Abstract
Purpose
Reduced capillary number in skeletal muscle due to disuse can hinder the delivery of insulin and amino acid delivery to muscle cells, diminishing insulin activity and muscle protein synthesis, ultimately contributing to anabolic resistance. However, it remains unknown whether mitigating capillary regression during inactivity improves anabolic resistance. This study aimed to investigate the effect of increasing capillary number through the administration of prazosin, which can increase blood flow and prevent capillary regression, on anabolic resistance in skeletal muscle induced by disuse.
Methods
Male Sprague Dawley rats were divided into control and hindlimb unloading (HU) groups, with half of each group receiving prazosin (50 mg/L) in their drinking water for 2 weeks. Histological analysis of the soleus muscles was conducted to measure the capillary-to-fiber (C/F) ratio, while western blotting was performed to measure the activation of the Akt/mTORC1 muscle protein synthesis pathway before and after insulin stimulation.
Results
The C/F ratios were significantly lower in the HU and HU + Prz groups than in the control group but were significantly higher in the HU + Prz group than in the HU group. Following insulin stimulation, the phosphorylation levels of Akt, p70S6K, and S6RP increased in all groups, with a significantly greater increase observed in the HU + Prz group compared to the HU group, indicating improved molecular signaling related to muscle protein synthesis.
Conclusion
Administration of prazosin during hindlimb unloading mitigated capillary regression and enhanced insulin-stimulated muscle protein synthesis response. These findings suggest that enhancing capillary number may reduce the anabolic resistance caused by muscle disuse.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-024-01454-y.
Keywords: Hindlimb unloading, Skeletal muscle, Prazosin, Muscle protein synthesis, Capillary regression, Insulin resistance
Introduction
Skeletal muscle mass is controlled by a balance of muscle protein turnover, which is the net difference between muscle protein synthesis (MPS) and muscle protein breakdown. Skeletal muscle anabolic resistance, characterized by a reduced response to anabolic stimuli such as protein intake, hormonal activation, or muscle contraction, impedes muscle growth and maintenance, especially during periods of aging, obesity, clinical illness, and periods of physical inactivity [1, 2], leading to significant muscle atrophy and functional decline. Notably, several factors, including changes in hormone sensitivity, nutrient signaling, and inflammatory responses, have been linked to anabolic resistance in skeletal muscles [2]. However, the specific factors contributing to this phenomenon during muscle disuse are not fully understood.
Insulin resistance is one of the primary causes of anabolic resistance [2, 3]. Studies have shown that insulin triggers anabolic responses in skeletal muscles by promoting protein synthesis, facilitating insulin binding to its receptors on muscle cell membranes, and increasing amino acid delivery to muscle cells, thereby activating the mammalian target of rapamycin complex 1 (mTORC1) anabolic signaling pathway [4, 5]. However, insulin resistance is believed to impair these processes, thereby reducing muscle protein anabolism [6]. Research on insulin sensitivity in skeletal muscles during disuse has primarily focused on insulin-induced glucose uptake. Ex vivo experiments have demonstrated that insulin stimulation increases insulin sensitivity and glucose uptake in disused skeletal muscles [7–9]. However, in vivo studies administering insulin intraperitoneally to animals in a disused state have reported no increase in skeletal muscle glucose uptake [10]. The discrepancy in these results might involve whether insulin reached the muscle cells directly or via the blood flow and blood vessel walls. Effective insulin-induced glucose uptake not only requires insulin binding to muscle cell membrane receptors and promotion of GLUT4 translocation but also adequate insulin influx into the muscle tissue and its capillary transit to reach muscle cells [11]. Studies have indicated that skeletal muscles undergo a reduction in capillary number [12, 13] and impaired endothelial function [14, 15] during disuse, suggesting that factors related to muscle blood flow significantly influence the anabolic response to insulin in skeletal muscle during disuse.
Capillaries are vital for facilitating the exchange of oxygen, nutrients, and waste products between the blood and muscle cells in skeletal muscle [16]. Increased capillarity in skeletal muscle enhances insulin-induced glucose uptake [17] and exercise capacity [18], leading to greater metabolic activity. Conversely, a decrease in capillary number reduces insulin sensitivity [19] and muscle endurance [20], lowering metabolic activity. However, the limitations in substance delivery are not solely due to a decline in capillary numbers but also to reduced insulin-dependent vascular responses [21]. Insulin has been shown to promote capillary recruitment and transendothelial insulin delivery [11]. However, conditions such as obesity may impair these responses, negatively impacting insulin transport to muscle cells and glucose uptake [14]. Recent research has suggested that impaired insulin-dependent endothelial response may hinder amino acid delivery to muscle cells, contributing to anabolic resistance [22]. These findings indicate that capillary number and endothelial response to insulin may influence the anabolic response during periods of disuse, although definitive evidence is still lacking.
Capillary growth in skeletal muscles occurs through sprouting and non-sprouting angiogenesis [23]. Endurance exercise promotes sprouting angiogenesis, while increased blood flow induced by α-adrenergic receptor blockers, as prazosin, stimulates non-sprouting angiogenesis [23, 24], ultimately expanding the capillary bed and improving substance exchange between muscle cells and blood. Prazosin has been shown in numerous in vivo studies to selectively promote angiogenesis in skeletal muscles [25–27], improving capillary number and insulin sensitivity in both healthy rats [17] and those treated with glucocorticoids [28]. However, the effects of prazosin on capillarity and insulin sensitivity in disused skeletal muscles remain unknown.
