Abstract
The Taenia crassiceps recombinant antigen KETc7 has been shown to be effective as a vaccine against experimental murine cysticercosis, a laboratory model used to test potentially promising molecules against porcine Taenia solium cysticercosis. Based on the deduced amino acid sequence of this proline-rich polypeptide, three fragments, GK-1, GK-2, and GK-3, were chemically synthesized in linear form. Of the three peptides, only GK-1 induced sterile protection against T. crassiceps cysticercosis in 40 to 70% of BALB/cAnN male mice. GK-1 is an 18-amino-acid peptide which contains at least one B-cell epitope, as demonstrated by its ability to induce an antibody response to the peptide and T. crassiceps antigen without need of a carrier protein. Immunofluorescence studies revealed that anti-GK1 antibodies strongly react with the native protein in the tegument of T. crassiceps and also with anatomical structures of T. solium eggs, oncospheres, cysticercus, and tapeworm. GK-1 also contains at least one T-cell epitope, capable of stimulating the proliferation of CD8+ and to a lower extent CD4+ T cells primed either with the free peptide or T. crassiceps total antigen. The supernatant of the stimulated cells contained high levels of gamma interferon and low levels of interleukin-4. Similar results were obtained with T cells tested for intracellular cytokine production, an indication of the peptide’s capacity to induce an inflammatory response. The remarkable protection induced by GK-1 immunization, its physicochemical properties, and its presence in all developmental stages of T. solium point to this synthetic peptide as a strong candidate in the construction of a synthetic vaccine against T. solium pig cysticercosis.
Taenia solium cysticercosis is highly prevalent in humans and pigs in Latin America, Asia, and Africa (24) and has serious health and economic consequences (10). Although cysticercosis has been practically eradicated in developed countries, it is a major concern in the developing world and of consideration as a reemerging disease in the United States because of immigration from areas where the disease is endemic (20). Moreover, a recent publication indicates that European countries may not be totally rid of human neurocysticercosis caused by T. solium (26). The life cycle of this parasite includes a larval (cysticercus) phase affecting both pigs and humans after ingestion of eggs present in human feces. The eggs are produced by the adult tapeworm localized in the gut of humans who ingested live cysticerci present in improperly cooked pork meat. The tapeworm produces millions of eggs that are passed to the environment. Transmission is thus clearly related to prevailing low sanitary standards in personal hygiene and environmental control and also with rustic rearing of pigs in impoverished sectors of the rural population. Control of transmission by general improvement of the social, economic, and educational status of developing countries is not within reach in the near future. But since the pig is an indispensable intermediate host, transmission could be reduced by lowering the prevalence of pig cysticercosis through vaccination. Development of an effective vaccine for use in pigs is being pursued by a number of scientists (14, 16, 23).
Because experimentation leading to a vaccine against porcine cysticercosis is hampered by the high cost and slow data retrieval involved in testing pigs, another cestode, Taenia crassiceps, which exhibits extensive antigen similarities with T. solium and whose metacestodes easily and rapidly develop in the peritoneal cavity of mice (3, 7, 10), has been used as an experimental model to test and screen promising antigens before testing them in pigs (11, 12, 22, 28). Thus, we have shown that total T. crassiceps antigens can partially protect pigs against T. solium cysticercosis: however, the effects of vaccination with antigen extracts depended on the dose used, some being protective while others led to facilitation of the infection (23), a finding that oriented our research to the identification of individual protective antigens and their peptidic epitopes (11, 12, 28). We identified and cloned four recombinant T. crassiceps antigens (KETc1, -4, -7, and -12) which conferred to mice different levels of resistance to murine cysticercosis (12). The antigenicity profile of the deduced 100-amino-acid sequence of the KETc7 clone was structurally assessed to detect potentially immunologically active epitopes (8). Three of the peptide candidates of KETc7 (GK-1, GK-2, and GK-3) were chemically synthesized, and their antigenicity was tested with sera from T. solium- and T. crassiceps-infected hosts (humans, pigs, and mice). Since the three peptides were extensively reactive with these sera (8), we assessed their protective capacity and studied the immune response that they elicit in immunized mice. We also searched for the peptide’s presence in T. solium specimens to obtain indications as to its potential inclusion in a vaccine against porcine cysticercosis, especially if found in oncospheres and early larvae, the parasite’s developmental stages most vulnerable to immunological attack by antibodies (17). Also, the peptide’s physicochemical properties and structural characteristics were studied to understand its immunological functions.
MATERIALS AND METHODS
Peptides.
The peptides GK-1 (amino acids [aa] 69 to 85; GYYYPSDPNTFYAPPYS[A]), GK-2 (aa 55 to 66; [KK]MPPYPTGGPPPV[K]), and GK-3 (aa 35 to 50; PPYAPNPGPPPPYTGA) were manually prepared by stepwise solid-phase synthesis with N-tert-butyloxycarbonyl derivatives of l-amino acids on phenylacetamidomethyl resin (Sigma Chemical Co., St. Louis, Mo.). All peptides were 95% pure as judged by high-pressure liquid chromagraphy on analytical C18 reversed-phase columns (3.9 by 150 mm; Delta Pak; Waters). The correct amino acid sequence of each peptide was confirmed by protein sequencing on a pulsed-liquid-phase protein sequencer (Applied Biosystems) at the National Institute of Cardiology, Mexico City, Mexico. GK-1 was coupled to bovine serum albumin (BSA) by standard procedures (25) using glutaraldehyde. Also, GK-1 was prepared as MAP (multiple-antigen peptide), containing eight copies of the GK-1 sequence coupled to a core matrix comprising oligomeric lysine (25).
Mice.
A syngenic BALB/cAnN strain of mice, previously characterized as susceptible to cysticercosis (22), was used for vaccine trials. Original stocks were purchased from M. Bevan (University of Seattle) and then bred and kept in our animal facilities by the single-line breeding system for 20 generations. All mice used were males of 5 to 7 weeks of age at the beginning of the experiments. The experiments reported herein were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals (1a).
Immunization of mice and collection of sera.
Groups of 5 to 10 BALB/cAnN mice each were immunized subcutaneously with different doses (0.5, 10, and 50 μg/mouse) of each peptide (GK-1, GK-2, and GK-3) emulsified in Freund’s complete adjuvant (FCA) prepared as previously reported (28). GK-1 (10 μg/mouse) as well as MAP–GK-1 and BSA–GK-1 (each at 50 μg/mouse) were prepared in saponin (Sigma) at a concentration of 100 μg/mouse as reported elsewhere (13). This concentration of peptide was determined as optimal when saponin was used as the adjuvant in collateral experiments (data not shown). Ten days later, the mice were given a booster with the same immunizing dose of the same peptide in the same adjuvant as used before. Immune sera were obtained from each mouse before each immunization and stored at −70°C until individually tested for the presence of specific antibodies.
