Abstract
Myeloid sarcoma (MS) is a rare extramedullary tumor originating from immature bone marrow cells. MS of the breast is an extremely uncommon disease with non-specific clinical and radiological features. The present case report describes a distinctive case of MS of the breast, which posed diagnostic challenges due to the absence of typical imaging characteristics at the time of presentation. The patient was a 58-year-old woman who presented with a breast mass. Further examination and testing confirmed the diagnosis of MS in the right breast, with metastases to the right iliac, pubic and ischial regions. Immunohistochemical analysis identified metastatic tumors distinguished by the expression of a number of markers, including Ki-67, myeloperoxidase and cluster of differentiation 43. The patient underwent six cycles of chemotherapy with a regimen comprising etoposide, methylprednisolone, cytarabine and cisplatin, and 28 cycles (56 cGy each) of consolidation radiotherapy. Extensive examination and long-term follow-up revealed no further tumor recurrence or metastasis. Myeloid sarcomas of the breast typically manifest as palpable masses requiring diagnostic imaging. However, due to the rarity of MS of the breast without any signs of leukemia, its diagnosis and treatment are challenging. The present case report highlights the importance of maintaining high clinical, radiological and pathological standards when diagnosing this disease. Additionally, a comprehensive review of the literature on breast MS is provided. This highlights the necessity for clinicians to consider this rare diagnosis in patients presenting with a breast mass, to facilitate the appropriate treatment and prevent unnecessary procedures such as mastectomies.
Keywords: breast neoplasms, acute myeloid leukemia, sarcoma
Introduction
Myeloid sarcoma (MS) of the breast is an extremely rare disease, occurring as a manifestation in only 0.12% of patients with acute myeloid leukemia (AML) (1). It is an extramedullary tumor comprising immature cells derived from the myeloid lineage. While MSs can affect various organs, including the breast, skin, lymph nodes, intestines, bones and central nervous system, the isolated involvement of the breast is extremely rare, with only a few reported cases in the literature (2,3). The clinical manifestations of breast MS are often nonspecific, which makes the initial clinical assessment challenging, particularly in patients who have exhibited no prior indication of myeloid lesions or involvement of breast tissue. Although breast imaging plays a crucial role in the initial evaluation of this disease, the imaging features of MS may be similar to those of breast cancer or lymphoma, rendering it challenging to distinguish between these conditions (4). Currently, the diagnosis of breast MS can only be confirmed using immunohistochemical examinations and other histological tests. Therefore, it is necessary for suspected malignant breast masses to be confirmed through needle puncture pathology. In the context of MS, a variety of chemotherapeutic regimens, including idarubicin, high-dose cytarabine, cyclophosphamide and cisplatin, have been used to induce remission (5). Idarubicin is a DNA topoisomerase inhibitor that disrupts protein synthesis and hampers DNA repair, thereby promoting cell death. Cytarabine acts by inhibiting DNA polymerase during the DNA synthesis phase, which interrupts DNA replication and reduces tumor cell growth. Cyclophosphamide is metabolized within tumor cells to form the potent phosphamide mustard inside, which alkylates DNA and creates cross-links between DNA strands that inhibit the growth and reproduction of tumor cells. Cisplatin is a cytotoxic agent that is effective throughout the cell cycle, and is capable of killing tumor cells at various stages of their growth (6). In addition, radiation therapy may be used to reduce the risk of local recurrence (7).
The present case report describes a case of MS originating from a single breast, its clinical and pathological characteristics and therapeutic management.
Case report
A 58-year-old woman was admitted to The Affiliated Hospital of Inner Mongolia Medical University (Hohhot, China) in April 2022 after the discovery of a lump in her right breast within the previous 2 months. The patient was generally healthy with no notable medical history. Physical examination revealed symmetrical breasts with no nipple retraction, discharge or ‘orange peel’ appearance. A hard lump measuring ~25×20×15 mm was detected at the 10 o'clock position in the right breast, ~2 cm away from the nipple. The lump had an irregular surface and unclear boundaries. However, it was movable and not adherent to the chest wall; in addition, no tenderness was observed. No masses were detected in the left breast, and no enlargement of the lymph nodes was observed in either armpit.
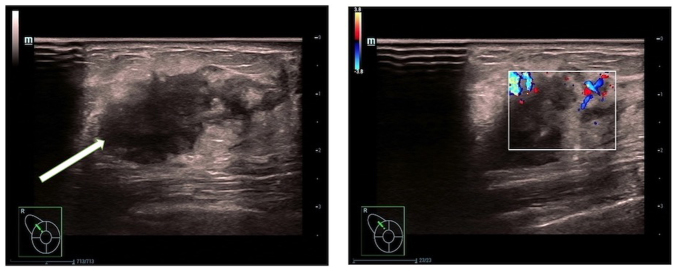
Routine blood examination was within normal limits, revealing a white blood cell count of 6.0×109 cells/l, with neutrophil and lymphocyte percentages of 65.1 and 28.2%, respectively, a hemoglobin level of 128 g/l and platelet count of 247×109 cells/l. Mammography revealed the presence of uneven dense glandular tissue in both breasts. A nodular high-density shadow with unclear boundaries, measuring ~21.6×14.7 mm, was evident in the upper outer quadrant of the right breast (Fig. 1). Breast ultrasonography (Fig. 2) showed a hypoechoic nodule measuring ~22.1×14.4 mm at the 11 o'clock position in the right breast, approximately one finger breadth away from the nipple. The nodule was irregularly shaped with unclear boundaries and surrounding spicules. Rich linear blood-flow signals were observed peripherally.
Figure 1.
