) | 7 (100) | <0.001 |
| MELD score, median (range) | 7.9 (6–15) | 18.6 (11–26) | <0.001 |
| CTCAE severity score, n (%) | |||
| Grade 3 | 73 (78.5) | 0 | <0.001 |
| Grade 4 | 20 (21.5) | 7 (100) | |
| DILI‐N severity score, n (%) | |||
| Non severe | 86 (92.5) | 0 | <0.001 |
| Severe | 7 (7.5) | 7 (100) | |
| Hepatitis treatment, n (%) | |||
| Both steroids and UDCA | 23 (24.7) | 3 (42.9) | 0.37 |
| Steroids only | 43 (46.2) | 4 (57.1) | 0.70 |
| UDCA only | 15 (16.1) | 0 | 0.59 |
| No treatment | 10 (10.8) | 0 | 0.99 |
| Second‐line immunosuppressant | 5 (5.5) | 4 (57.1) | 0.001 |
| ICI rechallenge, n (%) | 52 (57.1) | 0 | 0.004 |
| Response to cancer treatment (RECIST), n (%) | |||
| Progressive disease | 15 (17.7) | 0 | 0.68 |
| Stable disease | 19 (22.4) | 1 (20) | |
| Complete or partial response | 48 (56.4) | 4 (57.1) | |
| Days until hepatitis resolution, mean (±SD) | 55.7 (±42.5) | 51 (±30.8) | 0.95 |
Abbreviations: ANA, antinuclear antibodies; ASMA, anti‐smooth muscle antibodies; CTCAE, Common Terminology Criteria for Adverse Events; DILI‐IEWG, drug‐induced liver injury international working group; DILI‐N, drug‐induced liver injury Network; ICI, immune checkpoint Inhibitor; RECIST, response evaluation criteria in solid tumour; UDCA, ursodeoxycholic acid. Bold values are significant values.
Head and neck cancer, hepatocellular carcinoma, colorectal cancer, oesophageal cancer, breast cancer, intestinal T‐cell lymphoma.
3.3. Liver‐related outcome: ALI
ALI occurred in seven patients (7%), among them 5 patients had hepatic encephalopathy (i.e. ALF). The characteristics of patients with ALI are summarised in Table S2: CHILI pattern was mostly hepatocellular (n = 5), five patients developed ALF, and four patients received a second‐line treatment with MMF. There was a difference regarding ALI between CTCAE G3 and G4 (p = 0.002), and between non‐severe and severe hepatitis according to DILI‐N (p < 0.0001) and DILI‐IEWG (p < 0.0001) (Figure 2). At 6 months, the ALI‐free survival rates were 92.9% (95% CI: 59.1–99.0) for G3, and 40% (95% CI: 12.3–67.0) for G4 according to CTCAE (Figure 2A). According to DILI‐N, the ALI‐free survival rate at 6 months was 100% (95% CI: 100.0–100.0) for non‐severe and 12.5% (95% CI: 0.7–42.3) for severe hepatitis (Figure 2B). In the DILI‐IEWG, the 6‐month ALI‐free survival rate was 94.4% (95% CI: 66.6–99.2) for non‐severe and 0.0% (95% CI: 0.0–0.0) for severe (Figure 2C). Hyperbilirubinemia was significantly associated with hospitalisation (n = 12, 46.2%; p < 0.001) and UDCA treatment (n = 16, 61.5%; p = 0.03). Jaundice was significantly associated with hepatic encephalopathy (n = 5, 26.3%; p < 0.001), second‐line immunosuppressant therapy (n = 6, 31.6%; p = 0.001), plasmapheresis (n = 2, 10.5%; p = 0.04), and 3‐months mortality (n = 3, 15.8%; p = 0.006). ALI and hyperbilirubinemia, including jaundice, were not significantly associated with any histological lesion.
FIGURE 2.