Parasites.
The ORF strain of T. crassiceps (Zeder 1800) Rudolphi 1810, isolated by Freeman in 1962 (7) and supplied by B. Enders (Behringwerke, Marburg, Germany) in 1984 has been maintained by serial passage in BALB/cAnN female mice for 14 years in our animal facilities. Cysticerci for infection were harvested from the peritoneal cavity of mice 1 to 3 months after inoculation of 10 nonbudding small cysticerci (2 to 3 mm in diameter) per animal. Whole T. solium cysticerci were dissected from skeletal muscle of highly infected pork carcasses between 2 and 4 h after slaughter in an abattoir in Zacatepec, Morelos, Mexico. Segments from T. solium tapeworm and eggs were obtained from the feces of an infected child in the state of Puebla, Mexico. The tapeworm was recovered after the child’s treatment with a single oral dose (2 g) of niclosamide (Yomesan; kindly supplied by Bayer, Mexico City, Mexico). After washing in saline plus antibiotics (penicillin [100 U/per ml] plus streptomycin [100μg/ml]) streptomycin), several gravid proglottids were separated for immunofluorescence assays; eggs were obtained by cutting the proglottids with fine sharp scissors and then teasing the fragments. The eggs were then washed in saline before inclusion to immunolocalization studies.
T. crassiceps cysticercal antigens.
Soluble antigens were recovered from T. crassiceps cysticerci by the procedure previously described (9). Briefly, cysticerci recovered 1 to 3 months after infection were collected and placed in ice-cold phosphate-buffered saline (PBS). Cysticerci were then suspended in a minimal amount of buffer and centrifuged at 25,000 rpm for 60 min at 4°C. Afterwards, the cysts were ruptured by centrifugation, and the supernatant, which included the mixture of soluble T. crassiceps antigens, was recovered.
ELISA for antibody measurements.
T. crassiceps antigens obtained as previously described (10) were used as antigens in enzyme-linked immunosorbent assay (ELISA) to measure the antibody response induced by peptide immunization as described elsewhere (19). Briefly, 96-well flat-bottomed microtitration plates (Nunc, Roskilde, Denmark) were coated with the respective antigen preparation (1 μg/per well) and incubated overnight at 4°C. Sera were used at 1:100 dilution in PBS containing 1% BSA. Bound mouse immunoglobulins (Igs) were detected by using alkaline phosphatase-conjugated sheep anti-mouse IgG (whole molecule; Sigma) diluted 1:1,000 for 1 h at 37°C. The substrate used was detected by using p-nitrophenyl phosphate (Sigma) in diethanolamine buffer for 10 min at room temperature. The reaction was stopped with 2 N NaOH. Readings of optical density at 405 nm (OD405) were carried out in a Humareader ELISA processor (Human Gessellschaft Für Biochemica und Diagnostica, Taunusstein, Germany).
Proliferation assay.
Spleen cells from nonimmunized (injected only with saponin) or GK-1-immunized mice were harvested 15 days after the second immunization with saponin or GK-1 plus saponin, respectively, and cultured in RPMI 1640 medium supplemented with l-glutamine (0.2 mM), nonessential amino acids (0.01 mM), penicillin (100 U/ml), streptomycin (100 μg/ml), and fetal bovine serum (FBS; 10%). Cells were cultured with the appropriate concentration of concanavalin A (CoA) (5 μg/ml) or GK-1 or T. crassiceps antigens (10 μg/ml) and incubated at 37°C in a 5% CO2 humidified atmosphere, in flat-bottomed microtiter plates, at a cell concentration of 2 × 105 per 200 μl of final volume. Considering previous results in which higher levels of proliferation were obtained when peritoneal cells were included in the assay (data not shown), 104 peritoneal cells recovered from the same mice were added to each well at a volume of 50 μl. Peritoneal cells were obtained by ex vivo lavage with 5 ml of RPMI 1640. The cells were sedimented by centrifugation at 800 × g for 10 min. The pellets were resuspended in an additional 3 ml of supplemented RPMI 1640 medium and adjusted in volume to contain 2 × 105 cells/ml. After 72 h, the cultured cells were pulsed (1 μCi per well) for a further 18 h with [methyl-3H]thymidine (Amersham Life Science, Little Chalfont, England). Then all cells were harvested, and the amount of incorporated label was measured by counting in a model 1205 β-plate spectrometer (Wallac). All assays were performed in triplicate with at least four individual mice.
Spleen cell phenotype analysis.
After 3 days of in vitro culture with different doses of mitogen, antigen, or peptide, splenocytes were harvested and CD8/CD25 and CD4/CD25 expression was determined by staining with two-color cytometry using fluorescein isothiocyanate (FITC)-conjugated anti-CD8 (Pharmingen, San Diego, Calif.), FITC-conjugated anti-CD4 (Pharmingen), and phycoerythrin (PE)-conjugated anti-CD25. The percentage of CD3+ cells was determined by single-color cytometry using FITC-conjugated anti-CD3 (Boehringer, Mannheim, Germany) as previously described (6). Parallel samples of the cells were stained with the corresponding isotype control to account for nonspecific staining of the cells. Briefly, cells were washed with PBS containing 10% of gamma globulin-depleted FBS plus 0.02% NaN3 and incubated with the indicated antibodies at 4°C for 30 min. After washing, splenocytes were resuspended in cold 3% formaldehyde in isotonic solution and analyzed by FACScan (Becton Dickinson, Palo Alto, Calif.). Results were expressed as percentage of positive cells.
Cytokine measurements.
Supernatant from spleen cells described above were harvested after 72 h. The solid-phase ELISAs for measurement of interleukin-4 (IL-4) and gamma interferon (IFN-γ) were used as previously described (27) and as instructed by the manufacturer (Pharmingen). The pairs of cytokine-specific monoclonal antibodies and recombinant cytokines were all obtained from Pharmingen.