Building upon these findings, we hypothesized that prazosin-induced capillary growth in muscle would enhance blood flow and insulin sensitivity in disused skeletal muscles, thereby fostering an insulin-driven anabolic response. Additionally, muscle disuse not only leads to muscle atrophy but also triggers various changes in the skeletal muscles. To investigate this, hindlimb unloading (HU), a widely recognized rodent model for studying the effects of muscle unloading [29–31], was employed. The soleus muscle, known for its high oxidative metabolism and susceptibility to disuse effects, exhibits significant changes, including reductions in capillary number, diameter, and tortuosity [13, 31–35]. Therefore, this study aimed to explore the effect of prazosin-induced capillary increases on insulin-stimulated anabolic responses in the soleus muscle of hindlimb-unloaded rats.
Methods
Experimental design
Overall, 24 male Sprague-Dawley (SD) rats (age: 12 weeks; Japan SLC, Hamamatsu, Japan) were used in this study. The animals were randomly divided into two groups: normal or hindlimb-unloaded (HU) conditions. Within each group, the rats were further subdivided based on their water intake, receiving either plain water or water with prazosin hydrochloride (50 mg/L) ad libitum [26]. This resulted in four experimental groups: control + water (Con, n = 6), control + prazosin (Con + Prz, n = 6), hind limb unloading + water (HU, n = 6), and hind limb unloading + prazosin (HU + Prz, n = 6). HU was performed through tail suspension, as previously described [36, 37]. Each rat was kept in a separate cage at a room temperature of 22 ± 2 °C, under a 12-hour light/dark cycle, with lights on from 07:00 to 19:00.
This study received approval from the Institutional Animal Care and Use Committee and adhered to the Prefectural University of Hiroshima Animal Experimentation Regulations (Mihara, Japan). All experimental and animal care procedures were performed in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Muscle preparation
The muscle was prepared as previously reported [34, 35]. At the end of HU period, the 6-h fasted rats were deeply anesthetized by inhalation of 2% isoflurane gas using an anesthetic mask. To investigate the anabolic signaling triggered by insulin stimulation, muscle samples were excised before and after insulin stimulation following established procedures with slight modifications [28]. Briefly, the left soleus muscle was excised, and insulin (3 U/kg body mass) was intraperitoneally administered. Twenty minutes post-insulin injection, the right soleus muscle was excised. This protocol enables the evaluation of insulin stimulation in skeletal muscle. Euthanasia was performed after the collection of the right muscle specimen through exsanguination and heart removal. Subsequently, both excised soleus muscles were promptly frozen in dry-ice-cooled acetone and stored at -80 °C until analysis.
Histological analyses
Muscle transverse sections were prepared as previously described [34, 38]. The mid-belly of the soleus muscle was mounted on a specimen chuck with Tissue-Tek O.C.T. compound (Sakura Finetechnical, Tokyo, Japan), and 12 μm thick transverse sections were cut using a cryostat microtome (CM-1510 S; Leica Microsystems, Heidelberg, Germany). These sections were immunohistochemically stained with a dystrophin monoclonal antibody (D8043, Sigma Aldrich, St. Louis, MO, USA), as previously described [39, 40], to identify muscle fibers and measure muscle fiber cross-sectional area (CSA). To visualize capillaries, additional sections were stained for alkaline phosphatase (AP), as detailed in earlier studies [35, 41]. The capillary-to-fiber (C/F) ratio was calculated by counting capillaries and myofibers in each cryosection using microscopic images of the AP staining. All measurements were conducted with ImageJ (National Institutes of Health, Bethesda, MD, USA).
Western blot analysis
Western blot analysis was conducted according to previously described with slight modifications [34, 35, 42]. Each soleus muscle sample (approximately 20 mg) was homogenized in RIPA lysis buffer with a protease inhibitor cocktail (RIPA Lysis Buffer System, sc-24,948; Santa Cruz Biotechnology, Dallas, TX, USA) and centrifuged at 10,000 ×g for 10 min at 4 °C, followed by supernatant collection. Protein concentration in the supernatant was measured using a protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA). Samples were mixed with 2 × Blue Loading Buffer and boiled at 95 °C for 5 min. Proteins (20–40 µg per lane) were then loaded onto and separated on 7.5%, 10%, or 12.5% sodium dodecyl sulfate-polyacrylamide gels. Subsequently, the proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and blocked for 5 min with EveryBlot blocking buffer (Bio-Rad Laboratories). Next, the membranes were incubated with antibodies against p-Akt (Ser473, #9271, Cell Signaling Technology [CST], Danvers, MA, USA), Akt (#9272, CST), p-p70S6K (Thr389, #9234, CST), p70S6K (#2708, CST), p-S6RP (Ser240/244, #2215, CST), S6RP (#2217, CST), p-eNOS (Ser1117, #9571, CST), eNOS (#32,027, CST), p-AMPK (#2535, CST), and AMPK (#5831, CST) overnight at 4 °C, followed by a 1-h incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000 in PBST). Proteins were detected using chemiluminescent reagents (Clarity™ Western ECL Substrate, Bio-Rad Laboratories, Hercules, CA, USA). The images were then analyzed with the Image Quant™ LAS 500 system (Cytiva, Marlborough, MA, USA). Ponceau S staining was used to ensure equal loading across lanes and for normalization purposes. Band intensities were measured using Image Quant™ TL software (Cytiva).