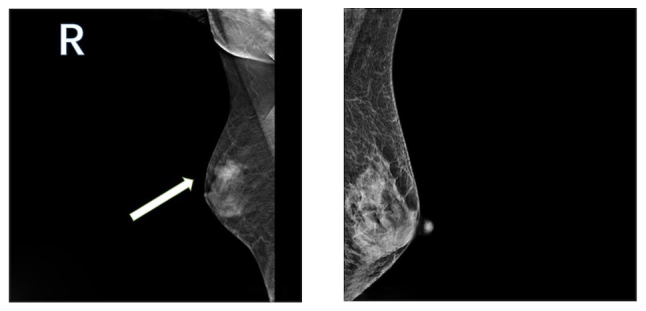
Mammography of bilateral breast glands showing uneven dense glandular tissue. A nodular high-density shadow, measuring ~21.6×14.7 mm and characterized by an unclear border, is visible in the upper outer quadrant of the right breast (left panel). The arrow indicates the mass detected in the breast. The skin and nipple shadows on both breasts appear normal. No lymph node shadows are visible in either armpit. The imaging diagnosis is nodules in the upper outer quadrant of the right breast, classified as BI-RADS 4a. The BI-RADS is a standardized system of reporting breast pathology encountered on mammography, ultrasound and magnetic resonance imaging (41). BI-RADS, Breast Imaging-Reporting and Data System; R, right.
Figure 2.
Ultrasound images of the breast lesion. A low-echo nodule measuring ~22.1×14.4 mm is observed in the right breast, at the 11 o'clock position, approximately one finger-width away from the nipple and 5 mm from the skin surface (left panel). The nodule has an irregular shape and unclear boundary. Color Doppler flow imaging shows linear blood-flow signals within the nodule (right panel). These ultrasound findings suggest a solid nodule in the right breast, falling under Breast Imaging-Reporting and Data System classification 4a. A biopsy was recommended.
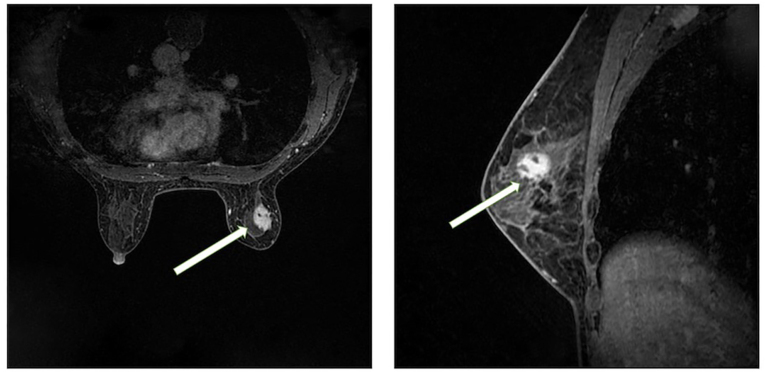
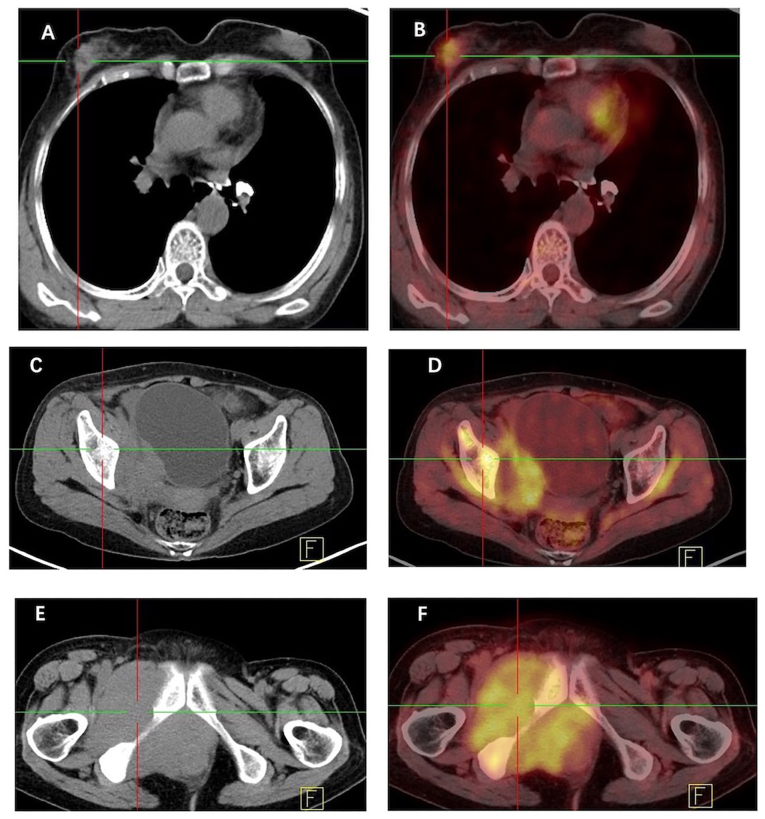
Breast magnetic resonance imaging (MRI; Fig. 3) showed a slightly elongated T2 signal in the upper outer quadrant of the right breast, which exhibited high signal intensity on diffusion-weighted imaging. The lesion measured ~18.5×14 mm and was observed to have irregular edges, lobulation and spicules. Doubly deprotonated-diethylenetriamine penta-acetic acid-enhanced MRI scanning revealed markedly uneven enhancement of the lesion accompanied by a dynamic curve with a plateau-shaped profile. Furthermore, [18F] fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT; Fig. 4) indicated heightened metabolism within a nodular soft tissue shadow in the upper outer quadrant of the right breast, suggesting the presence of a malignant lesion. Additionally, bone destruction was observed in the right ilium, pubic bone and ischium, accompanied by masses in the surrounding soft tissue, indicating the presence of metastatic tumors in these locations.
Figure 3.
Magnetic resonance imaging of the right breast. A circular shape with a slightly longer T2 signal is evident in the upper outer quadrant of the right breast, which. exhibits high signal intensity on diffusion-weighted imaging (left panel). The lesion is ~18.5×14 mm in size, and has irregular margins with visible lobulations and spicules. Contrast-enhanced doubly deprotonated-diethylenetriamine penta-acetic acid scanning (right panel) shows marked heterogeneous enhancement of the lesion, and the dynamic curve displays a plateau shape. The arrows indicate the lesion. Based on this imaging, the diagnosis was a lesion in the upper outer quadrant of the right breast, classified as Breast Imaging-Reporting and Data System 4b.