For the detection of intracellular cytokines in spleen cells treated with medium, GK-1 or T. crassiceps antigens were cultured for 60 h. To inhibit cytokine secretion, brefeldin A (2 μM) was added to cell cultures 10 h before the assay. At harvest, the cells were centrifuged at 500 × g for 10 min and washed twice in ice-cold PBS containing 10% globulin-depleted FBS plus 0.02% NaN3. CD3 and interleukin expression was determined by two-color fluorescence-activated cell sorting (FACS) as previously described (4). Briefly, cells were stained with the FITC-conjugated anti-CD3 monoclonal antibody (Pharmingen). Intracellular cytokines were assayed by using a cytoStain TM kit (Pharmingen) to fix and permeabilize the cells. To stain intracellular cytokines, fixed and permeabilized cells were incubated with monoclonal rat anti-IL-2–PE, anti-IL-4–PE, anti-IL-10–PE, or anti-IFN-γ–PE (all from Pharmingen). Parallel samples of the cells were stained with an isotype control to account for nonspecific staining of the cells. Ten thousand cells were analyzed with a CD3+ lymphocyte gate as defined by light scatter in a FACScan (Becton Dickinson). The percentage of cells in each quadrant is indicated in the dot plot. Quadrant statistics were set on the basis of the corresponding isotype controls.
Experimental challenge.
Metacestodes used in challenge infections were harvested from BALB/cAnN female mice carrying the ORF strain of T. crassiceps cysticerci. Ten small (diameter of ca. 2 mm), nonbudding larvae were suspended in 0.5 M NaCl–0.01 M sodium phosphate buffer (pH 7.2) and injected intraperitoneally into each mouse, using a 27-gauge needle. Mice were killed 30 days after infection, and the cysts found inside the peritoneal cavity were counted. In this form of infection, the parasites do not migrate to another location in the host. We attribute variation in individual parasite intensities within groups to differences in the infectivity of each parasite inoculum, which also varies between the different parasite harvests used in challenge infection. In consequence, experimental designs measuring levels of immunity by parasite intensity must include in each vaccination session a session-specific measurement of each inoculum infectivity in nonvaccinated control mice.
Immunolocalization of GK-1 protein.
T. crassiceps cysticerci and T. solium specimens (cysticerci, eggs, and tapeworm segments) were placed on ice into a 50-ml conical plastic-bottom centrifuge tube with ice-cold PBS. The vesicular fluid was removed from cysticerci by slicing the cyst walls and letting the fluid drain into a sterile centrifuge tube and stored in the cold (4°C) until use. The tissues were then incubated for 30 s with glycine-chloride buffer (50 mM glycine-HCl [pH 2.5], 0.1% Triton X-100, 0.15 mM NaCl) to reduce contamination of host protein, and the pH was restored by adding Tris-HCl (pH 9). After further washing, tissues were included in Tissue-Tek O.C.T. compound (Miles, Inc.), frozen at −70°C, and sectioned 6 μm thick. Sections were placed on poly-l-lysine-treated microslides, air dried for 30 min, fixed in acetone for 10 min, and dried for 15 min at room temperature. The slides were rehydrated and blocked with 1% BSA in PBS plus 0.1% Triton X-100 (pH 7.2) for 1 h. In cysticercal tissue sections, a second blocking was performed with sheep anti-mouse IgG (whole antibody; Amersham) diluted 1:100 in PBS plus 0.1% BSA and incubated for 1 h at 4°C. Slides of T. solium tapeworms and eggs were incubated for 1 h at 4°C with horse serum diluted 1:100 in PBS plus 0.1% BSA as a second blocking agent. Solutions were removed, and the slides were overlaid with the appropriate sera (diluted 1:10,000 in PBS–0.1% BSA) from noninfected (negative control), T. crassiceps-infected (positive control), or anti-GK1-immunized mice, incubated overnight at 4°C, and then washed twice in PBS (pH 7.2). Finally, sections were incubated with FITC-labeled goat anti-mouse IgG (Zymed, San Francisco, Calif.) diluted 1:50 for 1 h at room temperature. Slides were washed twice and mounted with Aquatek polyvinyl alcohol (Merck, Darmstadt, Germany). Preparations were observed with an Olympus BH2-RFCA epifluorescence microscope.
Statistical analysis.
Statistical comparison of individual parasite intensities between groups was performed by the Wilcoxon ranked sum test, because many mice in the immunized groups bore no parasites and because parasite intensity is a discontinuous variable (i.e., 0, 1, 2, … n parasites). Data were considered statistically significant at P < 0.05. Statistical analysis of the difference between mean values of binding activity in ELISA, flow cytometry, and proliferation assays was carried out by Welch’s unpaired t test (alternative t test). All statistical analyses were performed by the Instat software program (GraphPad, San Diego, Calif.).
Computational methods in peptide structural analysis.
Theoretical chemistry calculations of GK-1 started with optimization of its geometry by methods based on molecular mechanics (1). Subsequently, the peptide was submitted to a single-point calculation with the Austin model 1 semiempirical quantum chemistry method (2). In this way, the electrostatic charges, electron density, electrostatic potentials, and dipole moment of the molecule were obtained. Additionally, the log octanol/water partition coefficient and distributed hydrophobicity of GK-1 were calculated. The software used consisted of SPARTAN 4.0 (Wave Function Inc., Irvine, Calif.), Insight II (Biosym/MSI, San Diego, Calif.), and Chem Plus (Hypercube, Inc., Ontario, Canada).
RESULTS
Protective effect of peptide immunization against T. crassiceps cysticercosis.
The effects of peptide immunization on the number of cysticerci recovered from mice immunized with GK-1, GK-2, and GK-3 at different doses (0.5, 10, and 50 μg per mice) in FCA are shown in Table 1. Immunization with the GK-2 and GK-3 peptides did not confer protection, whereas three of five mice immunized with GK-1 were completely protected at a dose of 50 μg per mice. To further evaluate this protective capacity, free GK-1 as well as BSA–GK-1 and MAP–GK-1 emulsified in saponin were used for immunization in several repeated experiments. Table 2 confirms, in several instances, the high level of protection induced by GK-1 when used as an immunogen either free of carrier or conjugated to BSA. Mice immunized with MAP–GK-1 did not show reduced mean parasite intensity, although some mice were totally protected.
TABLE 1.