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using two-way analysis of variance (ANOVA) (HU × prazosin or insulin × prazosin). When interactions were detected, a Bonferroni multiple-comparison test was conducted. All statistical analyses were carried out using GraphPad Prism software (version 7.0; GraphPad Inc., San Diego, CA, USA). Statistical significance was defined as P < 0.05.
Results
Body weight and soleus muscle mass
Table 1 shows data on body weight and soleus muscle mass. Final body weight decreased after HU treatment. Both absolute and relative soleus muscle masses significantly decreased post-HU; however, prazosin treatment resulted in a significant increase in these parameters (main effects of HU and prazosin at P < 0.05) (Table 1).
Table 1.
Body weight and soleus muscle mass
| Con | Con + Prz | HU | HU + Prz | |
|---|---|---|---|---|
| Initial body weight (g) | 373 ± 3 | 367 ± 3 | 377 ± 2 | 368 ± 3 |
| Final body weight (g) | 431 ± 6 | 416 ± 6 | 358 ± 5a | 363 ± 6a |
| SOL mass (mg) | 176 ± 9 | 182 ± 9b | 80 ± 1a | 114 ± 8ab |
| SOL/BW (mg/100 g ) | 40.9 ± 1.8 | 43.6 ± 1.9b | 22.4 ± 0.4a | 31.4 ± 2.4ab |
Values are expressed as mean ± SEM. Significant differences: a main effect of hindlimb unloading (P < 0.05); b main effect of prazosin (P < 0.05)
Capillary-to-fiber ratio
Figure 1A shows representative sections stained for AP across different groups. C/F ratio significantly declined following HU. Conversely, prazosin treatment significantly elevated the value (main effect of HU and prazosin at P < 0.05, respectively), as illustrated in Fig. 1B.
Fig. 1.
Representative microscopic images of transverse serial sections stained for AP of the soleus muscle in each group (A). Capillary-to-fiber (C/F) ratio (B). Scale bar = 100 μm. Values are expressed as mean ± SEM. n.s. indicates not significant
Myofiber cross-sectional area
Figure 2A presents representative sections stained with the anti-dystrophin antibody for each group. The distribution of the myofiber CSA in each experimental group is shown in Fig. 2B. Histogram representations of myofiber CSA in the Con and Con + Prz groups exhibited minimal variation. In contrast, the histogram representation for the HU + Prz group shifted towards a larger myofiber CSA compared to the HU group. Myofiber CSA was significantly reduced following HU; however, prazosin treatment resulted in a significant increase in these values in each experimental group (Fig. 2C). Additionally, prazosin treatment significantly increased myofiber CSA in the disused soleus muscle.
Fig. 2.
Representative microscopic images of transverse serial sections stained for Dystrophin of the soleus muscle in each group (A). Muscle fiber cross-sectional area (B). Distributions of fiber cross-sectional area for each experimental group. A total of > 1000 fibers per experimental group (> 200 fibers per muscle sample, n = 6) were analyzed from randomly chosen areas in each soleus muscle (C). Scale bar = 100 μm. Values are expressed as mean ± SEM. Significant differences: * between water and prazosin for each group (P < 0.05); # between Con and HU group for each treatment (P < 0.05). n.s. indicates not significant
Protein expression levels of anabolic pathway
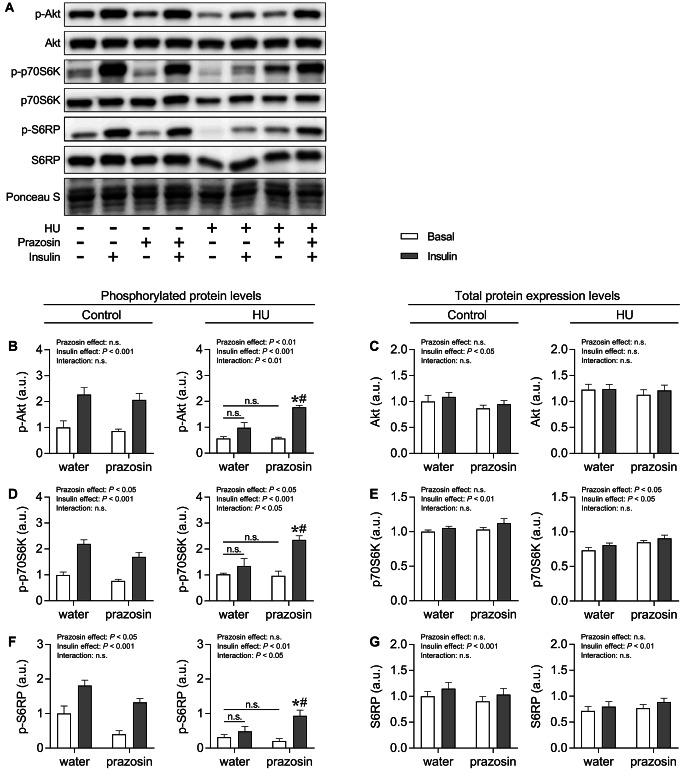
Figure 3A shows representative images of protein blots for anabolism-related signaling with or without insulin stimulation. The analysis of phosphorylated protein levels revealed that in the control group, insulin stimulation significantly increased p-Akt, p-p70S6K, and p-S6RP levels (Fig. 3B, D, and F). Conversely, prazosin significantly decreased p-p70S6K and p-S6RP levels. In the HU group, there were significant interaction effects between prazosin and insulin for p-Akt, p-p70S6K, and p-S6RP levels. Notably, in the water-treated HU group, insulin stimulation did not increase these phosphorylated protein levels. However, in the prazosin-treated HU group, insulin stimulation significantly increased these levels, with significantly higher levels compared to those of the water-treated HU group. Regarding total protein levels, insulin stimulation significantly increased Akt, p70S6K, and S6RP levels in the control group, and p70S6K and S6RP levels in the HU group (Fig. 3C, E, and G). Additionally, prazosin treatment significantly increased total p70S6K levels.