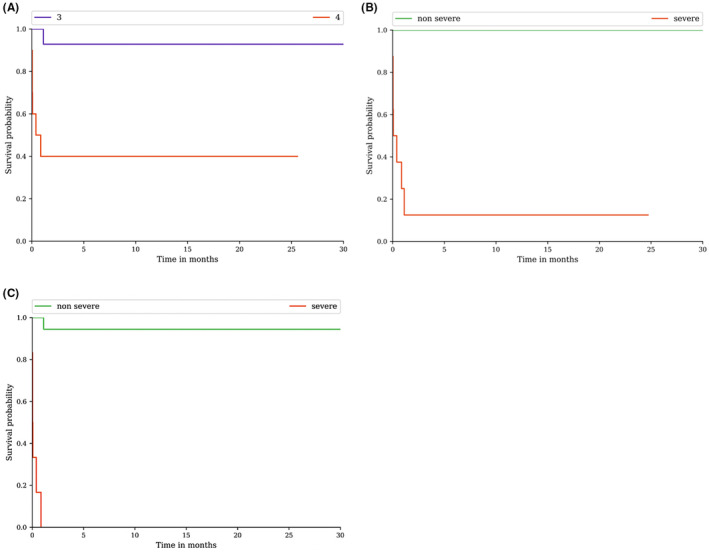
Acute liver injury estimated according to severity classifications CTCAE, DILI‐N and DILI‐IEWG. (A) Acute liver injury stratified by severity according to CTCAE: G3 vs. G4; p = 0.002. (B) Acute liver injury stratified by severity according to DILI‐N; p < 0.0001. (C) Acute liver injury stratified by severity according to DILI‐IEWG; p < 0.0001. Acute liver injury rate was estimated using the Kaplan–Meier method and Cox test regression in percentage. Non severe is grades 1 and 2; Severe is grades 3 and 4.
3.4. Secondary outcomes
Hospitalisation, hepatic encephalopathy, and second‐line immunosuppressant were significantly associated with the severity according to CTCAE (Table 2), DILI‐N (Table 3), and DILI‐IEWG (Table 4). The median hospital stay was 7 days (2–83), and the median delay between onset of CHILI and initiation of second‐line immunosuppressant was 27 days (8–483). Five patients had hepatic encephalopathy (5%), and lobular necrosis with bridge necrosis was significantly more frequent in those patients with hepatic encephalopathy (n = 2, 66.7%; p = 0.04). During the follow‐up, 22 patients died (22%), mostly after neoplasia evolution (n = 19, 86.4%), and three patients died from ALF (13.6%). Mortality at 3‐months was significantly associated with ALI and hepatic encephalopathy (n = 3, 100%; p < 0.001). Severity grade according to CTCAE was not significantly associated with 3‐months mortality (G3 1.4%, G4 7.7%; p = 0.17), in contrast to DILI‐N (G1 0%, G2 0%, G3 12.5%, G4 40%; p = 0.002) and DILI‐IEWG (G1 0%, G2 10%, G3 28.6%; p = 0.002). Overall mortality was not associated with severity according to CTCAE, DILI‐N, nor DILI‐IEWG.
3.5. Severity classifications performance
Compared with CTCAE classification, the DILI‐N and DILI‐IEWG classifications had a better performance in predicting liver dysfunction in severe hepatitis (p < 0.05) (Figure 3). The AUC difference between CTCAE classification and MELD score according to ALI was 0.09 (p < 0.01, z = 2.81). The area under the curve was 0.81 (95% CI: [0.67; 0.96]) for CTCAE to predict ALI, 0.90 (95% CI: [0.74; 1.06]) for MELD score, 0.98 (95% CI: [0.97; 1.0]) for DILI‐N and 0.98 (95% CI: [0.96; 1.0]) for DILI‐IEWG (Figure 3). The optimal cut‐offs to predict ALI were grade 4 by CTCAE, 11‐point threshold for MELD score, grade 3 by DILI‐N, and grade 2 by DILI‐IEWG. The area under the curve for total bilirubin to predict ALI was 0.79 (95% CI: [0.56; 1.0]), and the optimal cut‐off value for total bilirubin to predict ALI was 31.2 μmol/L.
FIGURE 3.
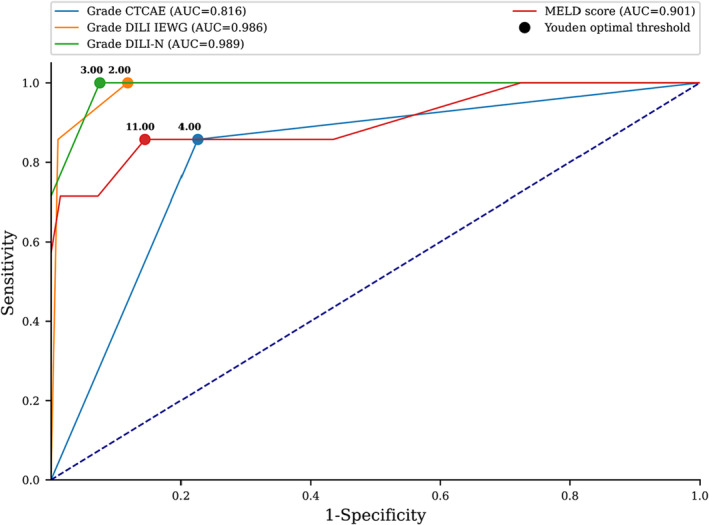
Correlation of severity classifications to predict acute liver injury. Graph shows receiver operating characteristic curve for predicting Acute Liver Injury. Solid blue line indicates Common Terminology Criteria for Adverse Events (CTCAE), solid red line indicates MELD (Model for End‐stage Liver Disease), solid green line indicates DILI‐N (Drug Induced Liver Injury‐Network), and solid orange line indicates DILI‐IEWG (Drug Induced Liver Injury‐International Expert Working Group).