Effects of immunization with three immunogenic peptides from T. crassiceps recombinant protective antigen KETc7 on individual parasite intensities
| Group | Mean intensity ± SD (no. of mice with no cysts)a
|
|||
|---|---|---|---|---|
| No peptide | Peptide (μg/mouse)
|
|||
| 0.5 | 10 | 50 | ||
| Control | 14 ± 8.19 | |||
| Immunized with: | ||||
| GK-1 | 9.4 ± 10.9 (0) | 12.4 ± 14.3 (1) | 3.8 ± 5.8b (3) | |
| GK-2 | 13.0 ± 7.9 (0) | 9..2 ± 4.9 (0) | 7.4 ± 2.7 (0) | |
| GK-3 | 14.4 ± 5.81 (0) | 6.7 ± 4.14 (0) | 7.4 ± 2.0 (0) | |
Individual parasite intensity (i.e., number of cysticerci in each mouse) in groups of five mice. Groups of five male mice each were immunized with FCA (controls) or the indicated peptides in FCA and challenged 15 days after the second immunization. Thirty days after challenge, mice were sacrificed and the parasite intensity was determined.
Statistically significant difference between control and immunized mice at 95% confidence interval (P < 0.05).
TABLE 2.
Protective immunity against murine T. crassiceps cysticercosis by immunization with cysticercal antigens and different forms of GK-1
| Antigen(s) | Group | No. of cysticerci in each mouse in group
|
|
|---|---|---|---|
| 1st trial | 2nd trial | ||
| Linear GK-1 | Control | 4, 5, 5, 6, 7, 7, 9, 12 (0/8b) | 27, 24, 20, 21, 29, 20, 24, 27, 34 (0/9) |
| Immunized | 0, 0, 0, 0, 1, 2, 2c (4/7) | 0, 0, 0, 0, 0, 0, 0, 2, 5, 6c (7/10) | |
| MAP–GK-1 | Control | 15, 19, 24, 28, 51 (0/5) | 10, 12, 21, 26, 64 (0/5) |
| Immunized | 0, 0, 25, 40, 60, 62, 65, 75, 75, 79 (2/10) | 0, 0, 0, 0, 2, 17, 24, 27, 62, 81 (4/10) | |
| BSA–GK-1 | Control | 42, 50, 55, 57, 62 (0/5) | 7, 13, 17, 48, 53 (0/5) |
| Immunized | 9, 12, 14, 15, 36c (0/5) | 0, 0, 0, 0, 0, 0, 0, 0, 4, 23c (8/10) | |
| All T. crassiceps | Control | 13, 15, 16, 21, 23 (0/5) | 9, 14, 17, 19, 25 (0/5) |
| Immunized | 0, 0, 0, 0, 3, 5c (4/6) | 0, 0, 0, 0, 0, 2, 9c (5/7) | |
Mice were immunized twice with soluble T. crassiceps cysticercal antigens (100 μg per mouce), GK-1 free of carrier (10 μg per mouce), or BSA–GK-1 orMAP–GK-1 (each at 50 μg per mouce) in saponin. Control mice were injected twice with saponin in saline. Mice were challenged 15 days after the booster and sacrificed 30 days later. The parasite intensity in each mouse was determined. Data shown are from two independent experiments.
Number of mice without a single parasite of the total tested in each group.
Statistically significant difference between control and immunized mice at the 95% confidence interval.
Determination of B-cell epitope(s) on GK-1.
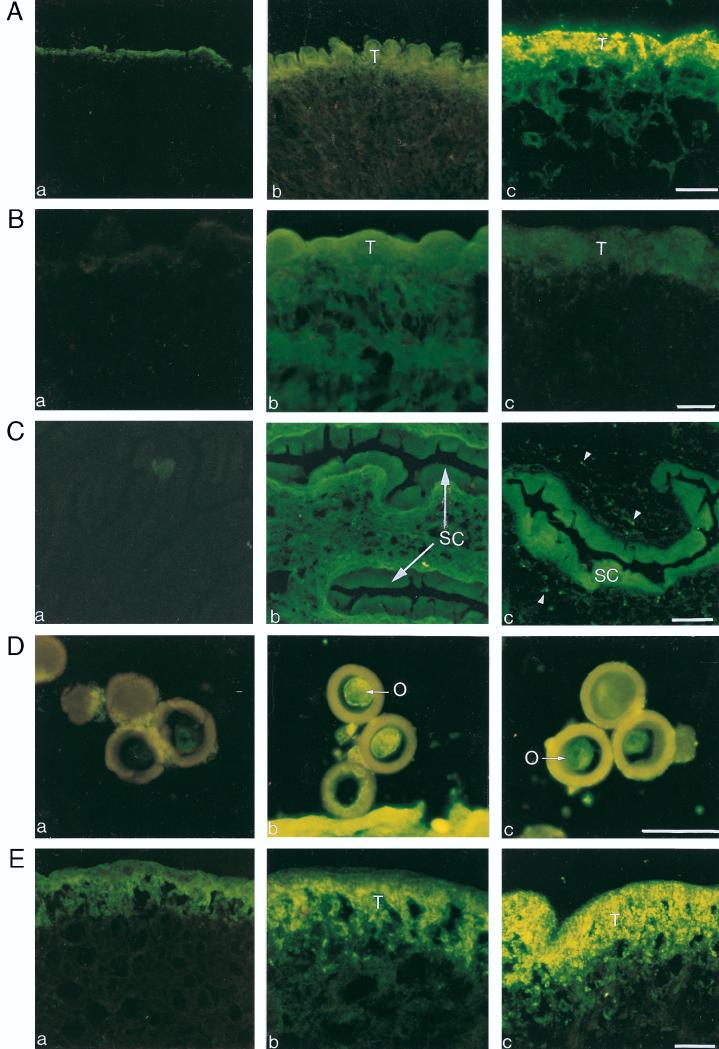
To test for the presence of a B-cell epitope(s) on the GK-1 peptide, we studied whether GK-1 immunization induced antibodies against the peptide and against whole T. crassiceps antigen by ELISA. Mice immunized with the monomeric nonconjugated form of GK-1 produced low but detectable levels of serum antibodies that reacted with GK-1 as well as with T. crassiceps in ELISA (Table 3). The examination of anti-GK-1 antiserum reactivity against histological sections of T. crassiceps revealed that these antibodies specifically react with T. crassiceps cysticerci at the tegument of the parasite. Furthermore, the anti-GK-1 antisera also reacted with all developmental stages of T. solium (Fig. 1). A clear reaction was detected in the oncosphere contained inside the eggs and also in the egg wall. In T. solium cysticerci the reacting protein is concentrated in the spiral canal, while in the tapeworm it is located throughout the distal tegument. The specificity of all of these antibody reactions in ELISA and immunofluorescence was demonstrated by specific preabsorption of antisera with free GK-1 and lack of reactivity of normal mouse serum.
TABLE 3.