Fig. 3.
A: Representative Western blots for p-Akt, Akt, p-p70S6K, p70S6K, p-S6RP, and S6RP in each group (A). Mean protein expression levels of p-Akt (B), Akt (C), p-p70S6K (D), p70S6K (E), p-S6RP (F), and S6RP (G) in the soleus muscle of each group. Values are expressed as mean ± SEM. Significant differences: * between basal and insulin-stimulated condition in each treatment (P < 0.05); # between water and prazosin treatment in each condition with or without insulin stimulation (P < 0.05). n.s. indicates not significant
Protein expression levels of eNOS and AMPK
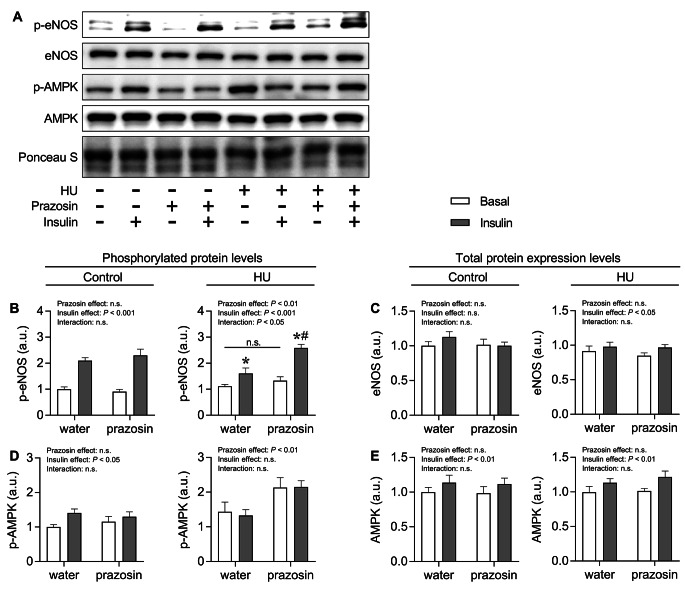
Figure 4A shows representative images of protein blots for phosphorylated and total eNOS and AMPK with or without insulin stimulation. The analysis of phosphorylated protein levels revealed that in the control group, insulin stimulation significantly increased both p-eNOS and p-AMPK levels (Fig. 4B and D). In the HU group, there was a significant interaction effect between prazosin and insulin for p-eNOS. Although insulin stimulation significantly increased p-eNOS levels in both the water- and prazosin-treated groups, the level in the prazosin-treated group was significantly higher than that in the water-treated group. On the other hand, prazosin significantly increased p-AMPK levels regardless of insulin stimulation. Regarding total protein levels, insulin stimulation significantly increased AMPK levels in the control group, and both eNOS and AMPK levels in the HU group (Fig. 4C and E).
Fig. 4.
A: Representative western blots for p-eNOS, eNOS, p-AMPK, and AMPK (A). Mean protein expression levels of p-eNOS (B), eNOS (C), p-AMPK (D), and AMPK (E) in the soleus muscle of each group. Values are expressed as mean ± SEM. Significant differences: * between basal and insulin-stimulated condition in each treatment (P < 0.05); # between water and prazosin treatment in each condition with or without insulin stimulation (P < 0.05). n.s. indicates not significant
Discussion
In this study, we investigated the effects of prazosin treatment on capillary regression in the soleus muscle during HU and its subsequent impact on anabolic signaling in response to insulin stimulation. Disuse-induced capillary regression in the soleus muscle significantly reduced the activation of anabolic signaling following insulin stimulation. However, prazosin administration counteracted capillary regression in the soleus muscle, enhancing the activation of anabolic signaling in response to insulin. These findings suggest that prazosin may mitigate muscle anabolic resistance by preserving capillaries during mechanical unloading.
Capillary regression in the soleus muscle due to disuse, as documented in this study, aligns with findings from previous studies [31, 33, 34, 43]. However, prazosin significantly inhibited capillary regression. Prazosin increases skeletal muscle blood flow, leading to increased blood flow that exerts wall shear stress on the vascular endothelium within the skeletal muscle, which in turn results in increased production of nitric oxide (NO) and enhanced expression of vascular endothelial growth factor (VEGF), thereby promoting capillary neoformation [23, 27]. Previous studies have shown that prazosin administration ameliorates muscle capillary regression in both healthy [27] and pathologically affected animals, such as those with diabetes [25] and those undergoing glucocorticoid treatment [26]. While the effects of prazosin in a disuse model had not been previously established, the inhibition of capillary regression in the soleus muscle by prazosin observed in this study likely occurred through a similar mechanism.
