Abstract
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive tumors, and the most common cause of cancer-related deaths. In the past, vascular infiltration of the tumor rendered the disease unresectable. However, today, venous or arterial involvement of a PDAC is classified as borderline resectable (BR) or locally advanced (LA) disease. Pancreaticoduodenectomy (PD) with vascular resections is a promising intervention intended for complete resection of BR- and LA-PDAC. This study aims to assess the overall survival of patients undergoing PD with vascular resections, compared to those without. A PubMed search was conducted for cohort studies that included patients with BR- or LA-PDAC treated with vascular resections. The retrieved publications were screened following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist. The study protocol was registered at the International Prospective Register for Systematic Reviews (PROSPERO). Sixteen cohort studies were included in our systematic review. Fourteen of them included patients undergoing PD with venous-only resections for PDAC. The 5-year overall survival rates ranged from 8.0% to 22.2% for vascular resection patients, and 4.0% to 24.3% for standard PD patients. Three cohorts included patients with PDAC and arterial and/or venous involvement who were treated with arterial resections. Their median overall survival ranged from 13.7 to 17.0 months, similar to that of patients who did not undergo vascular resections. PD with vascular resections in patients with BR- and LA-PDAC could lead to similar overall survival to that after standard PD.
Keywords: Pancreatic neoplasms, Surgical procedures, Pancreaticoduodenectomy, Mortality
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most common cause of cancer-related deaths worldwide with a survival rate of 12.5% at 5 years [1]. Over the last few years, the incidence of pancreatic cancer has increased, with the mean age of diagnosis being 71 years, while over 50% of the patients present with metastatic disease [2]. The stage of the disease is determined with imaging modalities, such as endoscopic ultrasound and computed tomography (CT) scanning. Of them, CT scanning represents the most useful modality to determine resectable (stage I or II), locally advanced (LA; stage III), or metastatic (stage IV) disease [3,4]. Advancements in imaging, such as the incorporation of Cinematic Rendering and 3D visualization, allow for better identification of vascular involvement of the tumor, thus enabling the preoperative categorization of the tumors as “borderline resectable”, or “locally advanced” [5].
According to the International Association of Pancreatology (IAP), borderline resectable pancreatic ductal adenocarcinoma (BR-PDAC) is defined as tumor contact of 180° or greater, or invasion of the superior mesenteric vein or portal vein (SMV/PV) with bilateral narrowing or occlusion, and not exceeding the inferior border of the duodenum. Regarding arterial involvement, a BR tumor is defined as the tumor contact with the superior mesenteric artery (SMA) and/or the celiac artery (CA) of less than 180°, without showing stenosis or deformity or tumor abutment of the common hepatic artery (CHA), and without showing tumor contact with the proper hepatic artery (PHA) and/or the CA. Tumor invasion of the CA of more than 180° without the involvement of the gastroduodenal artery is defined as LA-PDAC [6].
In the past, a pancreatic tumor with expansion to a surrounding vessel would have been classified as unresectable. According to the National Cancer Institute of National Institutes of Health, vascular infiltration co-exists in up to 25% of patients with pancreatic cancer [7]. Today, as research has shown, BR-PDAC can be resected, so long as negative margin (R0) resection is possible [8]. When vascular involvement is present, the role of neoadjuvant chemotherapy (NAT) is fundamental, as it tends to contain the disease, therefore increasing the candidates for complete resection [9,10]. Of note, the combination of pancreatectomy with vascular resection remains the only option for long-term survival [8]. Moore attempted the first successful pancreaticoduodenectomy (PD) with resection of the SMV in 1951, while Appleby reported the first CA resection in 1953 [11,12]. Over the years, evolution in chemotherapy regimens and surgical techniques has offered patients with BR disease the potential of a 5-year survival, comparable to that of resectable disease [13].
This study compares the survival and perioperative outcomes of patients with BR-PDAC or LA-PDAC who underwent venous or arterial resections with those of standard PD.
MATERIALS AND METHODS
Search strategy
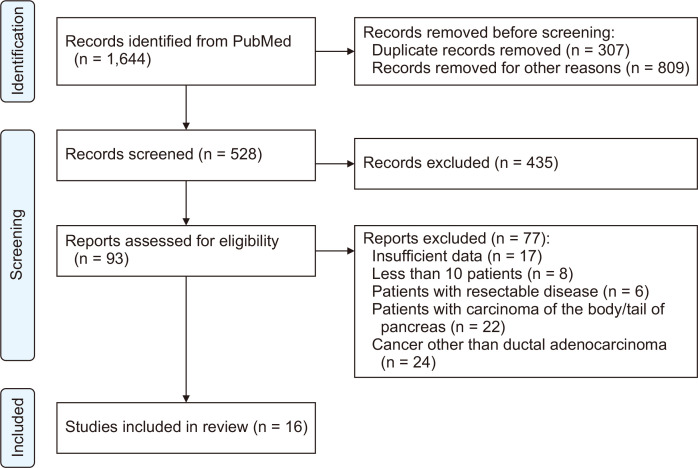
The PubMed literature was searched for the terms “pancreatic cancer”, “pancreatic adenocarcinoma”, “pancreatic ductal adenocarcinoma”, “pancreaticoduodenectomy”, “vascular resection”, “arterial resection”, “artery resection”, “vein resection”, and “venous resection”. The previous keywords were used in various combinations. Two of the reviewers (MP, SF) completed the search, which yielded 1,644 results. Duplicates and irrelevant studies were excluded by title and abstract screening, and the remaining 93 were further assessed for eligibility. Finally, 16 cohorts were included in our review (Preferred Reporting Items for Systematic Reviews and Meta-Analysis [PRISMA] flow diagram; Fig. 1) [14]. Any conflicts in the selection process were resolved through discussion. The review protocol was registered at the International Prospective Register for Systematic Reviews (PROSPERO ID CRD42022371194) of the National Institute of Health Researchers (NIHR).