Level of antibodies measured by ELISA during the immunization protocola
| Antibody against: | Mean antibody level (OD405) ± SD
|
||||
|---|---|---|---|---|---|
| Nonimmunized | Injected with:
|
||||
| Saponin (control)
|
Saponin + GK-1
|
||||
| 1st dose | 2nd dose | 1st dose | 2nd dose | ||
| GK-1 | 0.129 ± 0.007 | 0.146 ± 0.005 | 0.164 ± 0.010 | 0.164 ± 0.020b | 0.190 ± 0.020b |
| T. crassiceps antigens | 0.150 ± 0.01 | 0.189 ± 0.006 | 0.230 ± 0.027 | 0.247 ± 0.105b | 0.431 ± 0.131b |
Groups of 10 mice each immunized and tested 2 weeks after each immunization The serum antibody levels against GK-1 and T. crassiceps cysticercal antigens were determined by ELISA in GK-1-immunized, nonimmunized, and saponin-injected mice. Levels of murine antibodies raised during the course of immunization with GK-1 peptide are shown.
Considered statistically significant at P < 0.05.
FIG. 1.
Immunofluorescence staining of T. crassiceps (A) and T. solium (B and C) cysticerci and of eggs (D) and adult tegument (E) of T. solium, incubated with sera from normal mice (a), 30-day T. crassiceps-infected mice (b), and pooled sera obtained 15 days after the booster of GK-1 (c). The labeled epitope is clearly evident in structures accessible to the immune system. It is intensively expressed in the tegument (T) of T. crassiceps cysticerci (A, panel c) and weakly in the T. solium cysticerci (B, panel c). It is strongly expressed in the cuticular folds of the spiral canal (SC) (C, panel c), in the oncosphere (O) (D, panel c), and in the distal tegument (T) of the tapeworm (E, panel c). The arrowheads (C, panel c) indicate the protonehridia. Bars = 40 μm.
Assessment of T-cell epitopes on the GK-1 peptide.
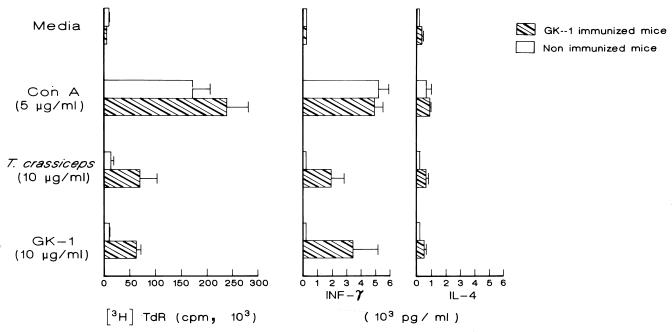
To identify the presence of a T-cell epitope(s) on the GK-1 peptide, we studied the proliferative response of spleen cells from mice treated with GK-1 or saponin alone. Spleen cells from mice injected in vivo with free peptide or saponin were stimulated in vitro with the same peptide (10 μg/ml), with T. crassiceps whole antigen (10 μg/ml), or with ConA (5 μg/ml) as a positive control. Results show that in vitro stimulation with GK-1 as well as with cysticercal antigens induced a strong proliferative response in cells from GK-1 immunized mice (Fig. 2). Cells from mice injected with saponin (nonimmunized mice) showed no proliferative response above background levels. These results confirm the presence in GK-1 of T-cell epitope(s).
FIG. 2.
T-cell proliferative response of spleen cells from nonimmunized (injected with saponin alone) and immunized mice determined by [3H]thymidine incorporation on day 3 of culture. Data presented are means ± standard deviations for four individual mice separately assayed. Cytokine (IFN-γ and IL-4) production was determined in collected cultured supernatant obtained 72 h poststimulation. Data are the means for four mice and are representative of two experiments performed in duplicate. Significantly increased proliferative response and IFN-γ levels were achieved when cells from immunized mice were stimulated both with T. crassiceps antigens and GK-1 peptide.
As Table 4 shows, the proportion of CD3+ T cells from GK-1-immunized mice increased from 39.9 to 58.7 or 54.7% and the proportion of CD8+ cells increased from 12.2 to 19.3 or 20.4% when cells were activated with GK-1 or antigen, respectively. The proportion of CD4+ cells increased only from 28.3 to 32.6 or 32.7%. Interestingly, most of the T-stimulated cells (CD8+ and CD4+) from immunized mice were also CD25+.
TABLE 4.
Flow cytometer analysis of spleen cells from nonimmunized and GK-1-immunized micea
| Incubation with: | Mean % positive cellsb
|
|||||
|---|---|---|---|---|---|---|
| Controls
|
Immunized mice
|
|||||
| CD3+ | CD4+ | CD8+ | CD3+ | CD4+ | CD8+ | |
| Medium | 38.8 ± 2.7 | 26.4 ± 0.8 (5.7) | 10.9 ± 0.8 (5.1) | 399 ± 4.0 | 28.3 ± 1.2 (5.8) | 12.2 ± 1.2 (4.5) |
| GK-1 | 38.5 ± 2.2 | 26.6 ± 2.08 (4.9) | 11.7 ± 1.0 (4.3) | 58.7 ± 4.9b | 32.6 ± 2.6b (91.1) | 19.3 ± 2.3b (93.9) |
| Cysticercal antigens | 35.2 ± 2.1 | 24.3 ± 0.9 (4.7) | 10.5 ± 0.6 (3.9) | 54.7 ± 6.5b | 32.7 ± 2.9b (86.6) | 20.4 ± 3.7b (89.5) |
Flow cytometer analysis was performed on spleen cells of nonimmunized and GK-1-immunized mice after 3 days of culture without or with GK-1 or T. crassiceps cysticercal antigens. Each value represents the percentage of positive cells from four individual mice and is representative of two experiments performed in duplicate. The percentage of CD4+ or CD8+ cells that also expressed CD25+ is given in parentheses.
Significant increase in the percentage of CD8+, CD4+, and CD3+ cells in specifically stimulated immune mice compared with nonimmunized mice (P < 0.05).