The activation of the mTORC1 pathway is crucial for increased MPS and skeletal muscle growth [44–46]. HU is suggested to lead to a blunted activation of mTORC1-related factors in response to feeding in the soleus muscle, indicating HU-induced anabolic resistance to anabolic stimuli [47]. Consistent with previous findings, insulin stimulation activated anabolic signals such as Akt, P70S6K, and S6RP in the control group. However, insulin resistance, where the effectiveness of insulin is diminished under various pathological conditions such as aging, leads to reduced anabolic signaling in muscle proteins, thereby impacting skeletal muscle mass maintenance [48, 49]. Moreover, recent evidence suggests that physical inactivity play a role in weakening the anabolic response to insulin stimulation [1, 2]. Consistent with this, our findings showed that anabolic signaling stimulated by insulin was also diminished in the HU group, effectively replicating the insulin and anabolic resistance observed during disuse.
Capillary regression in skeletal muscles contributes to insulin resistance by diminishing the efficacy of insulin in muscle cells. Positive correlations between skeletal muscle capillarization and insulin sensitivity have been established [17, 50, 51], as capillaries ensure efficient insulin delivery to muscle cells [52]. HU results in decreased capillary number and alterations in capillary structure within the soleus muscle [31, 33, 34, 43], potentially inhibiting substance delivery from the blood to muscle cells. Our findings showed a decrease in capillary number due to HU along with attenuated Akt activation following insulin stimulation, suggesting that disuse-induced reduction in the capillary network may hinder insulin delivery to muscle cells. Conversely, preserving the capillary network facilitates the efficient delivery of insulin and amino acids necessary for muscle synthesis. Previous studies have indicated that prazosin administration in animals experiencing muscle capillary thinning due to glucocorticoid treatment prevents capillary thinning and enhances Akt activation following insulin stimulation [28]. Likewise, we found that prazosin increased capillary number in the soleus muscle and activated downstream anabolic signals following insulin stimulation, implying that capillary network maintenance aids in ensuring the efficient delivery of insulin and amino acids to muscle cells. Thus, the concordance between the changes in capillary dynamics and insulin-induced Akt/mTORC1/p70S6K activation supports an integral relationship between insulin-induced anabolic responses and skeletal muscle capillaries. Furthermore, Snider et al. [53] highlighted the effect of skeletal muscle capillary number on the hypertrophic effects of resistance exercise on muscle cells, underscoring the role of skeletal muscle capillaries in the delivery of factors that regulate exercise-induced anabolic responses in muscle cells. Therefore, enhancing muscle capillaries through nutritional and physical training interventions may maximize the anabolic effects on muscles by promoting hormone and amino acid delivery to muscle cells. Therefore, enhancing muscle capillaries could maximize the anabolic effects induced by nutritional and physical training interventions on muscle, by facilitating the delivery of hormones and amino acids to muscle cells. This could serve as a valuable intervention strategy to overcome anabolic resistance, which is often observed in states of muscle disuse due to physical inactivity.
Another potential explanation for the enhanced insulin-induced anabolic signaling induced by prazosin treatment in disused muscles may be the increase in NO production. Insulin delivery to muscle cells is determined not only by the number of capillaries but also by an increase in muscle blood flow due to capillary recruitment during insulin stimulation and an increase in transendothelial insulin transport [14]. Consuming both amino acids and glucose results in higher MPS than consuming amino acids alone [49]. Additionally, the significant attenuation of insulin-induced MPS by the inhibition of NO production using L-NAME suggests that changes in blood flow induced by insulin are determining factors for insulin-induced anabolic responses [54]. These reports suggest that the insulin-induced increase in skeletal muscle blood flow is responsible for activating anabolic responses. In the present study, activated p- eNOS was observed in response to insulin stimulation in the HU + Prz group. Phosphorylated eNOS plays a pivotal role for NO production [55]. Thus, it is likely that a potential increase in NO production, along with an increase in intramuscular vascular blood flow due to increased wall shear stress. Therefore, prazosin treatment of disused muscle may preserve the ability of skeletal muscle capillaries to recruit and transendothelially transport insulin, thus efficiently delivering insulin to muscle cells. Conversely, in the HU group, the activation of eNOS by insulin stimulation was attenuated, suggesting a reduction in insulin-induced capillary recruitment and transendothelial transport. It has been reported that NO-induced increases in muscle blood flow do not diminish in disused muscles [56]. Hence, the attenuation of NO production following insulin stimulation and the difference in eNOS activity with or without prazosin treatment could be evidence of the differences in insulin-induced NO production and muscle blood flow changes between the conditions. Together, these results indicate that prazosin can multifacetedly improve skeletal muscle anabolic responses by enhancing capillary number and NO production. Additionally, our study highlights the potential of targeting capillary growth and normalizing endothelial function as potential therapeutic targets to prevent and treat muscle atrophy and anabolic resistance.
Insulin resistance induced by physical inactivity remains less explored compared to that triggered by dietary factors [57]. In this study, the suppression of capillary regression and enhancement of insulin-stimulated eNOS activation by prazosin were shown to promote Akt activation upon insulin stimulation. This finding implies that the dramatic negative shifts in vascular factors due to disuse could significantly affect insulin sensitivity. Improvements in muscle capillarity and endothelial function during disuse might have facilitated the efficient transport of oxygen, hormones, and nutrients to muscle cells via the blood flow. Notably, in the HU + Prz group, the myofiber CSA was significantly greater than in the HU group, highlighting prazosin’s efficacy in mitigating muscle atrophy. Nonetheless, the precise mechanisms behind these observations warrant further investigation.