Figure 4.
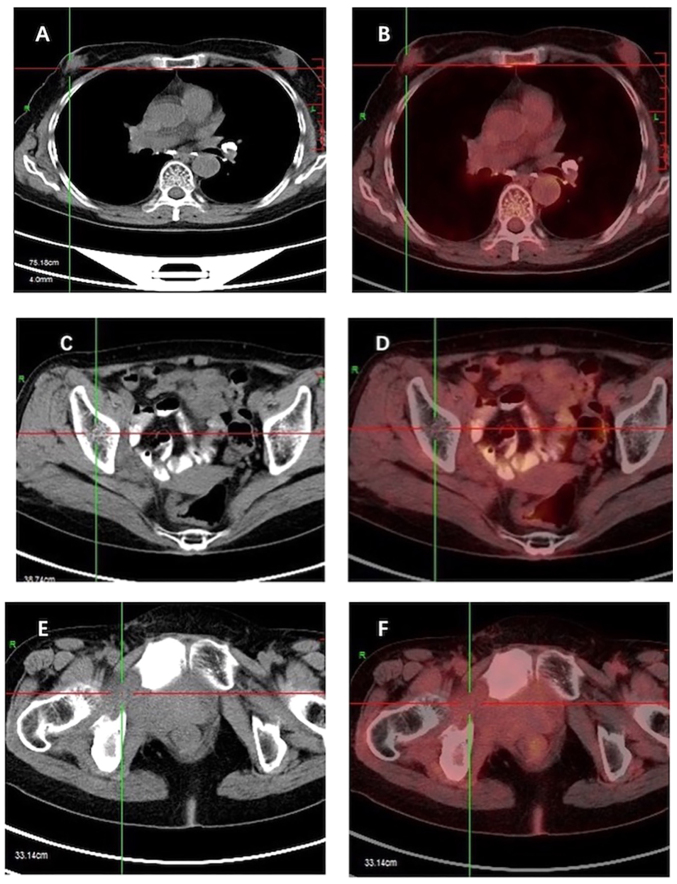
CT and [18F] FDG PET-CT scans of the breast mass. (A) Non-contrast axial CT scan reveals a large irregular mass in the right breast, and (B) [18F] FDG PET-CT reveals that the mass is metabolically active. (C) Non-contrast axial CT scan demonstrates tumor metastasis in the iliac region, and (D) [18F] FDG PET-CT demonstrates its metabolic activity. (E) Non-contrast axial CT scan shows bone destruction in the ischium with a surrounding soft tissue mass, and (F) [18F] FDG PET-CT demonstrates metabolic activity of the ischium and soft tissue mass. CT, computed tomography; [18F] FDG, fluorine-18 fluorodeoxyglucose; PET, positron emission tomography.
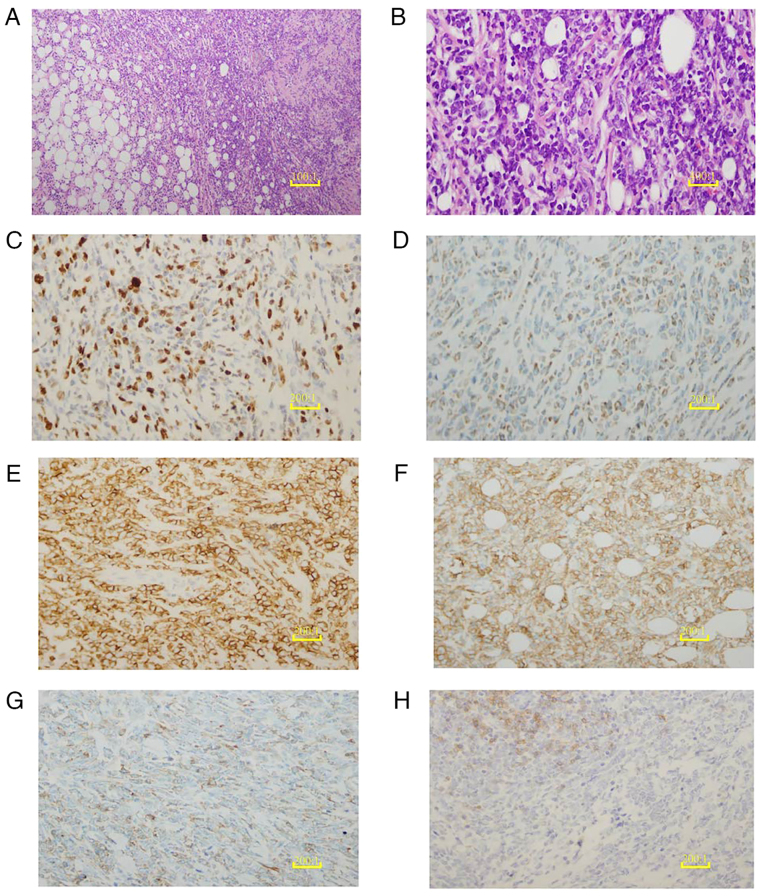
Hematoxylin and eosin staining of the right breast mass biopsy revealed numerous compressed cells with distinct morphological features (Fig. 5A and B) indicating the high likelihood of B-lymphoblastic lymphoma/leukemia. However, following needle biopsy, it was decided that an excisional biopsy was necessary as the small size of the needle aspiration biopsy sample and the challenges in accurately determining the nature of the tumor rendered the results inconclusive. Consequently, the patient underwent surgery to excise the right breast mass and an intraoperative frozen section examination was performed. During surgery, the tumor was found within the breast tissue. It exhibited an incomplete capsule and the cut surface had an appearance resembling that of fish flesh. The frozen section examination suggested the presence of breast lymphoma; therefore, further evaluations using paraffin sections and immunohistochemistry were recommended. The final pathology report indicated the widespread infiltration of immature tumor cells, with only a few remaining small ducts and acini. Scattered eosinophils were also reported.