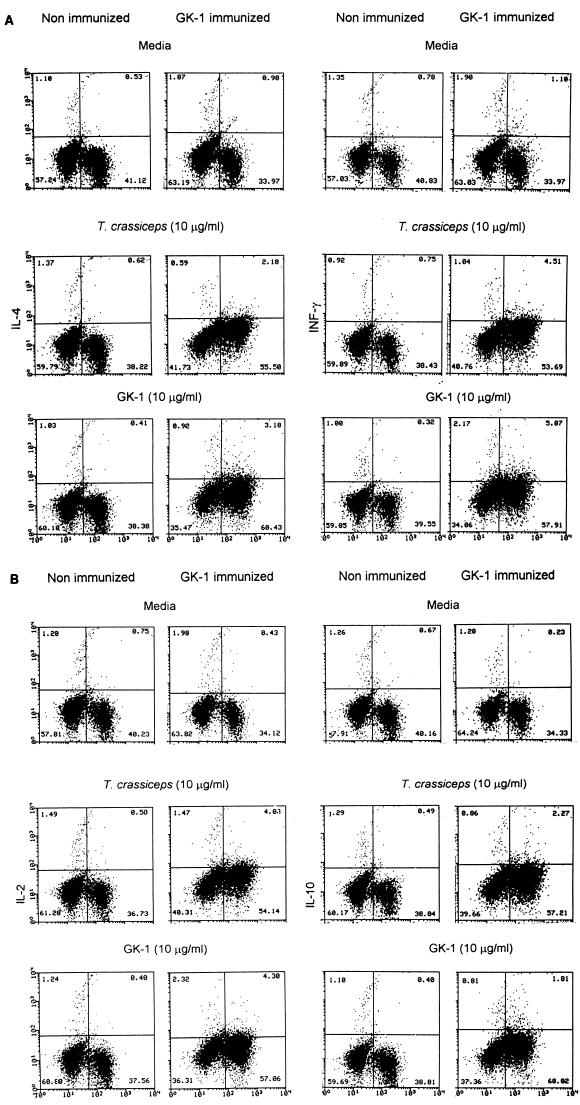
Next, we determined the level of secreted cytokines in the supernatant of in vitro-stimulated spleen cells. Splenocytes from nonimmunized control mice produced a small amount of IL-4 and IFN-γ that increased only after stimulation with ConA. In contrast, a clearly increased amount of IFN-γ and a low amount of IL-4 were found after stimulation of the splenocytes of GK-1-immunized mice both with GK-1 and with whole cysticercal antigens (Fig. 2). The frequency of cells capable of producing IL-4, IFN-γ, IL-2, and IL-10 was also determined by FACS after intracellular staining for cytokines. The increased percentage of cells producing IFN-γ and IL-4 determined by FACS was found to be consistent with ELISA analysis of the supernatants. Figure 3 shows that frequencies of cells producing IFN-γ and IL-2 were significantly higher among T. crassiceps antigen- or GK-1-stimulated cells from immunized mice than among cells from nonimmunized mice; levels of IL-4 and IL-10 were also increased, albeit to lesser extents.
FIG. 3.
Spleen lymphocytes from nonimmunized (injected with saponin alone) and GK-1-immunized mice 60 h poststimulation were analyzed for intracellular cytokines (IFN-γ and IL-4 [A] and IL-2 and IL-10 [B]) and surface CD3+ staining by FACS. Cells had been dually stained with FITC (abscissa) and PE (ordinate). The percentage of cells in each quadrant of the dot plot is indicated. The data are representative of three experiments using different mice.
GK-1 physicochemical properties.
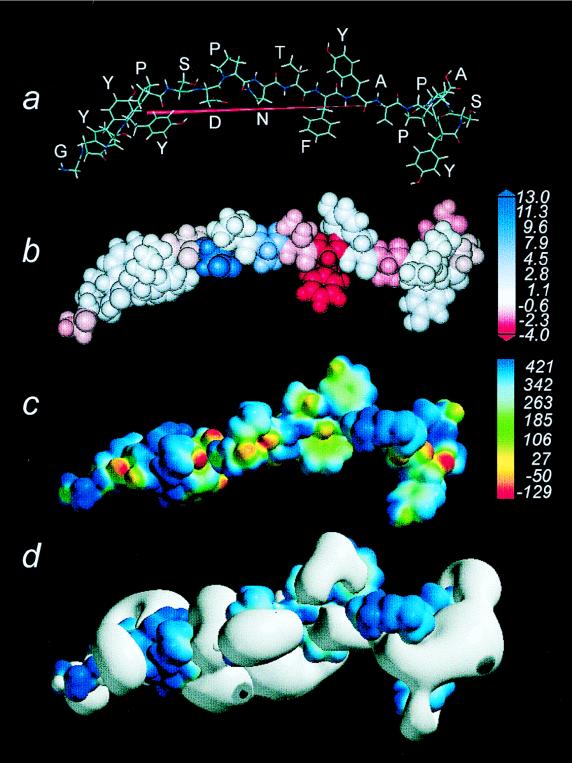
The dipole moment was directed toward the tyrosine end of GK-1. The octanol/water partition coefficient of the peptide was 7.92. However, its hydrophobicity was distributed in zones in accordance with the amino acid composition. Thus, the phenylalanine region was the most hydrophobic, while alanine was the most hydrophilic and the glycine end was more hydrophobic than the tyrosine end. Planar rings from the tyrosine and phenylalanine showed high electron density. However, the higher electron density was observed for the oxygen atoms belonging to the carbonyl and hydroxy functional groups. Interestingly, the electrostatic potentials were displayed emerging mostly from these functional groups. Figure 4 shows the physicochemical properties of GK-1.
FIG. 4.
(a) Amino acid arrangement of GK-1 and its optimized geometry. The red arrow shows the dipole moment pointing the vector’s negative end toward tyrosine. (b) Space-filling model of GK-1, showing different degrees of hydrophobicity (red) or hydrophilicity (blue). (c) The encoded electronic density elicited by the molecule and its calibration bar at the right, where oxygen atoms display red while negative zones corresponding to lone pairs of electrons in atoms show as yellow. (d) The bulk of electrostatic potential emerging mostly from the negative zones of the electron density surface.
DISCUSSION
High levels of sterile immunity to experimental T. crassiceps cysticercosis were conferred to male mice immunized with a synthetic 18-amino-acid peptide (GK-1) from the recombinant protein KETc7 of the parasite (12). The proportion of totally protected mice varied in experiments performed on different occasions from 40 to 70%, while the average decrement in the immunized group’s parasite intensity was 85 to 95% of that expected from challenged control male mice. Variation in parasite intensity within experimental groups and between experimental sessions is a common finding in this form of cysticercosis due to factors not fully identified but that we attribute to variation in infectivity of each parasite harvest and inoculum. The statistical validity of the inferences drawn from these experiments is, however, not weakened if each experimental session includes its own internal control. Coupling of GK-1 to BSA or rearranging the peptide in an eight-pointed MAP construct did not result in an increased immunogenicity of the peptide and may in fact have reduced it somewhat.
