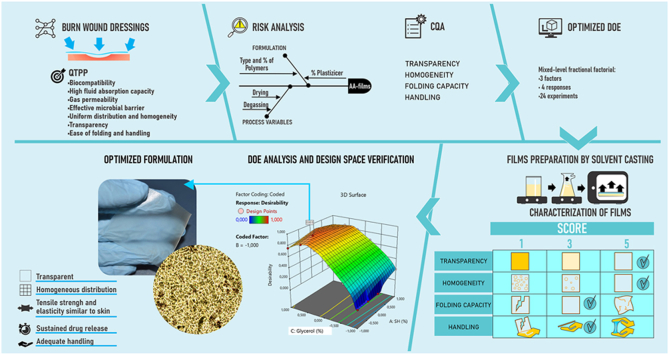
Abstract
The management of wounds primarily revolves around pain relief, effective infection control and the promotion of tissue regeneration to prevent complications like chronic skin wounds. While polymeric bioactive films are innovative alternatives to conventional wound dressings, there exists a dearth of guidance regarding their quality control. This underscores the imperative need to establish precise critical quality attributes, a task undertaken within this study using an antibiotic-anesthetic film as a model. The aim was to establish the influence of critical composition and process parameters and optimize the formula.
First, the quality target product profile was defined, and critical quality attributes were identified. Our material selection included ciprofloxacin hydrochloride (an antimicrobial), lidocaine hydrochloride (an anesthetic), as well as excipients, such as sodium alginate, sodium hyaluronate, carbomer and glycerol. The critical components were identified through a risk assessment matrix, and their influence on film composition was determined by experimental verification using Design-Expert® software. A full factorial design was employed to assess the effects of sodium hyaluronate, carbomer and glycerol (as independent variables) on transparency, homogeneity, folding capacity and handling. Following this, an optimized formulation was achieved and subjected to further characterization.
These optimized antibiotic-anesthetic films exhibited uniform micro-distribution of components, ensuring dosage uniformity. Both ciprofloxacin hydrochloride and lidocaine hydrochloride displayed sustained release profiles, suggesting potential therapeutic benefits for skin wounds. Furthermore, the resistance and elongation properties were similar to those of human skin.
Utilizing a QbD approach, we successfully developed an optimized antibiotic-anesthetic film that adhered to the essential critical quality attributes. This films exhibits potential utility as a wound dressing. The findings presented in this study establish a fundamental framework for delineating the critical quality attributes of dressing films and refining their formulation to optimize wound treatment.
Keywords: Film, Polyelectrolytes, Polymeric wound dressings, Quality by design, Risk analysis, Design space, Quality control
Graphical abstract
Highlights
-
•
Development of QbD-based burn wound dressing with natural polyelectrolytes, an antibiotic and an anesthetic agent.
-
•
Critical quality attributes such as transparency, homogeneity, folding capacity, and ease of handling were identified.
-
•
The influence of polymer type and concentration, as well as the percentage of glycerol as a plasticizer, was evaluated.
-
•
The optimized formulation was characterized for its mechanical, physicochemical, and biopharmaceutical properties.
1. Introduction
Burn injuries represent a significant global health challenge due to their high incidence, associated morbidity and potential for severe complications, including infections and scarring [1]. Despite ongoing research, there are no clinically approved dressings that effectively control infections and pain relief, while also facilitating wound healing and preventing permanent disfigurement or loss of skin functionality. Developing a single material for topical application that combines all these requirements remains a challenge [2,3].
Hydrogels based on biopolymeric carriers are considered promising for the development of wound dressings due to their biocompatibility, hydrophilic nature and ability to mimic many properties of native tissue, potentially promoting the wound healing process [[4], [5], [6]]. An evolution of hydrogel technology is the development of thin films, which retain the beneficial properties of hydrogels while offering additional advantages, such as accurate dosing, ease of administration and minimally invasive application [7,8]. These features have made films increasingly popular as an alternative wound dressing compared to traditional options like creams or gauzes [9].