Figure 5.
Histopathological and immunohistochemical analysis of the breast mass. (A) H&E staining of the breast mass biopsy tissue reveals diffuse, sheet-like growth with disrupted ductal structures (scale bar, 10 µm). (B) The H&E-stained breast mass biopsy tissue is extensively infiltrated by medium-sized malignant cells with round follicular nuclei containing finely dispersed chromatin and small nucleoli (scale bar, 2.5 µm). (C) Ki-67 shows a proliferation index of 50%, serving as a marker for cancer cells. (D) Myeloid differentiation of the tumor cells is illustrated with a myeloperoxidase immunohistochemical stain. (E) Positive expression of CD43 is revealed by immunohistochemical staining, supporting the diagnosis of myeloid sarcoma. (F) Tumor cells surrounding the breast ductal epithelium are positive for CD117 immunohistochemical staining, indicating their myeloid origin. (G) The hematopoietic progenitor origin of the tumor cells is indicated by weakly positive CD34 immunohistochemical staining. (H) Weak CD19 immunohistochemical staining indicates the reduced or abnormal expression of B-cell markers in the tumor cells. (C-H), scale bar, 5 µm. H&E, hematoxylin and eosin; CD, cluster of differentiation.
Immunohistochemical analyses were conducted on 4-mµm sections of whole tumor tissues containing in situ and/or invasive regions, using autostainers such as the Ultra System from Roche Diagnostics or the Autostainer Link 48 from DakoCytomation following the manufacturer's instructions. Immunohistochemical analyses revealed the expression of paired box 5, cluster of differentiation (CD)43, CD34, CD99, myeloperoxidase (MPO) and CD117 in the tumor cells. In addition, ~50% of the tumor cells tested positive for Ki-67, while no expression of cytokeratin, CD20, CD3, CD5 and CD4 was detected. These findings were consistent with the diagnosis of MS, as shown in Fig. 5. As the pathological examination indicated MS, the patient underwent a bone marrow biopsy and peripheral blood examination to confirm the diagnosis and assess the extent of the disease. These did not identify any blast cells (Fig. 6). As the results from these procedures revealed no abnormalities, the possibility that the tumor had metastasized from the bone marrow to the breast was excluded. Thus, a diagnosis of primary MS of the breast with concurrent malignant bone metastasis was made.
Figure 6.
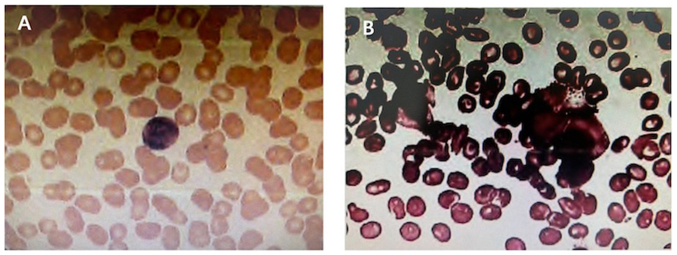
Bone marrow and peripheral blood analysis. (A) Bone marrow biopsy cell morphology examination and (B) cytological examination of the peripheral blood revealed no obvious abnormalities.
Although there is no specific treatment for MS, the current systemic chemotherapy regimens for AML are considered to be suitable first-line treatment approaches for MS. These mainly comprise cytarabine combined with anthracyclines or homoharringtonine, sometimes used in combination with etoposide (8). Considering that the cells observed in the pathological examination were predominantly primitive marrow cells and included some cells indicative of B-cell lymphoblastic lymphoma, a decision was made to use the ESHAP chemotherapy regimen, based on National Comprehensive Cancer Network guidelines (9). The ESHAP regimen is a second-line treatment option that includes etoposide, methylprednisolone, cytarabine and cisplatin. These drugs work synergistically to collectively target Ewing's sarcoma and B-cell lymphoblastic lymphoma without cross-resistance. Following surgery, the patient was transferred to the oncology department at the hospital to undergo six cycles of ESHAP regimen chemotherapy and 28 cycles of radiation therapy, each at a dose of 56 cGy. A follow-up contrast-enhanced [18F] FDG PET-CT scan at 12 months did not show any tumor residue or recurrence (Fig. 7). At the 2-year follow-up, the patient was thriving and disease-free.
Figure 7.
CT and [18F] FDG PET-CT scans of the breast mass after treatment. (A) Non-contrast axial CT scan demonstrates a clear reduction in the mass in the right breast following chemotherapy, and (B) [18F] FDG PET-CT demonstrates that the metabolism in the right breast has markedly decreased compared with that before treatment. (C) Non-contrast axial CT scan demonstrates increased bone density in the ilium, and (D) [18F] FDG PET-CT demonstrates reduced metabolism in the ilial region. (E) Non-contrast axial CT scan demonstrates the soft tissue mass around the ischium has decreased, and (F) [18F] FDG PET-CT demonstrates decreased metabolism of soft tissue mass around the ischium. CT, computed tomography; [18F] FDG, fluorine-18 fluorodeoxyglucose; PET, positron emission tomography.
Discussion
In the current case, a tumor infiltrating the breast parenchyma and comprising medium-sized immature cells with rounded nuclei surrounded by a thin rim of eosinophilic cytoplasm was identified and assessed. The tumor cells exhibited positive immunostaining for MPO, CD43 and CD117, suggesting their myeloid nature. Follow-up bone marrow biopsy and peripheral blood examination did not identify any blast cells, leading to the conclusion that this tumor was a primary MS of the breast. MS, which is composed of malignant immature cells, is a rare extramedullary tumor mainly associated with AML. According to the World Health Organization classification, MS is a major subgroup of myeloid neoplasms and acute leukemia (10). Typically, it is detected in patients who are diagnosed with myeloid leukemia; it may manifest concurrently with AML or as an initial presentation of relapse in patients with previously treated AML (11).