Sterile immunity is seldom induced in this form of cysticercosis by purified, natural, or recombinant antigens (10, 22, 28); however, GK-1 induced levels of protection higher than those observed with the whole KETc7 recombinant protein published elsewhere (12). Research into the reasons why this peptide is so effective relative to other forms of antigen preparation, including the complete recombinant antigen KETc7 from which the peptide is derived (12), could perhaps reveal general principles of immunogenicity applicable to this and other vaccine preparations. Assuming that the binding properties of GK-1 to antibodies and cellular receptors relate to its immunogenicity, and since these depend on its stereoelectronic properties, the high dipole moment and the asymmetry in the electronic distribution of GK-1 are noteworthy. Moreover, GK-1 showed high hydrophobic areas alternating with hydrophilic ones (Fig. 4b); this dual hydrophilic-hydrophobic property offers interesting possibilities of water and lipid interaction that may facilitate the peptide’s reaction with B and T membrane-bound receptors. The external distribution of the hydroxyl groups favors water or hydrogen bonding, judging from the rich and complex electrostatic potential of these hydrophilic groups, while the abundance of aromatic amino acids in GK-1 defines steric regions with high noncovalent electrostatic interactions capable of enhancing binding affinity that could also favor the peptide presentation by antigen-presenting cells (18). The alteration of peptide immunogenicity by the structural changes imposed by chemical coupling to BSA and the MAP construction also points to a strong structure dependence of its biological functions. GK-1’s electronic polarity, adequately positioned anchor motifs, and similarities to motifs reactive with class I major histocompatibility complex molecules may explain the peptide’s ability to induce a CD8+ proliferative response (18). The involvement of a B-cell response after immunization is documented by the presence of serum-specific anti-GK-1 antibodies in immunized mice. Immune reactivity against the whole-parasite antigens was greater than that against the GK-1 peptide itself, probably because of loss of reactivity of GK-1 once bound to the plate. T-cell involvement is shown by the in vitro proliferative assays with spleen cells from GK-1-immunized mice, which strongly responded to both GK-1 and cysticercal antigens probably favored by the increased percentage of T cells producing IL-2. The composition of the resultant lymphocyte population was most significantly enriched in CD8+ cells. Although the direct participation of a cytotoxic response in the control of the parasite’s reproduction remains to be thoroughly elucidated, the immune response elicited by this peptide features a prominent CD8+ T-cell response. Other factors contributing to parasite damage may be related to IFN-γ, a cytokine that plays a central role in cell-mediated effector mechanisms in the protection observed in mice vaccinated against other parasites (5). The large amount of IFN-γ detected by ELISA, as well as the increased percentage of CD3+ cells that produced this cytokine, could induced the inflammatory response and the activation of macrophages at the parasite’s vicinity (15). All of these data are also consistent with the low levels of antibodies induced by GK-1 immunization, which can be the consequence of low levels of IL-4 and IL-10. As Table 4 shows, the existence of a specific T-cell response in GK-1-immunized mice and not in those immunized with saponin alone was also demonstrated by the increased expression of a cell surface activation marker (CD25+) following antigen- or peptide-specific reactivation in vitro. The immune protection induced by GK-1 immunization and the polarized cytokine phenotype induced are in keeping with recent trends in opinion about immune resistance to metacestode diseases, which place Th1 cells in the forefront of protection (27), and add to well-established views that stress the role of antibody only in the destruction of early larvae developing from egg infection (21). These two mechanisms are, of course, not incompatible, and GK-1’s high protective efficiency may well result from the synergic action of its capacity to trigger both B- and T-cell immune responses.
Another feature of GK-1 that deserve mention is that it is represented in an antigen fraction of 56 kDa in T. crassiceps cysticerci which induces high levels of protection against T. solium pig cysticercosis (28). This GK-1 peptide is also recognized by sera from T. solium-infected humans (8). Furthermore, the identification of GK-1 by immunofluorescence at all stages of T. solium—infecting egg, hexacanth embryo, metacestode, and tapeworm—make GK-1 a likely effective target for immune attack and an interesting candidate for a vaccine against T. solium cysticercosis.
ACKNOWLEDGMENTS
This investigation was supported by Dirección General de Asuntos de Personal Académico IN208395 and IN212798, Universidad Nacional Autónoma de México, and CONACYT G25955m, México, Fundación Miguel Alemán.
We thank Felipe Massó for performing the peptide sequence analysis, Nelly Villalobos for obtaining the T. solium tapeworm, and Carlos Castellanos and Mercedes Baca for technical support. Isabel Pérez Montfort aided in translation of the manuscript.