Fig. 1.
PRISMA flowchart.
Inclusion and exclusion criteria
A study should meet the following inclusion criteria: involve adult patients, patients with PDAC, LA or BR disease, patients undergoing PD with vascular (arterial or venous) resection, outcomes of survival and morbidity, studies in the English language with more than 10 patients, and published after 2010.
The reasons for a study to be excluded were: pancreatic malignancies other than ductal adenocarcinoma, metastatic disease, case reports, pilot studies, commentaries and responses to author comments, surveys, author reflections, and conference debates (Table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Adult patients | Malignancies other than ductal adenocarcinoma |
| Patients with ductal adenocarcinoma of the head/neck of pancreas | Metastatic disease |
| Locally advanced or borderline resectable disease | Case reports and case series |
| Patients undergoing vascular resections for PDAC | Pilot studies |
| Outcomes on survival and morbidity | Author comments, responses and reflections |
| Studies including more than 10 patients | Surveys |
| English language | Conference debates |
| Published after 2010 |
PDAC, pancreatic ductal adenocarcinoma.
Data extraction
The following data were extracted in a predetermined datasheet form: title, year of publication, first author, number, mean age and sex of patients, perioperative characteristics, type of surgery, resected vessels, resection margin, median survival, 1-, 3-, and 5-year overall survival (OS), mortality, morbidity, and complications. The perioperative characteristics were specifically: neoadjuvant chemoradiotherapy, body mass index, ASA score, albumin, hemoglobin, bilirubin, CA19-9, TNM staging, type of operation, and length of hospital stay.
Risk of bias and quality assessment
The Cochrane Tool to Assess Risk of Bias in Cohort Studies was used to assess the risk of bias for each study. The Tool consists of eight questions on eight domains (selection, exposure, outcome presence before interventions, group matching, prognostic factors, follow-up, and co-interventions), and was performed by two independent reviewers (MP, SF) for each of the included studies. Any disagreement was resolved by consulting a third reviewer (AG).
We used the Newcastle–Ottawa Scale for cohort studies to evaluate the quality of each study [15]. This consists of questions about study selection, comparability, and outcome. A star is awarded for every item on the checklist, as shown in Table 2. Again, the quality assessment was completed by the two reviewers.
Table 2.
Newcastle–Ottawa scale scores for the studies included
| Study | Selection | Comparability | Outcome | Total | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of the exposed cohort | Selection of the non exposed cohort | Ascertainment of exposure | Outcome of interest not present at the start of the study | Assessment of outcome | Length of follow-up | Adequacy of follow-up | |||
| Addeo et al. [16], 2017 | * | * | * | * | * | * | * | * | 8/8 |
| Bachellier et al. [17], 2014 | * | * | * | * | * | * | * | 7/8 | |
| Flis et al. [18], 2016 | * | * | * | * | * | * | * | * | 8/8 |
| Han et al. [19], 2021 | * | * | * | * | * | * | * | * | 8/8 |
| Malleo et al. [20], 2017 | * | * | * | * | * | * | * | * | 8/8 |
| Martin et al. [21], 2018 | * | * | * | * | * | * | * | 7/8 | |
| Ravikumar et al. [22], 2014 | * | * | * | * | * | * | * | * | 8/8 |
| Xie et al. [23], 2017 | * | * | * | * | * | * | 6/8 | ||
| Bachellier et al. [24], 2020 | * | * | * | * | * | * | * | * | 8/8 |
| Elberm et al. [25], 2015 | * | * | * | * | * | * | * | 7/8 | |
| Lapshyn et al. [26], 2016 | * | * | * | * | * | * | * | * | 8/8 |
| Murakami et al. [27], 2013 | * | * | * | * | * | * | * | * | 8/8 |
| Turley et al. [28], 2012 | * | * | * | * | * | * | * | * | 8/8 |
| Fang et al. [29], 2017 | * | * | * | * | * | * | * | 7/8 | |
| Hwang et al. [30], 2015 | * | * | * | * | * | * | 6/8 | ||
| Jeong et al. [31], 2015 | * | * | * | * | * | * | * | * | 8/8 |
RESULTS
Sixteen cohort studies were included in our review [16-31]. Table 3 shows patient demographics and perioperative characteristics, while Table 4 shows the chemotherapy regimens administered to each patient group. The survival after PD with venous or arterial resections in patients with PDAC was compared to that of patients who were treated with conventional PD. A meta-analysis was not possible due to the heterogeneity of data regarding the resected vessels, the use of vascular grafts, the resection margins, or the administration of adjuvant chemotherapy. The results are separated into two categories for the reader’s convenience: patients undergoing venous resections only, and patients undergoing arterial resections with concomitant venous resections.
Table 3.