This study has few limitations. First, in this study, we used the soleus muscle, a slow-twitch muscle, from male rats exclusively. There are known differences in vascular adaptations to disuse between fast-twitch and slow-twitch muscles [12]. Additionally, there are sex differences in the molecular phenotypes related to skeletal muscle capillarity [58] and angio-adaptation in response to external stimuli [59]. However, our study did not examine the differences between fast-twitch and slow-twitch muscles or the impact of sex differences. Future studies should investigate these aspects, taking into account muscle fiber types and sex differences. Second, although HU is a well-established model for studying muscle disuse, it does not fully replicate the complexities of human muscle disuse conditions, such as those seen during prolonged bed rest or immobilization. Consequently, this limitation may affect the direct applicability of our findings to human clinical scenarios. Third, our study only investigated the molecular responses in skeletal muscle to insulin stimulation at a single time point, 20 min post-stimulation, focusing specifically on the Akt/mTOR/p70S6K pathway. Insulin signaling is complex and involves multiple pathways beyond those investigated in this study. To obtain a more accurate understanding of molecular responses, future studies should measure the MPS rate using the SUnSET method [60], assess insulin sensitivity directly, and include longitudinal assessments over extended periods. Future studies will also explore additional pathways to provide a more comprehensive understanding of insulin resistance and anabolic responses. Finaly, we did not measure NO production following insulin stimulation. Future studies should assess serum NOx levels after insulin stimulation and muscle blood flow or verify if these changes are mitigated by L-NAME-induced inhibition of NO synthesis.
In conclusion, our findings reveal that prazosin administration can attenuate disuse-induced capillary regression in the soleus muscle of HU rats. Furthermore, prazosin-induced capillary maintenance in disused muscles may enableinsulin-stimulated protein synthesis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported in part by JSPS KAKENHI (21K17652 and 23K10942).
Abbreviations
- ANOVA
Analysis of variance
- AP
Alkaline phosphatase
- C/F
Capillary-to-fiber
- CSA
Cross-sectional area
- HU
Hindlimb unloading
- mTORC1
Mammalian target of rapamycin complex 1
- MPS
Muscle protein synthesis
- NO
Nitric oxide
- PBST
Phosphate buffer saline with Tween 20
- PVDF
Polyvinylidene difluoride
- RIPA
Radioimmunoprecipitation assay
- SD
Sprague-Dawley
- SEM
Standard error of the mean
- SOL
Soleus
- VEGF
Vascular endothelial growth factor
Funding
This study was supported in part by JSPS KAKENHI (21K17652 and 23K10942).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request from a qualified researcher.
Declarations
Ethical approval
This study was approved by the Institutional Animal Care and Use Committee and followed the Prefectural University of Hiroshima Animal Experimentation Regulations (Mihara, Japan). All experimental and animal care procedures were conducted according to the National Institute of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Disclosure of conflicts of interest
The authors declared that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Masayuki Tanaka and Miho Kanazashi contributed equally to this work.
References
- 1.Paulussen KJM, McKenna CF, Beals JW, Wilund KR, Salvador AF, Burd NA. Anabolic resistance of muscle protein turnover comes in various shapes and sizes. Front Nutr. 2021;8:615849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morton RW, Traylor DA, Weijs PJM, Phillips SM. Defining anabolic resistance: implications for delivery of clinical care nutrition. Curr Opin Crit Care. 2018;24:124–30. [DOI] [PubMed] [Google Scholar]
- 3.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol. 2016;229:R67–81. [DOI] [PubMed] [Google Scholar]
- 4.Greiwe JS, Kwon G, McDaniel ML, Semenkovich CF. Leucine and insulin activate p70 S6 kinase through different pathways in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;281:E466–71. [DOI] [PubMed] [Google Scholar]
- 5.Suryawan A, Davis TA. The abundance and activation of mTORC1 regulators in skeletal muscle of neonatal pigs are modulated by insulin, amino acids, and age. J Appl Physiol (1985). 2010;109:1448-54. [DOI] [PMC free article] [PubMed]
- 6.Rasmussen BB, Fujita S, Wolfe RR, Mittendorfer B, Roy M, Rowe VL, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonen A, Elder GC, Tan MH. Hindlimb suspension increases insulin binding and glucose metabolism. J Appl Physiol (1985). 1988;65:1833-9. [DOI] [PubMed]
- 8.O’Keefe MP, Perez FR, Sloniger JA, Tischler ME, Henriksen EJ. Enhanced insulin action on glucose transport and insulin signaling in 7-day unweighted rat soleus muscle. J Appl Physiol (1985). 2004;97:63–71. [DOI] [PubMed]
- 9.Henriksen EJ, Tischler ME, Johnson DG. Increased response to insulin of glucose metabolism in the 6-day unloaded rat soleus muscle. J Biol Chem. 1986;261:10707–12. [PubMed] [Google Scholar]