MS is rarely an isolated finding preceding myeloid leukemia of the blood or bone marrow, and MS of the breast is even rarer (12). Although MS can develop in individuals aged between 5 months and 89 years, these tumors predominantly affect younger individuals and children, with no distinct difference in the incidence rates between males and females (13). According to a study conducted by Viadana et al (14), among 503 patients with leukemia who underwent biopsies, only four of 235 patients with AML exhibited breast involvement. In addition, Naamo et al (15) reported the case of a 27-year-old female who presented with a palpable right breast lump, the biopsy of which showed breast tissue with diffuse infiltration of blasts compatible with MS. Furthermore, a study conducted by Amiraian et al (16) identified MS in both breasts of a 63-year-old woman with relapsed AML. To identify further cases, comprehensive searches in the PubMed, Embase and Cochrane Library databases for the years 2013–2023 were conducted. These yielded the reports of 14 patients with MS of the breast, excluding the patient presented in the present case report. The detailed clinical characteristics of these patients are presented in Table I (15–27). It may be observed that, while the clinical and pathological characteristics of these cases of breast MS are quite similar, the treatment plans varied considerably.
Table I.
Clinical characteristics of patients with breast myeloid sarcoma from 2014 to 2022.
| First author/s, year | Patient | Age, years | First symptom | Tumor location | Tumor size | AML link | CD43, CD34 | CD117, MPO | Treatment | Survival | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Naamo, 2022 | P1 | 27 | Painless breast mass | Right breast upper | 65×34×64 mm | No | (+), (+) | (+), (+) | Chemotherapy with Cytosar, Cerubidine and Mylotarg | Alive, 12 months | (15) |
| Amiraian, 2022 | P2 | 63 | Rapidly enlarging breast masses | Multiple lumps in both breasts | - | Yes | (++), (++) | -, (+) | Salvage chemotherapy, allogeneic bone marrow transplant | Deceased, 2 years 9 months | (16) |
| Ding, 2022 | P3 | 34 | Painless breast mass | Right breast | 31×1.5 mm | Yes | -, - | -, (+) | Chemotherapy after surgery and radiotherapy | Alive, 7 years | (17) |
| Huang, 2021 | P4 | 34 | Rapidly enlarging breast masses | Multiple lumps in both breasts | - | Yes | (+++), (+++) | (+), (+) | Chemotherapy | Deceased, 1 year | (18) |
| Varol, 2021 | P5 | 31 | Painless breast mass | Multiple lumps in both breasts | - | Yes | (+), (+) | (+), (+) | Systemic chemotherapy | Alive, 4 years | (19) |
| Kim and Kim, 2019 | P6 | 24 | Painless breast mass | Upper right breast | 43 mm | Yes | -, - | -, (+) | - | Alive, 1 year 6 months | (20) |
| Wu, 2019 | P7 | 42 | Painless breast mass | Upper right breast | 20×18 mm | No | -, - | -, (+) | 2 cycles of IA regimen chemotherapy after surgery | Alive, 1 year | (21) |
| Gomaa, 2018 | P8 | 29 | Painless breast mass | Upper left breast | 15×12×4 mm | No | -, (+) | (+), (−) | Radiotherapy and chemotherapy | Alive, 6 months | (22) |
| Uhida, 2016 | P9 | 28 | Painless breast mass | Right breast | - | No | -, - | -, - | Induction chemotherapy and stem cell transplantation | Deceased, 1 year | (23) |
| Stewart, 2015 | P10 | 46 | Enlarging, tender breast mass | Upper right breast | 44 mm | Yes | -, (+) | (+), (+) | - | N/A | (24) |
| Nalwa, 2015 | P11 | 33 | Painless breast mass | Left breast | - | No | -, (+) | (+),- | High dose cytarabine (HDAC) regimen | Alive, 1 year | (25) |
| Gunduz, 2014 | P12 | 33 | Painless breast mass | Upper right breast | 33 mm | Yes | -, - | -, - | Ara-C, etoposide and idarubicin chemotherapy | Deceased, 1 year 6 months | (26) |
| Fu and Luo, 2014 | P13 | 59 | Painless breast mass | Upper left breast | 18×12 mm | No | -, - | -, (+) | Chemotherapy with the MA regimen and consolidation chemotherapy | Alive, 4 years | (27) |
| Fu and Luo, 2014 | P14 | 39 | Painless breast mass | Lower left breast | 20×15 mm | No | -, - | -, (+) | Succumbed to fungal pneumonia without systemic chemotherapy | Deceased, 4 months | (27) |
Ara-C, cytarabine; HDAC, high-dose Ara-C; MA, mitoxantrone and Ara-C; N/A, not available.
MS of the breast lacks specific clinical features and typically appears as palpable nodules in one or both breasts, which may or may not be painful. These nodules can be mistaken for primary breast cancer (11). In this scenario, mammography typically identifies large, irregular, non-calcified masses with poorly defined borders (11), while ultrasonography commonly reveals hypoechoic lesions that are either homogeneous or heterogeneous and hypervascularized on color Doppler scans (12,20). Additionally, CT and MRI are often used for tumor localization, as these techniques are helpful in distinguishing MS from other masses. Specifically, MRI can be an effective diagnostic tool, which reveals MS of the breast as hypointense lesions on T1-weighted images and hyperintense lesions on T2-weighted images, with inhomogeneous enhancement (18). Furthermore, [18F] FDG-PET-CT imaging has emerged as a valuable tool for studying and monitoring extramedullary acute myelocytic leukemia (28). However, given that typical imaging characteristics of MS of the breast are lacking, it can lead to a misdiagnosis of primary breast malignancy, lymphoma, other neoplasm or inflammation (12,18). Although these findings are non-specific, they are suspicious and necessitate a biopsy. Accordingly, it is imperative to pay close attention to the characteristic clinical, radiographic and pathological findings when diagnosing isolated cases of breast MS.