Patient demographics and perioperative characteristics
| Author | Population | Vascular resection | Sex | Age | Staging (TNM) | CA 19-9 (median) | NAT | Adjuvant therapy (CT/RT/CRT) | Hospital length of stay (day) |
|---|---|---|---|---|---|---|---|---|---|
| Addeo et al. [16] | 181 | 91 | 106 M; 75 F | 68.0 ± 9.0 | T3N0, T3N1 | 132.0 | No | CT; VR: 83 (91.2); SR: 80 (88.9) | ND |
| Bachellier et al. [17] | 15 | 11 | 9 M; 6 F | 65.0 ± 18.0 | Stage II, stage III | Increased in n = 9 (> 37) | 15 (100.0) | CT: 14 (93.3) | ND |
| Flis et al. [18] | 133 | 22 | 62 M; 71 F | 65.4 ± 8.6 | T1, T2, T3, T4 | Increased in n = 94 (> 30) | ND | ND | ND |
| Han et al. [19] | 557 | 106 | 338 M; 219 F | 69.0 ± 10.2 | IA, IB, IIA, IIB, III, IV | 1,102.7 | No | CT: 343 (61.6) | 16.6 ± 20.0 |
| Malleo et al. [20] | 651 | 81 | 357 M; 294 F | 63.5 | ND | 87.7 | 61 (9.4) | CT; VR: 68 (84.0); SR: 477 (83.7) |
ND |
| Martin et al. [21] | 58 | 29 | 31 M; 27 F | 69.0 ± 10.2 | T1, T2, T3, T4 | VR: 715.0; SR: 262.0 | ND | CT; VR: 26 (90.0); SR: 27 (93.0) |
VR: 17.0; SR: 15.0 |
| Ravikumar et al. [22] | 1,588 | 230 | 856 M; 732 F | 66.0 | ND | ND | ND | ND | VR: 14.0; SR: 13.0; SB: 9.0 |
| Xie et al. [23] | 158 | 158 | 100 M; 58 F | 61.5 ± 9.2 | ND | VR: 116.3; SB: 380.6 | ND | CT: 158 (100.0) | VR: 21.0; SB: 12.4 |
| Bachellier et al. [24] | 118 | 118 | 61 M; 57 F | 62.0 | T3 | ND | 89 (85.4) | CT: 75 (63.5) | 22.0 |
| Elberm et al. [25] | 1,070 | 230 | 583 M; 487 F | 66.0 | T3, T4 | ND | ND | ND | ND |
| Lapshyn et al. [26] | 86 | 86 | 38 M; 48 F | 66.0 | ND | ND | VR: 2 (5.0); SR: 4 (9.0) | ND | ND |
| Murakami et al. [27] | 125 | 61 | 65 M; 60 F | 70.0 | IA, IB, IIA, IIB, III, IV | ND | ND | CT: 87 (69.6) | ND |
| Turley et al. [28] | 204 | 42 | 103 M; 97 F | 32.0–87.0 (range) | T2, T3, T4 | ND | VR: 23 (55.0); SR: 73 (45.0) |
ND | VR: 13.0; SR: 12.0 |
| Fang et al. [29] | 83 | 31 | 55 M; 28 F | 70.0 ± 5.0 | T1, T2, T3, T4 | VR: 613.7; SR: 393.5 | VR: 2 (6.5); SR: 1 (1.9) |
CT; VR: 8 (25.8); SR: 19 (36.5) | VR: 18.5 ± 8.3; SR: 20.2 ± 8.5 |
| Hwang et al. [30] | 137 | 17 | 82 M; 55 F | 56.8 ± 10.7 | ND | VR: 761.6; SR: 537.6 | VR: 2 (5.0); SR: 10 (9.3) |
CT; VR: 75 (70.1); SR: 26 (65.0) |
ND |
| Jeong et al. [31] | 276 | 46 | 159 M; 117 F | 61.0 | ND | ND | VR: 0 (0); SR: 2 (0.8) |
CT; VR: 32 (69.6); SR: 149 (64.8) | VR: 17.0; SR: 14.0 |
Values are presented as number only, mean ± standard deviation, or number (%).
NAT, neoadjuvant chemotherapy; CRT, chemoradiotherapy; M, male; F, female; ND, not determined; VR, vascular (venous or arterial) resection group; SR, standard resection group; SB, surgical bypass; CT, chemotherapy; RT, radiotherapy.
Table 4.
Chemotherapy regimens used in each studya)
| Author | Regimen |
|---|---|
| Addeo et al. [16] | Gemcitabine-based |
| Bachellier et al. [17] | FOLFIRINOX (n = 8) GEMOX (n = 7) |
| Flis et al. [18] | Gemcitabine-based |
| Han et al. [19] | 5-FU or capecitabine |
| Malleo et al. [20] | Gemcitabine ± oxaliplatin followed by FOLFIRINOX |
| Martin et al. [21] | NS |
| Ravikumar et al. [22] | NS |
| Xie et al. [23] | 5-FU + cisplatin/oxaliplatin + gemcitabine |
| Bachellier et al. [24] | FLOFIRINOX or GEMOX |
| Elberm et al. [25] | NS |
| Lapshyn et al. [26] | Gemcitabine-based |
| Murakami et al. [27] | Gemcitabine ± S-1 |
| Turley et al. [28] | NS |
| Fang et al. [29] | NS |
| Hwang et al. [30] | NS |
| Jeong et al. [31] | 5-FU or capecitabine |
NS, not stated.
a)Regimens used in the majority of the patients in each study. Other combinations were also utilized but were not reported in the original studies.