4. DISCUSSION
This retrospective study included 100 patients with severe CHILI according to CTCAE and compared different validated severity classifications to assess liver outcomes. CHILI pattern was mostly hepatocellular, median RUCAM score was 8 and median MELD score was 7. According to the CTCAE classification, 27 patients had a life‐threatening hepatitis, whereas only 14 patients and seven patients were severe according to DILI‐N and DILI‐IEWG classifications respectively. Although, the last CTCAE classification v5, 10 including total bilirubin, appears to accurately predict liver dysfunction accurately, but is insufficient in predicting 3‐months mortality, contrary to DILI‐N (23.1% vs. 0%, p = 0.002) and DILI‐IEWG (28.6% vs. 1.1%, p = 0.01) classifications. Indeed, we found that the DILI classifications (DILI‐N and DILI‐IEWG) had a better performance liver dysfunction (respectively p < 0.05), compared with the CTCAE classification. These findings show that the DILI classifications have a higher performance than the CTCAE classification in predicting ALI, ALF, and death at 3 months in patients with severe CHILI. Indeed, these results are consistent with a recent study by Atallah E et al who pointed out the discrepancy between the CTCAE classification and liver dysfunction, 7 with 99% of patients CTCAE grades 3 and 4, while no patient was DILI‐IEWG severe. The same results were reported by Parlati L et al, who reported 86 severe CHILI according to CTCAE, with no patients developing liver failure. 23 Additionally, steroids remain the recommended first‐line treatment for severe CHILI according to international recommendations, 9 , 11 , 26 but non‐treated hepatitis seem to be correlated with non‐severe CHILI according to DILI classifications in our study. Several studies have already reported cases with hepatitis grade ≥3 improvement without steroids, or with other therapeutics, such as UDCA. 27 , 28 Miller et al. had compared steroid‐treated and untreated patients with CHILI, and found that patients on steroids took more time for hepatitis improvement compared to grade 1 (23 vs. 14 days, p = 0.043). 26 Li et al. also showed that high dose of steroids (≥1.5 mg/kg) did not impact the incidence of steroid‐refractory hepatitis nor the time to ALT normalisation, compared to lower‐dose regimen, but induced more steroid adverse events. 29 Recently, Riveiro‐Barciela et al. proposed the use of corticosteroids depending on the degree of necroinflammation observed on liver biopsy. 30 Furthermore, we observed here a median delay of approximately 1 month before initiating a second‐line immunosuppressant. This leads us to reconsider the definition of corticoresistance, which is defined as a lack of response to corticosteroid therapy after a 3‐days period. 31 Still debating guidelines for the management of CHILI, ICI rechallenge after severe hepatitis is not recommended beyond CTCAE grade 3. Resuming ICI is an oncological challenge for patients with neoplasia, since patients experiencing irAEs often have partial or complete oncological response to ICIs. Several studies have already shown the feasibility of resuming ICI after grade ≥3 hepatitis, as there is no systematic recurrence, and the recurrent hepatitis is not worse. 