Pathologically, MS typically exhibits either a diffuse growth pattern or a single-cell infiltrating growth pattern. Based on the proportion of immature granulocytes at different stages of differentiation, MS can be categorized into three pathological types, namely blast cell, partially differentiated and differentiated (29). The blast cell type predominantly comprises myeloblasts with only a few differentiated promyelocytes, while the partially differentiated subtype is characterized by both myeloblasts and promyelocytes, and the differentiated subtype is predominantly composed of promyelocytes and granulocytes in later stages of maturity. Notably, eosinophilic granulocytes are prominent in the differentiated subtype. Nevertheless, the accurate diagnosis of MS using routine histological slices is challenging. This renders immunohistochemical assessment necessary to prevent the misdiagnosis of diffuse large B-cell lymphoma (30). In addition to these diagnostic complexities, specific genetic alterations such as t (8;21) (q22; q22.1), inv (16) (p13.1q22) or t (16;16) (p13.1; q22), as well as nucleophosmin 1 mutations, have been associated with MS (31).
Immunohistochemistry is crucial in the diagnosis of MS. Among the various markers, MPO is the most effective for distinguishing MS and is expressed in as many as 93% of myeloid tumors. However, its expression levels vary depending on the degree of differentiation (31). Nevertheless, a panel including MPO, CD43 and CD20 as markers has been shown to effectively differentiate >96% of MS cases (32). Notably, CD43 exhibits high sensitivity but poor specificity, as it is expressed in almost all cases of MS. Therefore, if tumor cells of unknown origin express CD43 but are negative for CD3, MS should be considered. By contrast, CD117 is mainly expressed in immature myeloid tumors and is absent in lymphomas, rendering it a sensitive indicator of myeloid tumors (33). Although CD20 is a characteristic differentiation antigen of B cells, most studies suggest that MS is CD20-negative, while other studies have reported a CD20 expression rate of 13% (34,35). Therefore, it is crucial to select the appropriate antibody combination for use in the immunohistochemical examination of MS.
The treatment approaches for primary MS of the breast include surgical resection, local radiotherapy and systemic chemotherapy. However, it has been noted that surgical resection and local radiotherapy alone are not effective in delaying the transformation of MS into AML or improving its prognosis (36). Therefore, primary MS is considered a systemic disease and requires systemic treatment. The administration of systemic chemotherapy is recommended for all solitary MS lesions in patients who have undergone surgical resection. A variety of chemotherapy regimens that induce AML remission have been used in the context of MS, including idarubicin and cytarabine; fludarabine, high dose cytarabine, idarubicin and granulocyte-colony stimulating factor (G-CSF); cyclophosphamide, cytarabine, topotecan and G-CSF; and daunorubicin and cytarabine (5).
In the present case, an integrated treatment approach for breast MS was used. This combined lumpectomy with systemic chemotherapy, which mirrors protocols typically used in AML, and was followed up with local radiotherapy aimed at achieving a cure. At 24 months post-treatment, the patient remained in good health without any signs of disease relapse. This case shares similarities with patients described in previous literature, such as the patient undergoing complete excision of the local tumor and receiving systemic chemotherapy predominantly consisting of cytarabine and doxorubicin. However, a notable difference is that the current case also underwent 28 cycles of radiotherapy following the completion of chemotherapy. Although the treatment methods for breast MS have not yet been standardized, the majority of studies have concluded that all patients should undergo either mastectomy or tumor resection surgery, along with standard systemic chemotherapy (5,37). The case described in the present study underwent tumor resection surgery and systemic chemotherapy, and one year later, no local recurrence of the breast was detected.
It has been suggested that anti-leukemia chemotherapy administered shortly after surgery aids in controlling the development of MS and improving its prognosis. For MS, the preferred treatment regimen uses anthracyclines in combination with cytarabine (38). Allogeneic hematopoietic stem cell transplantation has also been indicated to be an effective alternative treatment (39). In addition, molecular developments have facilitated the development of highly targeted therapies for patients with MS, including those associated with breakpoint cluster region-Abelson 1, Fms-like tyrosine kinase 3-internal tandem duplication and FIP1-like 1-platelet derived growth factor receptor a gene mutations, thereby yielding promising results (40).
In conclusion, MS of the breast is a rare malignant neoplasm of myeloid origin that is often misdiagnosed. It originates from myeloid cells and requires intensive systemic chemotherapy, allogeneic hematopoietic stem cell transplantation, surgical resection and/or radiotherapy for effective treatment. However, the lack of a standard treatment approach for breast MS poses a considerable challenge. Once MS has been diagnosed, the prompt initiation of induction chemotherapy is recommended. The study of additional cases is essential to enhance clinical practice and improve the outcomes of patients with MS. Future prospective multicenter studies are necessary to gain an improved understanding of MS and guide its diagnostic and treatment approaches.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MRI
magnetic resonance imaging
- FDG
fluorodeoxyglucose
- PET-CT
positron emission tomography-computed tomography
- CD
cluster of differentiation
- MPO
myeloperoxidase
- MS
myeloid sarcoma
- AML
acute myeloid leukemia
- G-CSF
granulocyte-colony stimulating factor
- FLAG
fludarabine + high dose cytarabine + G-CSF
- H&E
hematoxylin and eosin
Funding Statement
This study was supported by the Natural Science Foundation of Inner Mongolia Autonomous Region (grant no. 2023QN08047), the Youth Exploration Project of Affiliated Hospital of Inner Mongolia Medical University (grant no. 2022NYFYTS022) and the Science and Technology Program of Inner Mongolia Autonomous Region (grant no. 2023YFSH0039).
Availability of data and materials
The data generated in the present study maybe requested from the corresponding author.
Authors' contributions
ZZ, YC, RZ and ML contributed to study conception and design, and performed material preparation, data collection and analysis. The first draft of the manuscript was written by ZZ, and all authors commented on previous versions of the manuscript. ZZ and YC confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Inner Mongolia Medical University in accordance with the regulations of the ethics committee for the publication of case reports [ethics approval no. WZ (2023072)]. Written informed consent was obtained from the participant for inclusion in the study.