In 13 studies, 1,213 patients underwent PD with venous resection for BR- or LA-PDAC [16,18-23,25-29,31]. PV and/or SMV were the most resected vessels. The portal and SMV confluence was also resected when it was invaded by the tumor. Patients undergoing venous resections and those getting standard PD were compared in terms of their survival and postoperative complications.
Median OS after venous resection ranged from 12 to 28 months (Table 5) [20,31]. Accordingly, after conventional PD, the median survival was 15.2 to 27 months [16,18]. The 5-year overall survival rates ranged from 8.0% to 22.2% for vascular resection patients, and 4.0% to 24.3% for standard PD patients. Of all the studies that compared standard PD to PD with venous resections, the difference in survival was statistically significant in 3 [22,23,26], and nonsignificant in 6 [16,18,20,21,25,29] of 13 total reports. The highest reported mortality rate after venous resection was 10%, but no statistically significant difference between standard PD and PD with venous resections was reported (Table 6) [21].
Table 5.
Median and 5-year survival after venous resections versus conventional PD
| Author | Surgery type | p-value | |||
|---|---|---|---|---|---|
| PD with venous resections | Conventional PD | ||||
| Median OS (mon) | 5-year OS (%) | Median OS (mon) | 5-year OS (%) | ||
| Addeo et al. [16] | 22.0 | 21.0 | 27.0 | 21.0 | 0.28 |
| Flis et al. [18] | 16.1 | NS | 15.2 | 19.5 | 0.09 |
| Han et al. [19] | NS | 18.7 | NS | 24.3 | - |
| Malleo et al. [20] | 28.0 | 20.5 | 26.0 | NS | 0.635 |
| Martin et al. [21] | 15.2 | 10.0 | NS | 10.0 | 0.13 |
| Ravikumar et al. [22] | 18.2 | 0 | 18.0 | 4.0 | 0.0001 |
| Xie et al. [23] | 22.8 | NS | 7.3 (SB) | NS | < 0.001 |
| Elberm et al. [25] | 18.8 | NS | 18.5 | NS | 0.66 |
| Lapshyn et al. [26] | 14.0 | NS | 25.0 | NS | 0.042 |
| Murakami et al. [27] | 14.7 | 8.0 | 26.7 | 22.0 | - |
| Turley et al. [28] | 21.1 | NS | 20.0 | NS | - |
| Fang et al. [29] | 15.0 | 10.2 | 18.0 | 23.2 | 0.293 |
| Jeong et al. [31] | 12.0 | 22.2 | 16.0 | 18.6 | - |
PD, pancreaticoduodenectomy; OS, overall survival; NS, not stated; SB, surgical bypass.
Table 6.
Mortality after venous resections versus conventional PD
| Author | Mortality (30-day) | p-value | |
|---|---|---|---|
| PD with VR (%) | Conventional PD (%) | ||
| Addeo et al. [16] | 2.0 | 5.0 | - |
| Flis et al. [18] | 4.5 (60-day) | 4.5 (60-day) | - |
| Han et al. [19] | 0.9 | 1.1 | - |
| Malleo et al. [20] | 1.2 | 1.1 | - |
| Martin et al. [21] | 10.0 | 0 | 0.24 |
| Ravikumar et al. [22] | NS | NS | > 0.05 |
| Xie et al. [23] | 0 | 0 (SB) | - |
| Elberm et al. [25] | 4.3 | 3.9 | - |
| Lapshyn et al. [26] | 0 | 0 | - |
| Murakami et al. [27] | 0 | 0 | - |
| Turley et al. [28] | 4.7 | 3.7 | - |
| Fang et al. [29] | 0 | 0 | - |
| Jeong et al. [31] | 2.0 | 0.8 | - |
PD, pancreaticoduodenectomy; VR, venous resection; NS, not stated; SB, surgical bypass.
In 3 studies, 146 patients underwent arterial resections with or without synchronous venous resections for BR- or LA-PDAC [17,24,30]. Celiac axis was the most invaded structure by the tumor, while CHA and CA were the most resected vessels. SMA, PHA, and splenic artery resections were also reported. Lastly, NAT was administered in 116 patients (79.5%) with PDAC treated with PD and some type of arterial resection.
Median OS was 17.0, 13.7, and 14.8 months in the 3 studies, respectively, while only Hwang et al. [30] reported a median survival for the conventional PD group, which was statistically significantly longer than the venous resection counterpart (14.8 months vs. 18.0 months, p = 0.033). In two studies, the mortality rate was zero, while Bachellier et al. [24] described a 5.1% mortality rate (Table 7, 8).
Table 7.
Median survival and 5-year survival after arterial and/or concomitant venous resections vs. conventional PD
| Author | Surgery type | p-value | |||
|---|---|---|---|---|---|
| PD with AR ± VR | Conventional PD | ||||
| Median OS (mon) | 5-year OS (%) | Median OS (mon) | 5-year OS (%) | ||
| Bachellier et al. [17] | 17.0 | 11.0 (3-year) | NS | NS | |
| Bachellier et al. [24] | 13.7 | 11.8 | Not performed | ||
| Hwang et al. [30] | 14.8 | NS | 18.0 | NS | 0.033 |
PD, pancreaticoduodenectomy; OS, overall survival; NS, not stated; AR, arterial resection; VR, venous resection.