- 10.Xu PT, Song Z, Zhang WC, Jiao B, Yu ZB. Impaired translocation of GLUT4 results in insulin resistance of atrophic soleus muscle. Biomed Res Int. 2015;2015:291987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Liu Z. Muscle insulin resistance and the Inflamed Microvasculature: fire from within. Int J Mol Sci. 2019;20. [DOI] [PMC free article] [PubMed]
- 12.Roudier E, Gineste C, Wazna A, Dehghan K, Desplanches D, Birot O. Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J Physiol. 2010;588:4579–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanazashi M, Okumura Y, Al-Nassan S, Murakami S, Kondo H, Nagatomo F, et al. Protective effects of astaxanthin on capillary regression in atrophied soleus muscle of rats. Acta Physiol (Oxf). 2013;207:405–15. [DOI] [PubMed] [Google Scholar]
- 14.Kubota T, Kubota N, Kumagai H, Yamaguchi S, Kozono H, Takahashi T, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell Metab. 2011;13:294–307. [DOI] [PubMed] [Google Scholar]
- 15.Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol (1985). 2000;89:1483-90. [DOI] [PubMed]
- 16.Gudbjörnsdóttir S, Sjöstrand M, Strindberg L, Wahren J, Lönnroth P. Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab. 2003;88:4559–64. [DOI] [PubMed] [Google Scholar]
- 17.Akerstrom T, Laub L, Vedel K, Brand CL, Pedersen BK, Lindqvist AK, et al. Increased skeletal muscle capillarization enhances insulin sensitivity. Am J Physiol Endocrinol Metab. 2014;307:E1105–16. [DOI] [PubMed] [Google Scholar]
- 18.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–60. [DOI] [PubMed] [Google Scholar]
- 19.Bonner JS, Lantier L, Hasenour CM, James FD, Bracy DP, Wasserman DH. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olfert IM, Howlett RA, Tang K, Dalton ND, Gu Y, Peterson KL, et al. Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J Physiol. 2009;587:1755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiol Endocrinol Metab. 2011;301:E252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks NF, Rogers EM, Church DD, Ferrando AA, Jenkins NDM. The contributory role of vascular health in age-related anabolic resistance. J Cachexia Sarcopenia Muscle. 2022;13:114–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olfert IM, Baum O, Hellsten Y, Egginton S. Advances and challenges in skeletal muscle angiogenesis. Am J Physiol Heart Circ Physiol. 2016;310:H326–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2009;457:963–77. [DOI] [PubMed] [Google Scholar]
- 25.Dunford EC, Leclair E, Aiken J, Mandel ER, Haas TL, Birot O et al. The effects of voluntary exercise and prazosin on capillary rarefaction and metabolism in streptozotocin-induced diabetic male rats. J Appl Physiol (1985). 2017;122:492–502. [DOI] [PMC free article] [PubMed]
- 26.Mandel ER, Dunford EC, Trifonova A, Abdifarkosh G, Teich T, Riddell MC, et al. Prazosin can prevent glucocorticoid mediated Capillary Rarefaction. PLoS ONE. 2016;11:e0166899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baum O, Da Silva-Azevedo L, Willerding G, Wockel A, Planitzer G, Gossrau R, et al. Endothelial NOS is main mediator for shear stress-dependent angiogenesis in skeletal muscle after prazosin administration. Am J Physiol Heart Circ Physiol. 2004;287:H2300–8. [DOI] [PubMed] [Google Scholar]
- 28.Dunford EC, Mandel ER, Mohajeri S, Haas TL, Riddell MC. Metabolic effects of prazosin on skeletal muscle insulin resistance in glucocorticoid-treated male rats. Am J Physiol Regul Integr Comp Physiol. 2017;312:R62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shimkus KL, Shirazi-Fard Y, Wiggs MP, Ullah ST, Pohlenz C, Gatlin DM 3 et al. rd, Responses of skeletal muscle size and anabolism are reproducible with multiple periods of unloading/reloading. J Appl Physiol (1985). 2018;125:1456-67. [DOI] [PubMed]
- 30.Wagatsuma A, Kotake N, Kawachi T, Shiozuka M, Yamada S, Matsuda R. Mitochondrial adaptations in skeletal muscle to hindlimb unloading. Mol Cell Biochem. 2011;350:1–11. [DOI] [PubMed] [Google Scholar]
- 31.Fujino H, Kohzuki H, Takeda I, Kiyooka T, Miyasaka T, Mohri S et al. Regression of capillary network in atrophied soleus muscle induced by hindlimb unweighting. J Appl Physiol (1985). 2005;98:1407-13. [DOI] [PubMed]
- 32.Hirayama Y, Nakanishi R, Tategaki A, Maeshige N, Kondo H, Ishihara A et al. Enterococcus faecium strain R30 increases red blood cell velocity and prevents capillary regression in the soleus of hindlimb-unloaded rats via the eNOS/VEGF pathway. Microcirculation. 2017;24. [DOI] [PubMed]
- 33.Kanazashi M, Tanaka M, Murakami S, Kondo H, Nagatomo F, Ishihara A, et al. Amelioration of capillary regression and atrophy of the soleus muscle in hindlimb-unloaded rats by astaxanthin supplementation and intermittent loading. Exp Physiol. 2014;99:1065–77. [DOI] [PubMed] [Google Scholar]
- 34.Tanaka M, Kanazashi M, Kondo H, Fujino H. Time course of capillary regression and an expression balance between vascular endothelial growth factor-A and thrombospondin-1 in the soleus muscle of hindlimb unloaded rats. Muscle Nerve. 2022;65:350–60. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka M, Kanazashi M, Maeshige N, Kondo H, Ishihara A, Fujino H. Protective effects of Brazilian propolis supplementation on capillary regression in the soleus muscle of hindlimb-unloaded rats. J Physiol Sci. 2019;69:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol (1985). 2002;92:1367-77. [DOI] [PubMed]