Patient consent for publication
The patient provided written informed consent for publication of her data and images in this case report.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Goyal G, Bartley AC, Patnaik MM, Litzow MR, Al-Kali A, Go RS. Clinical features and outcomes of extramedullary myeloid sarcoma in the United States: Analysis using a national data set. Blood Cancer J. 2017;7:e592. doi: 10.1038/bcj.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitoz S, Atasoy C, Yavuz K, Gozdasoglu S, Erden I, Akyar S. Granulocytic sarcoma. Cranial and breast involvement. Clin Imaging. 2002;26:166–169. doi: 10.1016/S0899-7071(01)00388-6. [DOI] [PubMed] [Google Scholar]
- 3.Ngu IW, Sinclair EC, Greenaway S, Greenberg ML. Unusual presentation of granulocytic sarcoma in the breast: A case report and review of the literature. Diagn Cytopathol. 2001;24:53–57. doi: 10.1002/1097-0339(200101)24:1<53::AID-DC1009>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham I. A clinical review of breast involvement in acute leukemia. Leuk Lymphoma. 2006;47:2517–2526. doi: 10.1080/10428190600967022. [DOI] [PubMed] [Google Scholar]
- 5.Yamauchi K, Yasuda M. Comparison in treatments of nonleukemic granulocytic sarcoma: Report of two cases and a review of 72 cases in the literature. Cancer. 2002;94:1739–1746. doi: 10.1002/cncr.10399. [DOI] [PubMed] [Google Scholar]
- 6.Kewalramani T, Zelenetz AD, Nimer SD, Portlock C, Straus D, Noy A, O'Connor O, Filippa DA, Teruya-Feldstein J, Gencarelli A, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103:3684–3688. doi: 10.1182/blood-2003-11-3911. [DOI] [PubMed] [Google Scholar]
- 7.Miyazaki C, Shiozawa M, Koike R, Ogihara K, Sasaki Y, Shiba S, Nishida S, Sakuragi M, Mizunuma H, Fujita T, et al. Neoadjuvant chemotherapy for primary sarcoma of the breast: A case report. J Med Case Rep. 2019;13:289. doi: 10.1186/s13256-019-2197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu L, Li X, Su J, Chang C, He Q, Zhang X, Xu L, Song L, Pu Q. Effect of low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor priming regimen on patients with advanced myelodysplastic syndrome or acute myeloid leukemia transformed from myelodysplastic syndrome. Leuk Lymphoma. 2009;50:1461–1467. doi: 10.1080/10428190903096719. [DOI] [PubMed] [Google Scholar]
- 9.National Comprehensive Cancer Network, corp-author. NCCN clinical practice guidelines in oncology: Antiemesis. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf. doi: 10.6004/jnccn.2024.0029. Version 3.2024. [DOI] [PubMed] [Google Scholar]
- 10.Grantham JT, Howell DM, Bacaj PJ, Coad JE, Vos JA. Myeloid sarcoma of the bladder in the setting of refractory anemia with excess blasts-2 (RAEB-2) W V Med J. 2015;111:34–36. [PubMed] [Google Scholar]
- 11.Nicosia L, Latronico A, Farina M, Bozzini AC, Baratella P, Galimberti VE, Fiori S, Montesano M, Cassano E. Myeloid sarcoma of the breast: A pathology that should not be forgotten. Ecancermedicalscience. 2020;14:1160. doi: 10.3332/ecancer.2020.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thachil J, Richards RM, Copeland G. Granulocytic sarcoma-a rare presentation of a breast lump. Ann R Coll Surg Engl. 2007;89:W7–W9. doi: 10.1308/147870807X227827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu T, Xu G, Xu X, Yang J, Ding L. Myeloid sarcoma derived from the gastrointestinal tract: A case report and review of the literature. Oncol Lett. 2016;11:4155–4159. doi: 10.3892/ol.2016.4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viadana E, Bross ID, Pickren JW. An autopsy study of the metastatic patterns of human leukemias. Oncology. 1978;35:87–96. doi: 10.1159/000225262. [DOI] [PubMed] [Google Scholar]
- 15.Naamo S, Naamo S, Sarker S, Vasconez M, Froicu M. Breast manifestation of extramedullary myeloid sarcoma: A case report. Radiol Case Rep. 2022;17:4660–4665. doi: 10.1016/j.radcr.2022.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amiraian D, McDonough M, Geiger X. Bilateral myeloid sarcoma of the breast: A case report with radiological and pathological correlation. Cureus. 2022;14:e24731. doi: 10.7759/cureus.24731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Xi D, Chen Y, Gu W. Myeloid sarcoma of the breast as a first manifestation of acute myeloid leukemia: A case report. Asian J Surg. 2022;45:1622–1623. doi: 10.1016/j.asjsur.2022.03.056. [DOI] [PubMed] [Google Scholar]
- 18.Huang C, Fei S, Yao J, Chen P, Luo J, Wang Y, Li J, Wang W. Breast myeloid sarcoma presenting as a palpable breast lump after allogeneic stem cell transplantation for acute myelomonocytic leukemia: A rare case report. World J Surg Oncol. 2021;19:289. doi: 10.1186/s12957-021-02399-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varol E, Kiraz U, Guler SA, Vural Ç, Gülbaş Z, Utkan NZ. Breast recurrence of acute myeloid leukemia after bone marrow transplantation: A case report about myeloid sarcoma of the breast. Eur J Breast Health. 2021;17:292–295. doi: 10.4274/ejbh.galenos.2021.