Among the various techniques available for obtaining films, solvent-casting is the most widely used method due to its easy and cost-effective manufacturing process [4,7]. This method is commonly employed in industrial-scale production of transdermal patches and can be adapted for small-scale batches [10]. However, the properties of solvent-cast films can be significantly affected by environmental conditions and formulation processes. Variations in these factors can lead to issues such as foaming during mixing, cracking during cutting or sticking during packaging and handling [4,10,11].
Wound dressing films are generally classified as medical devices because their primary mechanism of action relies on the physical or mechanical interaction with the wound, rather than the pharmacological effects of any drugs they may contain. This classification aligns with regulatory standards like ISO 13485 and ISO 14971, which provide frameworks for quality assurance and risk management, respectively, in the development and manufacturing of medical devices [12]. Despite these standards, there remains a significant gap in the consistent establishment of critical quality attributes for wound dressing films. This lack of standardized criteria makes it difficult to effectively evaluate their safety, efficacy and overall performance [13,14].
Extensive research, as documented in numerous scientific articles and patents, has focused on characterizing wound dressing films and establishing their critical quality attributes. These studies identify a wide range of properties, including mechanical strength, fluid absorption capacity, in vitro release, surface morphology and wound healing capacity. While some authors have proposed acceptable ranges for attributes such as thickness, mechanical strength, elasticity and water vapor permeability, these recommendations lack consistency [4,15]. This inconsistency has led to cases where film quality failures are discovered in late development stages or even post-commercialization [11,16], underscoring the urgent need for more precise definitions and better risk management strategies for these critical quality attributes.
These gaps highlight the critical need for further research to establish clear specifications and quality attributes for wound dressing films. Applying rigorous risk analysis during the pre-formulation stage is essential when selecting biocompatible materials that can form films with the desired properties, such as high fluid uptake, impermeability to microorganisms, gas permeability and controlled drug release. Additionally, there is a need for standardized, affordable quality tests to assess key physical attributes of these films, including transparency, homogeneity, folding capacity and ease of handling.
Quality by Design (QbD) is “a systematic approach for product development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” [17]. QbD approaches have been used in pharmaceutical development for over two decades to enhance the consistency and quality of products, however, their application in the early development stages remains limited [18]. This approach could provide a structured methodology for defining films quality parameters of ultimately leading to more reliable and effective wound dressing solutions.
Polyelectrolytes are polymers that can form ionic complexes with drugs of opposite charge, allowing for the creation of new materials with controlled drug release properties. In these sense, a new antibiotic-anesthetic film (AA-films) based on natural polyelectrolytes sodium alginate (SA) and sodium hyaluronate (SH) was developed as a proof of concept for wound treatment [19]. These biopolymers were chosen due to their excellent biocompatibility and ability to form stable hydrogels that can effectively modulate drug release rates [12]. Their specific composition and the manufacturing method of these films have been protected by an Argentine patent (INPI Act N° 20190102232) [20].
The anesthetic drug, lidocaine, was rapidly released from AA-films into isotonic NaCl solutions, exhibiting a burst effect within the first 30 min. This provided a high drug concentration that is convenient for patients during dressing changes to manage pain. In contrast, the antibiotic ciprofloxacin was released from the AA-films at a significantly slower rate than lidocaine. This suggests a stronger interaction with the polyelectrolytes, allowing for the achievement of an effective antimicrobial concentration over an extended period [19].
Furthermore, in vitro efficacy against S. aureus and P. aeruginosa was demonstrated, indicating potential prevention of wound infection complications, such as delayed or non-healing wounds. Moreover, AA-films displayed several desirable properties, including transparency, compatibility with the pH of wounded skin, excellent water vapor permeability and a notable fluid absorption capacity [19]. These attributes contributed to improved wound healing in a deep second-degree burn model, and suggest that neither ciprofloxacin nor lidocaine interfered with the skin healing stages at the selected doses [21]. These in vivo studies were conducted in earlier stages of our research and are not part of the current work, serving as foundational data that informed the design and focus of this study. Nevertheless, the impact of composition attributes and the manufacturing process on AA-films, as guided by QbD strategies, requires further exploration.