27 , 32 Contraindications to the resumption of ICI might be reserved for patients with ALI. Finally, we propose in Figure 4 a strategy for the management of severe CHILI, to more clearly identify patients with severe hepatitis. Our statements regarding ICI rechallenge need more studies to be validated, but seem to challenge the over‐restrictive guidelines, as continuing ICI may be the only life‐prolonging option in CHILI patients with advanced neoplasia. Thus, there may be a bias in rechallenged patients as the most severe patients may be less likely to respond to corticosteroids and therefore less likely to be rechallenged. Further studies are needed to understand the mechanisms involved in the response to corticosteroid therapy in these patients. Our study has other limitations: (1) one patient had been transplanted but had discontinued immunosuppression therapy because of cancer, (2) six patients had possible causality grading assessed by the updated RUCAM, and (3) inclusion of patients from a special multidisciplinary meeting may have induced a selection bias. Beyond the limitations of the retrospective study design, we observed here a greater number of patients with ALI due to CHILI than in previous studies. Moreover, efficacy of the CTCAE was verified by cases assessed by the updated RUCAM, which helps to define characteristics of CHILI, not achieved by any other non‐validated procedure. Also, the MELD score, which has been previously studied in DILI patients, 22 , 33 is very interesting in our CHILI population, since it also seems to correlate with severity above the threshold of 11. In conclusion, the CTCAE classification is associated with severity, but combining it with the DILI classifications is more specific for predicting ALI, and therefore helps to identify the patients most at risk of liver dysfunction and death at 3 months (Figure 4). This approach would permit the more effective selection of patients with severe conditions that may require the administration of IV corticosteroids or second‐line immunosuppressant and avoid intensive treatment in non‐severe patients. Prognostic classifications DILI‐N and DILI‐IEWG might still be validated and based on the validated RUCAM, and even more specific prognostic scoring systems, based on the validated updated RUCAM, might be interesting in CHILI. Further studies are needed to accurately predict the response to corticosteroids in severe CHILI patients. The insights of this study highlight that current management guidelines may require revision, because they are likely to result in the overtreatment of patients with CHILI and to limit the possibility of ICI rechallenge.
FIGURE 4.
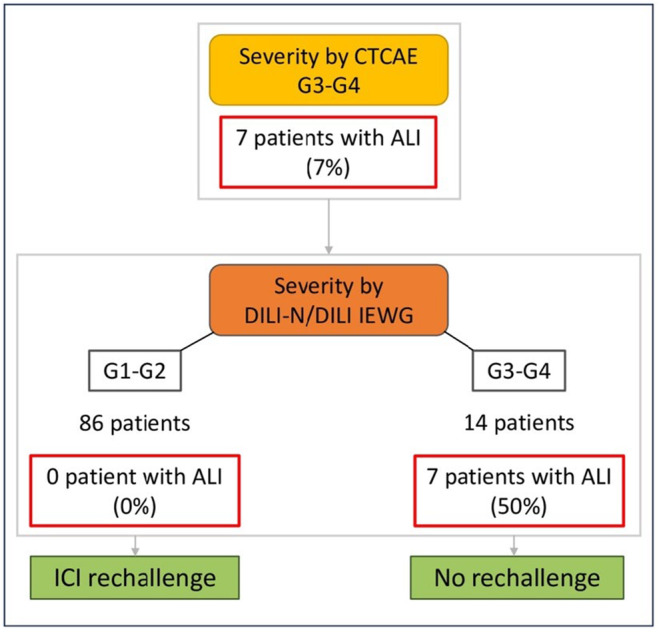
Proposed strategy for the management of patients with severe CHILI based on our results. Non severe is grades 1 and 2; Severe is grades 3 and 4. ALI, acute liver injury; CTCAE, Common Terminology Criteria for Adverse Events; DILI‐IEWG, Drug Induced Liver Injury‐International Expert Working Group; DILI‐N, Drug Induced Liver Injury‐Network.
AUTHOR CONTRIBUTIONS
Lina Hountondji: Conceptualization; investigation; writing – original draft; methodology; visualization; data curation; formal analysis; project administration; resources; validation; writing – review and editing. Stéphanie Faure: Resources; validation; writing – review and editing. Pascale Palassin: Resources; visualization. Philine Witkowski Durand Viel: Resources. Marie Dupuy: Resources. Dominique Larrey: Resources. Anouck Lamoureux: Resources. Cyrille Coustal: Resources. Dimitri Pureur: Resources. Candice Lesage: Resources. Éric Assenat: Resources. Benjamin Rivière: Resources. Jean‐Luc Faillie: Resources. Xavier Quantin: Resources. Georges‐Philippe Pageaux: Validation; visualization; writing – review and editing. Alexandre Thibault Jacques Maria: Methodology; resources; validation; visualization; writing – original draft. Lucy Meunier: Conceptualization; methodology; project administration; resources; supervision; validation; visualization; writing – review and editing; writing – original draft; formal analysis.
ACKOWLEDGEMENTS
MonRIO Group (Montpellier Reseau Immuno‐Oncology).