Table 8.
Mortality after arterial and/or concomitant venous resections vs. conventional PD
| Study | Mortality (30-day) | |
|---|---|---|
| PD with AR ± VR (%) | Conventional PD | |
| Bachellier et al. [17] | 0 | 0 |
| Bachellier et al. [24] | 5.1 | Not performed |
| Hwang et al. [30] | 0 | 0 |
PD, pancreaticoduodenectomy; AR, arterial resection; VR, venous resection.
The most common complications were postoperative pancreatic fistulae, post-pancreatectomy hemorrhage (PPH), and delayed gastric emptying (DGE). Table 9 shows all the complications that were reported in the vascular resection groups. Even though one study found that major complications occurred significantly more frequently in the vascular resection group (p = 0.004) [21], in the remainder of the included studies, complication rates were similar between the standard and the vascular resection groups.
Table 9.
Complications after PD with vascular resections
| Complications after PD with vascular resections | |||
|---|---|---|---|
| Complication | Number of cases | Complication | Number of cases |
| Pancreatic fistula | 331 (24.0) | Intra-abdominal abscess | 144 (10.6) |
| Biliary fistula | 30 (2.2) | Diarrhea | 4 (0.3) |
| Enteric fistula | 3 (0.2) | Intestinal iscemia | 1 (0.1) |
| PPH | 158 (11.6) | Wound infection | 35 (2.6) |
| Anastomotic leaka) | 68 (5.0) | Abdominal infection | 5 (0.4) |
| Chylous ascites | 3 (0.2) | Pancreatitis | 1 (0.1) |
| DGE | 211 (15.5) | Pleural effusion | 17 (1.3) |
| SMV/PV thrombosis | 1 (0.1) | Peritonitis | 1 (0.1) |
| Arterial thrombosis | 8 (0.6) | Wound dehiscence | 4 (0.3) |
Values are presented as number (%).
PD, pancreaticoduodenectomy; PPH, post-pancreatectomy hemorrhage; DGE, delayed gastric emptying; SMV/PV, superior mesenteric vein or portal vein.
a)Biliary or intestinal anastomotic leakage.
DISCUSSION
In this systematic review, it is shown that PD with venous resections for patients with LA- or BR-PDAC can have similar OS to that of the conventional PD with accepted mortality. Luketina et al. [3] came to similar conclusions, as they reported that venous resections do not increase perioperative morbidity and mortality. In contrast, Delpero and Sauvanet [8] found that mortality and morbidity were higher after PD with venous resections. Interestingly, in two of the included cohorts, the median OS after PD with vein resections was statistically significantly longer than the OS after standard PD [22,23]. In general, no significant differences regarding OS were presented, thus vascular resections should always be considered in patients with vascular invasion of the tumor, as the evidence shows that resecting the involved vessels may improve survival [32].
In contrast, data on arterial resections are not as evident. Only three studies with patients undergoing PD with arterial resections met our inclusion criteria, but a comparison between arterial and standard PD was not conducted. Of note, a systematic review by Małczak et al. [33] showed that PD with arterial resections may result in higher mortality than the conventional PD for PDAC. As data are not yet clear on arterial resections, to avoid them in patients with BR− and LA-PDAC, and consequently, to avoid the accompanying periprocedural mortality, new surgical techniques have recently been suggested. In the era of NAT, periarterial divestment, a technique initially described by Miao et al. in 2016, promises complete tumor removal without requiring arterial resection [34,35]. A circumferential dissection is performed alongside and between the vessel wall and the tumor in contact, which, particularly after NAT and tumor downstaging, allows for radical tumor resection [36]. Experience thus far has shown that periarterial divestments could be applied without a significant increase in postoperative mortality [37,38]. However, the feasibility, safety, and oncologic outcomes of periarterial divestments versus arterial resections need to be further evaluated with clinical trials in the future.
In 2017, Hackert et al. [39] introduced the TRIANGLE operation, which involves the resection of all soft tissue inside an anatomic triangle defined by the SMA, SMV/PV, and the CA. This technique implies the dissection of major vessels, followed by the resection of soft tissue in the prepared triangle, which increases the chances for negative resection margins, simultaneously avoiding the morbidity and mortality following an arterial resection [40-42]. The Triangle operation is safe, feasible, and particularly useful for patients with BR-PDAC [43,44]. However, its real clinical value has yet to be determined. Since December 2022, an ongoing large randomized controlled trial (the “TRIANGLE trial”) aims to compare the disease-free survival of patients randomly assigned to undergo the triangle operation or conventional PD, and hopefully offer clinical guidance on the role of this radical approach [45].
Complications after a major surgery such as PD with vascular resections occur frequently, and can range from diarrhea to multiple organ failure and death. In our study, the most common ones were DGE, pancreatic fistulae, PPH, and intra-abdominal abscesses. Giuliano et al. [46] also found DGE and pancreatic fistulae to be some of the most common post-PD complications. It was shown, however, that complications were similar among patients who underwent vascular resections, and those who did not. Fancellu et al. [47] in their meta-analysis also found no significant difference in complication rates. Consequently, performing vascular resections could be safe for patients with LA disease.