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SJ, Kim WG. Sonographic features of a myeloid sarcoma of the breast as a relapse of acute myeloid leukemia after stem-cell transplantation: A case report. Am J Case Rep. 2019;20:612–619. doi: 10.12659/AJCR.915453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HY, Liu L, Gu L, Luo YH. Clinical characteristics and management of primary granulocytic sarcoma of the breast: A case report. Medicine (Baltimore) 2019;98:e16648. doi: 10.1097/MD.0000000000016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomaa W, Ghanim A, Emam E, Bayoumi K, Ghanim A. Primary myeloid sarcoma of the breast: A case report and review of literature. J Microsc Ultrastruct. 2018;6:212–214. doi: 10.4103/JMAU.JMAU_15_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Uchida E, Watanabe K, Oshikawa G, Sakashita C, Kurosu T, Fukuda T, Arai A, Murakami N, Miura O, Yamamoto M. Refractory primary myeloid sarcoma of the breast with MLL-AF9 rearrangement. Rinsho Ketsueki. 2016;57:47–51. doi: 10.11406/rinketsu.57.47. [DOI] [PubMed] [Google Scholar]
- 24.Stewart RL, Dell CM, Samayoa L. Myeloid sarcoma of the breast misdiagnosed as poorly differentiated mammary carcinoma with lobular features. Breast J. 2015;21:192–193. doi: 10.1111/tbj.12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalwa A, Nath D, Suri V, Jamaluddin MA, Srivastava A. Myeloid sarcoma of the breast in an aleukemic patient: A rare entity in an uncommon location. Malays J Pathol. 2015;37:63–66. [PubMed] [Google Scholar]
- 26.Gündüz E, Akay MO, Karagülle M, Ak IS. isolated granulocytic sarcoma of the breast after allogeneic stem cell transplantation: A rare involvement also detected by 18FDG-PET/CT. Turk J Haematol. 2014;31:88–91. doi: 10.4274/Tjh.2012.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu J, Luo J. Granulocytic sarcoma of the breast in acute myeloid leukemia: Two case reports. Oncol Lett. 2014;7:145–147. doi: 10.3892/ol.2013.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avni B, Koren-Michowitz M. Myeloid sarcoma: Current approach and therapeutic options. Ther Adv Hematol. 2011;2:309–316. doi: 10.1177/2040620711410774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li JM, Liu WP, Zhang MH, Wei X, Gu JM, Han AJ, Wu WQ, Chen XY. Clinicopathologic and immunophenotypic analysis of myeloid sarcoma. Zhonghua Bing Li Xue Za Zhi. 35:606–611. (In Chinese) [PubMed] [Google Scholar]
- 30.Pileri SA, Ascani S, Cox MC, Campidelli C, Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero D, et al. Myeloid sarcoma: Clinico-pathologic, phenotypic and cytogenetic analysis of 92 adult patients. Leukemia. 2007;21:340–350. doi: 10.1038/sj.leu.2404491. [DOI] [PubMed] [Google Scholar]
- 31.Estey EH. Acute myeloid leukemia: 2019 update on risk-stratification and management. Am J Hematol. 2018;93:1267–1291. doi: 10.1002/ajh.25214. [DOI] [PubMed] [Google Scholar]
- 32.Traweek ST, Arber DA, Rappaport H, Brynes R. Extramedullary myeloid cell tumors. An immunohistochemical and morphologic study of 28 cases. Am J Surg Pathol. 1993;17:1011–1019. doi: 10.1097/00000478-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Audouin J, Comperat E, Le Tourneau A, Camilleri-Broët S, Adida C, Molina T, Diebold J. Myeloid sarcoma: Clinical and morphologic criteria useful for diagnosis. Int J Surg Pathol. 2003;11:271–282. doi: 10.1177/106689690301100404. [DOI] [PubMed] [Google Scholar]
- 34.Mourad W, Kfoury H, Al Husseini H. The value of CD34, myeloperoxidase and chloroacetate esterase (Leder) stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med. 2001;21:287–291. doi: 10.5144/0256-4947.2001.287. [DOI] [PubMed] [Google Scholar]
- 35.He J, Zhu L, Ye X, Li L, Zhu J, Zhang J, Xie W, Shi J, Zheng W, Wei G, et al. Clinical characteristics and prognosis of nonleukemic myeloid sarcoma. Am J Med Sci. 2014;347:434–438. doi: 10.1097/MAJ.0b013e31829ca859. [DOI] [PubMed] [Google Scholar]
- 36.Florou D, Katsara M, Feehan J, Dardiotis E, Apostolopoulos V. Anti-CD20 agents for multiple sclerosis: Spotlight on ocrelizumab and ofatumumab. Brain Sci. 2020;10:758. doi: 10.3390/brainsci10100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang XE, Li YJ, Zhou XD. Granulocytic sarcoma of the breast: A case report. Oncol Lett. 2015;10:2447–2449. doi: 10.3892/ol.2015.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widhalm G, Dietrich W, Müllauer L, Streubel B, Rabitsch W, Kotter MR, Knosp E, Roessler K. Myeloid sarcoma with multiple lesions of the central nervous system in a patient without leukemia. Case report. J Neurosurg. 2006;105:916–919. doi: 10.3171/jns.2006.105.6.916. [DOI] [PubMed] [Google Scholar]
- 39.Tsimberidou AM, Kantarjian HM, Wen S, Keating MJ, O'Brien S, Brandt M, Pierce S, Freireich EJ, Medeiros LJ, Estey E. Myeloid sarcoma is associated with superior event-free survival and overall survival compared with acute myeloid leukemia. Cancer. 2008;113:1370–1378. doi: 10.1002/cncr.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Védy D, Muehlematter D, Rausch T, Stalder M, Jotterand M, Spertini O. Acute myeloid leukemia with myeloid sarcoma and eosinophilia: Prolonged remission and molecular response to imatinib. J Clin Oncol. 2010;28:e33–e35. doi: 10.1200/JCO.2009.23.6976. [DOI] [PubMed] [Google Scholar]
- 41.Spak DA, Plaxco JS, Santiago L, Dryden MJ, Dogan BE. BI-RADS((R)) fifth edition: A summary of changes. Diagn Interv Imaging. 2017;98:179–190. doi: 10.1016/j.diii.2017.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study maybe requested from the corresponding author.