Declaration of personal interests: The authors declare no conflicts of interest related to this work.
FUNDING INFORMATION
The authors did not receive any funding for this research from agencies in the public, private, or non‐profit sectors.
AUTHORSHIP
Guarantor of the article: Lucy Meunier is the Guarantor of the article.
Supporting information
Table S1.
Table S2.
Hountondji L, Faure S, Palassin P, Viel PWD, Dupuy M, Larrey D, et al. Time to use the right classification to predict the severity of checkpoint inhibitor‐induced liver injury, as assessed for causality using the updated RUCAM . Aliment Pharmacol Ther. 2024;60:1561–1572. 10.1111/apt.18276
Alexandre Thibault Jacques Maria, and Lucy Meunier contributed equally.
The Handling Editor for this article was Dr Rohit Loomba, and it was accepted for publication after full peer‐review.
Contributor Information
Lina Hountondji, Email: l-hountondji@chu-montpellier.fr.
Lucy Meunier, Email: lucy-meunier@chu-montpellier.fr.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Sharma P, Allison JP. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell. 2015;161(2):205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. La‐Beck NM, Jean GW, Huynh C, Alzghari SK, Lowe DB. Immune checkpoint inhibitors: new insights and current place in cancer therapy. Pharmacotherapy. 2015;35(10):963–976. [DOI] [PubMed] [Google Scholar]
- 4. Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event‐free survival with Pembrolizumab in early triple‐negative breast cancer. N Engl J Med. 2022;386(6):556–567. [DOI] [PubMed] [Google Scholar]
- 5. Fife BT, Bluestone JA. Control of peripheral T‐cell tolerance and autoimmunity via the CTLA‐4 and PD‐1 pathways. Immunol Rev. 2008;224:166–182. [DOI] [PubMed] [Google Scholar]
- 6. De Martin E, Michot JM, Rosmorduc O, Guettier C, Samuel D. Liver toxicity as a limiting factor to the increasing use of immune checkpoint inhibitors. JHEP Rep. 2020;2:100170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Atallah E, Welsh SJ, O'Carrigan B, Oshaughnessy A, Dolapo I, Kerr AS, et al. Incidence, risk factors and outcomes of checkpoint inhibitor‐induced liver injury: a 10‐year real‐world retrospective cohort study. JHEP Rep. 2023;5(10):100851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta‐analysis. JAMA Oncol. 2018;4(12):1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haanen J, Obeid M, Spain L, Carbonnel F, Wang Y, Robert C, et al. Management of toxicities from immunotherapy: ESMO clinical practice guideline for diagnosis, treatment and follow‐up. Ann Oncol. 2022;33(12):1217–1238. [DOI] [PubMed] [Google Scholar]
- 10. Common Terminology Criteria for Adverse Events (CTCAE) v 5.0 . 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
- 11. Schneider BJ, Naidoo J, Santomasso BD, Lacchetti C, Adkins S, Anadkat M, et al. Management of immune‐related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073–4126. [DOI] [PubMed] [Google Scholar]
- 12. European Association for the Study of the Liver , Electronic address: easloffice@easloffice.eu; Clinical Practice Guideline Panel: Chair; Panel members; EASL Governing Board representative . EASL clinical practice guidelines: drug‐induced liver injury. J Hepatol. 2019;70(6):1222–1261. [DOI] [PubMed] [Google Scholar]
- 13. Fontana RJ, Liou I, Reuben A, Suzuki A, Fiel MI, Lee W, et al. AASLD practice guidance on drug, herbal, and dietary supplement‐induced liver injury. Hepatology. 2023;77(3):1036–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, et al. Drug‐Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Aithal GP, Watkins PB, Andrade RJ, Larrey D, Molokhia M, Takikawa H, et al. Case definition and phenotype standardization in drug‐induced liver injury. Clin Pharmacol Ther. 2011;89:806–815. [DOI] [PubMed] [Google Scholar]
- 16. Tzadok R, Levy S, Aouizerate J, Shibolet O. Acute liver failure following a single dose of Atezolizumab, as assessed for causality using the updated RUCAM. Case Rep Gastrointest Med. 2022;23(2022):5090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nogami A, Wada N, Muraoka E, Iwaki M, Kobayashi T, Matsumura M, et al. Mortality associated with the development of acute liver failure after a single dose of nivolumab. Clin J Gastroenterol. 2023;16(3):464–469. [DOI] [PubMed] [Google Scholar]