According to the International Study Group of Pancreatic Surgery (ISGPS), no evidence suggests NAT in BR-PDAC with isolated venous involvement, but NAT should be considered in the case of arterial infiltration in select patients, as it may result in better chances of negative margin resection (R0) of the tumor [48]. That is highly important when macroscopically complete resection is doubtful preoperatively. FOLFIRINOX and Gemcitabine-based regimens are widely used, but depending on the tumor’s biology and the patient’s clinical status, modifications may be applied, as well as additions to standard regimens. Broader utilization of NAT should be applied nowadays, since the benefits of a longer disease-free survival seem to outweigh the risks of its adverse effects [49].
Even though publications with high-quality evidence were included in our systematic review, certain limitations need to be addressed. Firstly, only cohort studies were included, which are prone to selection bias. Furthermore, the vessel reconstruction techniques varied, including end-to-end anastomosis or graft interposition. Additionally, the operations were performed by different teams in different centers with variable experience. Another limitation is that the chemotherapy regimens differed between the studies, or were not available, adding to data heterogeneity. Given the complexity of such operations, resolving all the above issues demands the referral of patients with pancreatic cancer with vascular involvement to experienced centers, in an attempt to standardize the therapeutic interventions and create universal guidelines.
CONCLUSION
PD with venous resections in patients with BR- and LA-PDAC could lead to similar OS to that after standard PD with similar complications and accepted mortality. Data on arterial resections are not conclusive; however, new artery-sparing techniques could offer the potential for complete resection of the artery involving tumors after NAT. To establish a standard protocol for the management of BR/LA-PDAC, future research should focus on clinical trials with larger patient populations treated in high-volume centers by experienced surgical teams.
Funding Statement
FUNDING None.
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Conceptualization: AG, DG. Data curation: SF. Methodology: MP, SF. Supervision: AG, DG. Writing - original draft: MP, SF, AM, EL. Writing - review & editing: AG, GC, AM, EL, LP, VNP.
References
- 1.All cancer sites combined recent trends in SEER age-adjusted incidence rates, 2000-2021 [Internet] SEER; 2023. [cited 2024 May 31]. Available from: https://seer.cancer.gov/statistics-network/explorer/application.html?site=1&data_type=1&graph_type=2&compareBy=sex&chk_sex_3=3&chk_sex_2=2&rate_type=2&race=1&age_range=1&hdn_stage=101&advopt_precision=1&advopt_show_ci=on&hdn_view=0&advopt_show_apc=on&advopt_display=2#resultsRegion0 . [Google Scholar]
- 2.Park W, Chawla A, O'Reilly EM. Pancreatic cancer: a review. JAMA. 2021;326:851–862. doi: 10.1001/jama.2021.13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luketina RR, Hackert T, Büchler MW. Vascular resection in pancreatic cancer. Indian J Surg. 2015;77:381–386. doi: 10.1007/s12262-015-1364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersson R, Vagianos CE, Williamson RC. Preoperative staging and evaluation of resectability in pancreatic ductal adenocarcinoma. HPB (Oxford) 2004;6:5–12. doi: 10.1080/13651820310017093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javed AA, Young RWC, Habib JR, Kinny-Köster B, Cohen SM, Fishman EK, et al. Cinematic rendering: novel tool for improving pancreatic cancer surgical planning. Curr Probl Diagn Radiol. 2022;51:878–883. doi: 10.1067/j.cpradiol.2022.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, et al. International consensus on definition and criteria of borderline resectable pancreatic ductal adenocarcinoma 2017. Pancreatology. 2018;18:2–11. doi: 10.1016/j.pan.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Previous version: SEER cancer statistics review, 1975-2009 (vintage 2009 populations) [Internet] SEER; 2012. [cited 2024 May 31]. Available from: https://seer.cancer.gov/archive/csr/1975_2009_pops09/index.html . [Google Scholar]
- 8.Delpero JR, Sauvanet A. Vascular resection for pancreatic cancer: 2019 French recommendations based on a literature review from 2008 to 6-2019. Front Oncol. 2020;10:40. doi: 10.3389/fonc.2020.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limbu Y, Regmee S, Ghimire R, Maharjan DK, Thapa PB. Arterial divestment and resection in post-neoadjuvant pancreatic adenocarcinoma. Cureus. 2021;13:e20275. doi: 10.7759/cureus.20275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strobel O, Hank T, Hinz U, Bergmann F, Schneider L, Springfeld C, et al. Pancreatic cancer surgery: the new R-status counts. Ann Surg. 2017;265:565–573. doi: 10.1097/SLA.0000000000001731. [DOI] [PubMed] [Google Scholar]
- 11.Moore GE, Sako Y, Thomas LB. Radical pancreatoduodenectomy with resection and reanastomosis of the superior mesenteric vein. Surgery. 1951;30:550–553. [PubMed] [Google Scholar]