REFERENCES
- 1.Burkert U, Allinger N L. Molecular mechanics. ACS monograph. Washington, D.C: American Chemical Society; 1982. [Google Scholar]
- 1a.Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Washington, D.C: Institute of Laboratory Animal Resources, National Research Council; 1996. [Google Scholar]
- 2.Dewar M J S, Zoebisch E G, Healy E F, Stewart J P. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc. 1985;107:3902–3909. [Google Scholar]
- 3.Dorais F J, Esch G W. Growth rates of two Taenia crassiceps strains. Exp Parasitol. 1969;25:395–398. doi: 10.1016/0014-4894(69)90086-1. [DOI] [PubMed] [Google Scholar]
- 4.Elson L H, Nutman T B, Metcalfe D D, Prussin C. Flow cytometric analysis for cytokine production identifies Th1, Th2 and Th0 cells within the human CD4+ CD27− lymphocyte subpopulation. J Immunol. 1995;154:4294–4301. [PubMed] [Google Scholar]
- 5.Ferru Y, Georges B, Estaquier J, Delacre M, Harn D A, Tartar A, Capron A, Grassmasse H, Auriault C. Analysis of the immune response elicited by a multiple antigen peptide (MAP) composed of two distinct protective antigens derived from the parasite Schistosoma mansoni. Parasite Immunol. 1997;19:1–11. doi: 10.1046/j.1365-3024.1997.d01-138.x. [DOI] [PubMed] [Google Scholar]
- 6.Fragoso G, Lamoyi E, Mellor A, Lomeli C, Hernández M, Sciutto E. Increased resistance to Taenia crassiceps murine cysticercosis in Qa-2 transgenic mice. Infect Immun. 1998;66:760–764. doi: 10.1128/iai.66.2.760-764.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freeman R S. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolphi, 1810 (Cestoda) Can J Zool. 1962;40:969–990. [Google Scholar]
- 8.Gevorkian G, Manoutcharian K, Larralde C, Hernández M, Almagro J C, Viveros M, Sotelo J, Garcia E, Sciutto E. Immunodominant synthetic peptides of Taenia crassiceps in murine and human cysticercosis. Immunol Lett. 1996;49:185–189. doi: 10.1016/0165-2478(96)02503-5. [DOI] [PubMed] [Google Scholar]
- 9.Larralde C, Montoya R M, Sciutto E, Díaz M L, Govezensky T, Coltorti E. Deciphering Western blots of tapeworm antigens (T. solium, E. granulosus and T. crassiceps) reacting with sera from neurocysticercosis and hydatidic disease patients. Am J Trop Med Hyg. 1989;40:282–290. doi: 10.4269/ajtmh.1989.40.282. [DOI] [PubMed] [Google Scholar]
- 10.Larralde C, Padilla A, Hernández M, Govezensky T, Sciutto E, Gutiérrez G, Tapia-Conyer R, Salvatierra B, Sepúlveda J. Seroepidemiología de la cisticercosis en México. Salud Pública Méx. 1992;34:197–210. [PubMed] [Google Scholar]
- 11.Manoutcharian K, Larralde C, Aluja A, Fragoso G, Rosas G, Hernández M, Villalobos N, Rodarte L F, Govezensky T, Baca M, Sciutto E. Advances in the development of a recombinant vaccine against Taenia solium pig cysticercosis. In: Chanock R M, Brown F, Ginsberg H S, Norrby E, editors. Vaccines 95. Cold Spring Harbor, N.Y: Cold Spring Harbor, Laboratory; 1995. pp. 63–68. [Google Scholar]
- 12.Manoutcharian K, Rosas G, Hernández M, Fragoso G, Aluja A, Villalobos N, Rodarte L F, Sciutto E. Cysticercosis: identification and cloning of protective recombinant antigens. J Parasitol. 1996;82:250–254. [PubMed] [Google Scholar]
- 13.McColm A A, Bomford R, Dalton L. A comparison of saponin with other adjuvants for the potentiation of protective immunity by a killed Plasmodium yoelii vaccine in the mouse. Parasite Immunol. 1982;4:337–347. doi: 10.1111/j.1365-3024.1982.tb00445.x. [DOI] [PubMed] [Google Scholar]
- 14.Molinari J L, Rodríguez D, Tato P, Soto R, Arechavaleta F, Solano S. Field trial for reducing porcine Taenia solium cysticercosis in Mexico by systematic vaccination of pigs. Vet Parasitol. 1997;69:55–63. doi: 10.1016/s0304-4017(96)01102-8. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T R, Li L, Subash S. Functions of CD8 T cells subset secreting different cytokine patterns. Semin Immunol. 1997;9:87–92. doi: 10.1006/smim.1997.0065. [DOI] [PubMed] [Google Scholar]
- 16.Nascimento E, Costa J O, Guimaraes M P, Tavares C A. Effective immune protection of pigs against cysticercosis. Vet Immunol Immunopathol. 1995;45:127–137. doi: 10.1016/0165-2427(94)05327-o. [DOI] [PubMed] [Google Scholar]
- 17.Parkhouse R M, Harrison L J. Antigens of parasitic helminths in diagnosis, protection and pathology. Parasitology. 1989;99:S5–S19. doi: 10.1017/s0031182000083384. [DOI] [PubMed] [Google Scholar]
- 18.Rammensee H G, Friede T, Stevanovic S. MHC ligands and peptide motifs: first listing. Immunogenetics. 1995;41:178–228. doi: 10.1007/BF00172063. [DOI] [PubMed] [Google Scholar]
- 19.Ramos-Kuri M, Montoya M R, Padilla A, Govezensky T, Diaz M, Sciutto E, Sotelo J, Larralde C. Immunodiagnosis of neurocysticercosis. Arch Neurol. 1992;49:633–636. doi: 10.1001/archneur.1992.00530300069012. [DOI] [PubMed] [Google Scholar]
- 20.Richards F, Jr, Schantz P. Laboratory diagnosis of cysticercosis. Clin Lab Med. 1991;11:1011–1028. [PubMed] [Google Scholar]
- 21.Rickard M E, Williams J F. Hydatidosis/cysticercosis: immune mechanisms and immunization against infection. Adv Parasitol. 1982;21:229–296. doi: 10.1016/s0065-308x(08)60277-8. [DOI] [PubMed] [Google Scholar]
- 22.Sciutto E, Fragoso G, Trueba L, Lémus D, Montoya R M, Díaz M L, Govezensky T, Lomeli C, Tapia G, Larralde C. Cysticercosis vaccine: cross-protecting immunity with T. solium antigens against experimental murine T. crassiceps cysticercosis. Parasite Immunol. 1990;12:687–696. doi: 10.1111/j.1365-3024.1990.tb00997.x. [DOI] [PubMed] [Google Scholar]
- 23.Sciutto E, Aluja A, Fragoso G, Rodarte L F, Hernández M, Villalobos M N, Padilla A, Keilbach N, Baca M, Govezensky T, Díaz S, Larralde C. Immunization of pigs against Taenia solium cysticercosis: factors related to effective protection. Vet Parasitol. 1995;60:53–67. doi: 10.1016/0304-4017(94)00781-7. [DOI] [PubMed] [Google Scholar]
- 24.Sotelo J, del Bruto O, Román G. Cysticercosis. Curr Clin Trop Infect Dis. 1996;16:240–259. [PubMed] [Google Scholar]
- 25.Tam J P. Immunization with peptide carrier complexes: traditional and multiple antigen peptide system. In: Wisdom G B, editor. Peptide antigens. New York, N.Y: Oxford University Press; 1994. pp. 83–115. [Google Scholar]
- 26.Tamburrini A, Gomez Morales M A, Pozio E. Development of an immunoenzyme test for the diagnosis of human cysticercosis using a heterologous antigen. Parassitologia. 1995;37:195–198. [PubMed] [Google Scholar]
- 27.Terrazas L I, Bojalil R, Govezensky T, Larralde C. Shift from an early protective TH1-type immune response to a late permissive TH2-type response in murine cysticercosis (Taenia crassiceps) J Parasitol. 1998;84:74–81. [PubMed] [Google Scholar]
- 28.Valdez F, Hernández M, Govezensky T, Fragoso G, Sciutto E. Immunization against Taenia crassiceps cysticercosis. Identification of the most promising antigens in the induction of protective immunity. J Parasitol. 1994;80:931–936. [PubMed] [Google Scholar]