- 18. Dibos M, Dumoulin J, Mogler C, Wunderlich S, Reichert M, Rasch S, et al. Fulminant liver failure after treatment with a checkpoint inhibitor for gastric cancer: a case report and review of the literature. J Clin Med. 2023;12(14):4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Renault T, Meunier L, Monet C. Acute liver failure following immune checkpoint inhibitors. Clin Res Hepatol Gastroenterol. 2023;47(8):102203. [DOI] [PubMed] [Google Scholar]
- 20. European Association for the Study of the Liver , Electronic address: easloffice@easloffice.eu; Clinical Practice Guidelines Panel , Wendon J, Cordoba J, Dhawan A, Larsen FS, et al. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–1081. [DOI] [PubMed] [Google Scholar]
- 21. Ghabril M, Gu J, Yoder L, Corbito L, Ringel A, Beyer CD, et al. Development and validation of a model consisting of comorbidity burden to calculate risk of death within 6 months for patients with suspected drug‐induced liver injury. Gastroenterology. 2019;157(5):1245–1252.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reike‐Kunze M, Zenouzi R, Hartel J, Krech T, Weidemann S, Sterneck M, et al. Drug‐induced liver injury at a tertiary care centre in Germany: model for end‐stage liver disease is the best predictor of outcome. Liver Int. 2021;41(10):2383–2395. [DOI] [PubMed] [Google Scholar]
- 23. Danan G, Teschke R. RUCAM in drug and herb induced liver injury: the update. Int J Mol Sci. 2016;17(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pavlovic Markovic A, Stojkovic Lalosevic M, Mijac DD, Milovanovic T, Dragasevic S, Sokic Milutinovic A, et al. Jaundice as a diagnostic and therapeutic problem: a general Practitioner's approach. Dig Dis. 2022;40(3):362–369. [DOI] [PubMed] [Google Scholar]
- 25. Parlati L, Sakka M, Retbi A, Bouam S, Hassani L, Meritet J‐F, et al. Burden of grade 3 or 4 liver injury associated with immune checkpoint inhibitors. JHEP Rep. 2023;5:100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cunningham M, Gupta R, Butler M. Checkpoint inhibitor hepatotoxicity: pathogenesis and management. Hepatology. 2024;79(1):198–212. [DOI] [PubMed] [Google Scholar]
- 27. Hountondji L, Ferreira De Matos C, Lebossé F, Quantin X, Lesage C, Palassin P, et al. Clinical pattern of checkpoint inhibitor‐induced liver injury in a multicentre cohort. JHEP Rep. 2023;5(6):100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miller ED, Abu‐Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A, et al. Clinical characteristics and adverse impact of hepatotoxicity due to immune checkpoint inhibitors. Am J Gastroenterol. 2020;115(2):251–261. [DOI] [PubMed] [Google Scholar]
- 29. Li M, Wong D, Vogel AS, Sack JS, Rahma OE, Hodi FS, et al. Effect of corticosteroid dosing on outcomes in high‐grade immune checkpoint inhibitor hepatitis. Hepatology. 2022;75(3):531–540. [DOI] [PubMed] [Google Scholar]
- 30. Riveiro‐Barciela M, Barreira‐Díaz A, Salcedo MT, Callejo‐Pérez A, Muñoz‐Couselo E, Iranzo P, et al. An algorithm based on immunotherapy discontinuation and liver biopsy spares corticosteroids in two thirds of cases of severe checkpoint inhibitor‐induced liver injury. Aliment Pharmacol Ther. 2024;59(7):865–876. [DOI] [PubMed] [Google Scholar]
- 31. Karim G, Reddy M, Mobin N, Weisberg I, Dinani A. Immunosuppression management for refractory checkpoint inhibitor‐related hepatotoxicity. Clin Liver Dis (Hoboken). 2024;23(1):e0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Riveiro‐Barciela M, Barreira‐Díaz A, Callejo‐Pérez A, Muñoz‐Couselo E, Díaz‐Mejía N, Díaz‐González Á, et al. Retreatment with immune checkpoint inhibitors after a severe immune‐related hepatitis: results from a prospective multicenter study. Clin Gastroenterol Hepatol. 2023;21(3):732–740. [DOI] [PubMed] [Google Scholar]
- 33. Wang Y, Zou C, Wee A, Liu J, Ma Z, Guo T, et al. Comparison of the prognostic models for mortality in idiosyncratic drug‐induced liver injury. Hepatol Int. 2023;17(2):488–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Table S2.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