- 12.Appleby LH. The coeliac axis in the expansion of the operation for gastric carcinoma. Cancer. 1953;6:704–707. doi: 10.1002/1097-0142(195307)6:4<704::AID-CNCR2820060410>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 13.Bratlie SO, Wennerblom J, Vilhav C, Persson J, Rangelova E. Resectable, borderline, and locally advanced pancreatic cancer-"the good, the bad, and the ugly" candidates for surgery? J Gastrointest Oncol. 2021;12:2450–2460. doi: 10.21037/jgo-2020-slapc-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells G, Shea B, O'Connell D, Robertson J, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis [Internet] Oxford (UK); 2011. [cited 2024 May 31]. Available from: http://www.evidencebasedpublichealth.de/download/Newcastle_Ottowa_Scale_Pope_Bruce.pdf . [Google Scholar]
- 16.Addeo P, Velten M, Averous G, Faitot F, Nguimpi-Tambou M, Nappo G, et al. Prognostic value of venous invasion in resected T3 pancreatic adenocarcinoma: depth of invasion matters. Surgery. 2017;162:264–274. doi: 10.1016/j.surg.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Bachellier P, Rosso E, Fuchshuber P, Addeo P, David P, Oussoultzoglou E, et al. Use of a temporary intraoperative mesentericoportal shunt for pancreatic resection for locally advanced pancreatic cancer with portal vein occlusion and portal hypertension. Surgery. 2014;155:449–456. doi: 10.1016/j.surg.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Flis V, Potrc S, Kobilica N, Ivanecz A. Pancreaticoduodenectomy for ductal adenocarcinoma of the pancreatic head with venous resection. Radiol Oncol. 2016;50:321–328. doi: 10.1515/raon-2015-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han S, Choi DW, Choi SH, Heo JS, Han IW, You YH. Long-term outcomes following en bloc resection for pancreatic ductal adenocarcinoma of the head with portomesenteric venous invasion. Asian J Surg. 2021;44:313–320. doi: 10.1016/j.asjsur.2020.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Malleo G, Maggino L, Marchegiani G, Feriani G, Esposito A, Landoni L, et al. Pancreatectomy with venous resection for pT3 head adenocarcinoma: perioperative outcomes, recurrence pattern and prognostic implications of histologically confirmed vascular infiltration. Pancreatology. 2017;17:847–857. doi: 10.1016/j.pan.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Martin D, Petermann D, Fontanella S, Pu Y, Halkic N, Demartines N, et al. Pancreatic adenocarcinoma with histologically proven portal vein infiltration: what is the outcome? Eur J Gastroenterol Hepatol. 2018;30:1507–1513. doi: 10.1097/MEG.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 22.Ravikumar R, Sabin C, Abu Hilal M, Bramhall S, White S, Wigmore S, et al. Portal vein resection in borderline resectable pancreatic cancer: a United Kingdom multicenter study. J Am Coll Surg. 2014;218:401–411. doi: 10.1016/j.jamcollsurg.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Xie ZB, Gu JC, Zhang YF, Yao L, Jin C, Jiang YJ, et al. Portal vein resection and reconstruction with artificial blood vessels is safe and feasible for pancreatic ductal adenocarcinoma patients with portal vein involvement: Chinese center experience. Oncotarget. 2017;8:77883–77896. doi: 10.18632/oncotarget.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bachellier P, Addeo P, Faitot F, Nappo G, Dufour P. Pancreatectomy with arterial resection for pancreatic adenocarcinoma: how can it be done safely and with which outcomes?: a single institution's experience with 118 patients. Ann Surg. 2020;271:932–940. doi: 10.1097/SLA.0000000000003010. [DOI] [PubMed] [Google Scholar]
- 25.Elberm H, Ravikumar R, Sabin C, Abu Hilal M, Al-Hilli A, Aroori S, et al. Outcome after pancreaticoduodenectomy for T3 adenocarcinoma: a multivariable analysis from the UK Vascular Resection for Pancreatic Cancer Study Group. Eur J Surg Oncol. 2015;41:1500–1507. doi: 10.1016/j.ejso.2015.08.158. [DOI] [PubMed] [Google Scholar]
- 26.Lapshyn H, Bronsert P, Bolm L, Werner M, Hopt UT, Makowiec F, et al. Prognostic factors after pancreatoduodenectomy with en bloc portal venous resection for pancreatic cancer. Langenbecks Arch Surg. 2016;401:63–69. doi: 10.1007/s00423-015-1363-2. [DOI] [PubMed] [Google Scholar]
- 27.Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Benefit of portal or superior mesenteric vein resection with adjuvant chemotherapy for patients with pancreatic head carcinoma. J Surg Oncol. 2013;107:414–421. doi: 10.1002/jso.23229. [DOI] [PubMed] [Google Scholar]
- 28.Turley RS, Peterson K, Barbas AS, Ceppa EP, Paulson EK, Blazer DG, 3rd, et al. Vascular surgery collaboration during pancreaticoduodenectomy with vascular reconstruction. Ann Vasc Surg. 2012;26:685–692. doi: 10.1016/j.avsg.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Fang JZ, Lu CD, Wu SD, Huang J, Zhou J. Portal vein/superior mesenteric vein resection in pancreatic cancer treatment in the elderly. Medicine (Baltimore) 2017;96:e7335. doi: 10.1097/MD.0000000000007335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang JW, Kim SC, Song KB, Yoon JH, Nam JS, Lee JH, et al. Significance of radiologic location and extent of portal venous involvement on prognosis after resection for pancreatic adenocarcinoma. Pancreas. 2015;44:665–671. doi: 10.1097/MPA.0000000000000309. [DOI] [PubMed] [Google Scholar]