- 37.Tanaka M, Kanazashi M, Maeshige N, Kondo H, Ishihara A, Fujino H. Protective effects of Brazilian propolis supplementation on capillary regression in the soleus muscle of hindlimb-unloaded rats. J Physiol Sci. 2018;69:223–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanazashi M, Tanaka M. Acute effect of electrical stimulation on muscle protein synthesis and break-down in the soleus muscle of hindlimb unloaded rats. Biomed Res. 2023;44:209–18. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi R, Hirayama Y, Tanaka M, Maeshige N, Kondo H, Ishihara A, et al. Nucleoprotein supplementation enhances the recovery of rat soleus mass with reloading after hindlimb unloading-induced atrophy via myonuclei accretion and increased protein synthesis. Nutr Res. 2016;36:1335–44. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi R, Tanaka M, Maeshige N, Kondo H, Roy RR, Fujino H. Nucleoprotein-enriched diet enhances protein synthesis pathway and satellite cell activation via ERK1/2 phosphorylation in unloaded rat muscles. Exp Physiol. 2021;106:1587–96. [DOI] [PubMed] [Google Scholar]
- 41.Hansen-Smith FM, Blackwell LH, Joswiak GR. Expression of muscle capillary alkaline phosphatase is affected by hypoxia. J Appl Physiol (1985). 1992;73:776– 80. [DOI] [PubMed]
- 42.Kanazashi M, Tanaka M, Maezawa T, Fujino H. Effects of reloading after chronic neuromuscular inactivity on the three-dimensional capillary architecture in rat soleus muscle. Acta Histochem. 2020;122:151617. [DOI] [PubMed] [Google Scholar]
- 43.Hirayama Y, Nakanishi R, Maeshige N, Fujino H. Preventive effects of nucleoprotein supplementation combined with intermittent loading on capillary regression induced by hindlimb unloading in rat soleus muscle. Physiol Rep. 2017;5. [DOI] [PMC free article] [PubMed]
- 44.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–13. [DOI] [PubMed] [Google Scholar]
- 45.Ogasawara R, Fujita S, Hornberger TA, Kitaoka Y, Makanae Y, Nakazato K, et al. The role of mTOR signalling in the regulation of skeletal muscle mass in a rodent model of resistance exercise. Sci Rep. 2016;6:31142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hornberger TA, Chien S. Mechanical stimuli and nutrients regulate rapamycin-sensitive signaling through distinct mechanisms in skeletal muscle. J Cell Biochem. 2006;97:1207–16. [DOI] [PubMed] [Google Scholar]
- 47.Roberson PA, Shimkus KL, Welles JE, Xu D, Whitsell AL, Kimball EM, et al. A time course for markers of protein synthesis and degradation with hindlimb unloading and the accompanying anabolic resistance to refeeding. J Appl Physiol (1985). 2020;129:36–46. [DOI] [PMC free article] [PubMed]
- 48.Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, et al. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80:415–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prior SJ, Blumenthal JB, Katzel LI, Goldberg AP, Ryan AS. Increased skeletal muscle capillarization after aerobic exercise training and weight loss improves insulin sensitivity in adults with IGT. Diabetes Care. 2014;37:1469–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusters YH, Barrett EJ. Muscle microvasculature’s structural and functional specializations facilitate muscle metabolism. Am J Physiol Endocrinol Metab. 2016;310:E379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Snijders T, Nederveen JP, Joanisse S, Leenders M, Verdijk LB, van Loon LJ, et al. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J Cachexia Sarcopenia Muscle. 2017;8:267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dickinson JM, Volpi E, Rasmussen BB. Exercise and nutrition to target protein synthesis impairments in aging skeletal muscle. Exerc Sport Sci Rev. 2013;41:216–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Just TP, Jendzjowsky NG, DeLorey DS. Hindlimb unweighting does not alter vasoconstrictor responsiveness and nitric oxide-mediated inhibition of sympathetic vasoconstriction. J Physiol. 2015;593:2213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reidy PT, Monnig JM, Pickering CE, Funai K, Drummond MJ. Preclinical rodent models of physical inactivity-induced muscle insulin resistance: challenges and solutions. J Appl Physiol (1985). 2021;130:537– 44. [DOI] [PMC free article] [PubMed]
- 58.O’Reilly J, Ono-Moore KD, Chintapalli SV, Rutkowsky JM, Tolentino T, Lloyd KCK, et al. Sex differences in skeletal muscle revealed through fiber type, capillarity, and transcriptomics profiling in mice. Physiol Rep. 2021;9:e15031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rudnicki M, Abdifarkosh G, Rezvan O, Nwadozi E, Roudier E, Haas TL. Female mice have higher angiogenesis in Perigonadal Adipose tissue than males in response to High-Fat Diet. Front Physiol. 2018;9:1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. Faseb j. 2011;25:1028–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request from a qualified researcher.