- 31.Jeong J, Choi DW, Choi SH, Heo JS, Jang KT. Long-term outcome of portomesenteric vein invasion and prognostic factors in pancreas head adenocarcinoma. ANZ J Surg. 2015;85:264–269. doi: 10.1111/ans.12502. [DOI] [PubMed] [Google Scholar]
- 32.Oba A, Bao QR, Barnett CC, Al-Musawi MH, Croce C, Schulick RD, et al. Vascular resections for pancreatic ductal adenocarcinoma: vascular resections for PDAC. Scand J Surg. 2020;109:18–28. doi: 10.1177/1457496919900413. [DOI] [PubMed] [Google Scholar]
- 33.Małczak P, Sierżęga M, Stefura T, Kacprzyk A, Droś J, Skomarovska O, et al. Arterial resections in pancreatic cancer - systematic review and meta-analysis. HPB (Oxford) 2020;22:961–968. doi: 10.1016/j.hpb.2020.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Miao Y, Jiang K, Cai B, Lu Z, Wu J, Gao W, et al. Arterial divestment instead of resection for locally advanced pancreatic cancer (LAPC) Pancreatology. 2016;16:S59. doi: 10.1016/j.pan.2016.05.201. [DOI] [Google Scholar]
- 35.Diener MK, Mihaljevic AL, Strobel O, Loos M, Schmidt T, Schneider M, et al. Periarterial divestment in pancreatic cancer surgery. Surgery. 2021;169:1019–1025. doi: 10.1016/j.surg.2020.08.030. [DOI] [PubMed] [Google Scholar]
- 36.Karamarković AR, Juloski JT. Current surgical concepts and future perspectives in the treatment of borderline resectable and potentially resectable locally advanced pancreatic cancer. Chirurgia (Bucur) 2022;117:385–398. doi: 10.21614/chirurgia.2770. [DOI] [PubMed] [Google Scholar]
- 37.Loos M, Kester T, Klaiber U, Mihaljevic AL, Mehrabi A, Müller-Stich BM, et al. Arterial resection in pancreatic cancer surgery: effective after a learning curve. Ann Surg. 2022;275:759–768. doi: 10.1097/SLA.0000000000004054. [DOI] [PubMed] [Google Scholar]
- 38.Cai B, Lu Z, Neoptolemos JP, Diener MK, Li M, Yin L, et al. Sub-adventitial divestment technique for resecting artery-involved pancreatic cancer: a retrospective cohort study. Langenbecks Arch Surg. 2021;406:691–701. doi: 10.1007/s00423-021-02080-5. [DOI] [PubMed] [Google Scholar]
- 39.Hackert T, Strobel O, Michalski CW, Mihaljevic AL, Mehrabi A, Müller-Stich B, et al. The TRIANGLE operation - radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB (Oxford) 2017;19:1001–1007. doi: 10.1016/j.hpb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 40.Masiak-Segit W, Rawicz-Pruszyński K, Skórzewska M, Polkowski WP. Surgical treatment of pancreatic cancer. Pol Przegl Chir. 2018;90:45–53. doi: 10.5604/01.3001.0011.7493. [DOI] [PubMed] [Google Scholar]
- 41.Klotz R, Hackert T, Heger P, Probst P, Hinz U, Loos M, et al. The TRIANGLE operation for pancreatic head and body cancers: early postoperative outcomes. HPB (Oxford) 2022;24:332–341. doi: 10.1016/j.hpb.2021.06.432. [DOI] [PubMed] [Google Scholar]
- 42.Dumitraşcu T, Popescu I. Pancreatico-duodenectomy for pancreatic ductal adenocarcinoma: from artery-first approaches to TRIANGLE operation. Chirurgia (Bucur) 2022;117:377–384. doi: 10.21614/chirurgia.2771. [DOI] [PubMed] [Google Scholar]
- 43.Zhai S, Huo Z, Wang Y, Qian H, Zhao S, Shi Y, et al. TRIANGLE operation for borderline resectable pancreatic cancer in total pancreatectomy. Transl Cancer Res. 2019;8:2416–2424. doi: 10.21037/tcr.2019.09.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosso E, Zimmitti G, Iannelli A, Garatti M. The 'TRIANGLE operation' by laparoscopy: radical pancreaticoduodenectomy with major vascular resection for borderline resectable pancreatic head cancer. Ann Surg Oncol. 2020;27:1613–1614. doi: 10.1245/s10434-019-08101-4. [DOI] [PubMed] [Google Scholar]
- 45.Heger P, Hackert T, Diener MK, Feißt M, Klose C, Dörr-Harim C, et al. Conventional partial pancreatoduodenectomy versus an extended pancreatoduodenectomy (triangle operation) for pancreatic head cancers-study protocol for the randomised controlled TRIANGLE trial. Trials. 2023;24:363. doi: 10.1186/s13063-023-07337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giuliano K, Ejaz A, He J. Technical aspects of pancreaticoduodenectomy and their outcomes. Chin Clin Oncol. 2017;6:64. doi: 10.21037/cco.2017.09.01. [DOI] [PubMed] [Google Scholar]
- 47.Fancellu A, Petrucciani N, Porcu A, Deiana G, Sanna V, Ninniri C, et al. the impact on survival and morbidity of portal-mesenteric resection during pancreaticoduodenectomy for pancreatic head adenocarcinoma: a systematic review and meta-analysis of comparative studies. Cancers (Basel) 2020;12:1976. doi: 10.3390/cancers12071976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2014;155:977–988. doi: 10.1016/j.surg.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 49.Brown ZJ, Cloyd JM. Trends in the utilization of neoadjuvant therapy for pancreatic ductal adenocarcinoma. J Surg Oncol. 2021;123:1432–1440. doi: 10.1002/jso.26384. [DOI] [PubMed] [Google Scholar]