Therefore, the purpose of this work is to develop and optimize AA-films using QbD strategies, aiming to establish critical quality attributes and standards for wound dressings. The study will also evaluate the impact of composition and process parameters on these attributes. This approach aims to provide foundational data for future proposals of quality acceptance criteria that could be applied to industrialized or compounded formulations.
2. Materials and methodology
2.1. Materials
Ciprofloxacin as hydrochloride was generously provided by Bago® laboratory (Argentina). Lidocaine hydrochloride was obtained from Parafarm® (Argentina). Sodium hyaluronate (SH) Parafarm® (Argentina, Batch: 11508; MW 1730 kDa) had a sodium content of 2.4 meq Na/g, determined by acid-base titration. Sodium alginate (SA) was purchased from Sigma Aldrich® (USA, Batch: MKBX6322V) and had a sodium content of 4.60 meq Na/g, determined using a sodium selective electrode (perfectION®, Mettler Toledo®, USA). Carbomer 934P NF (Cb) (Parafarm®, Argentina) had a carboxylic acid equivalents of 12 meq/g determined by acid-base titration. Anhydrous glycerol 99.5 % and glacial acetic acid 99.5 % were obtained from Cicarelli® (Argentina), and acetonitrile was acquired from Sintorgan® (Argentina). The phosphate-buffered saline (PBS) solution (pH = 7.4 ± 0.1) was prepared by dissolving the following salts (all from Parafarm®, Argentina) in water: 8 mg/mL NaCl, 0.201 mg/mL KCl, 0.204 mg/mL KH2PO4 and 2.9 mg/mL Na2HPO4•12H2O were adjusted to pH 7.40 ± 0.05 with NaOH 1 M or HCl 1 M (Anedra®, Argentina). The sterile 0.9 % NaCl solution was obtained from Braun® (Argentina). All the chemical reagents and solvents used were of pharmaceutical or analytical quality. Ultrapure water was obtained from a water purification system (Heal Force®, China).
2.2. Identification of quality target product profile (QTPP), critical quality attributes (CQA) and risk assessment
The methodology was conducted following the principles of QbD as outlined in the ICH Q8 (R2) and Q9 guidelines [22,23]. The development and optimization of the AA-films was guided by a clearly defined QTPP, which outlines the desired quality characteristics that the final product must meet to ensure safety, efficacy and patient compliance. The QTPP included attributes such as biocompatibility, high fluid absorption capacity, gas permeability, effective microbial barrier, uniform distribution and homogeneity, transparency and ease of folding and handling.
Based on the QTPP, CQAs were identified as the key measurable properties that have a direct impact on the quality, safety and efficacy of the AA-film. These CQAs included, among others, fluid uptake capacity, gas permeability, microbial barrier effectiveness, transparency, homogeneity, as well as, folding and handling properties. The selection of CQAs was informed by both regulatory requirements and intended clinical performance.
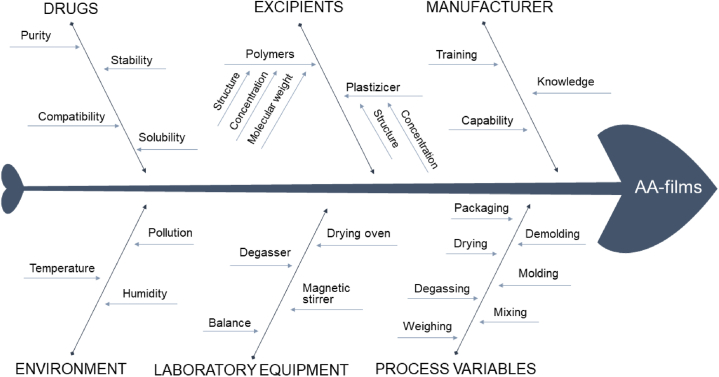
To ensure the CQAs were consistently achieved, a comprehensive risk assessment study was conducted. Initially, an Ishikawa fishbone diagram was used to qualitatively identify and categorize potential risk factors related to both critical material attributes (CMAs) and critical process parameters (CPPs) that could influence the CQAs.
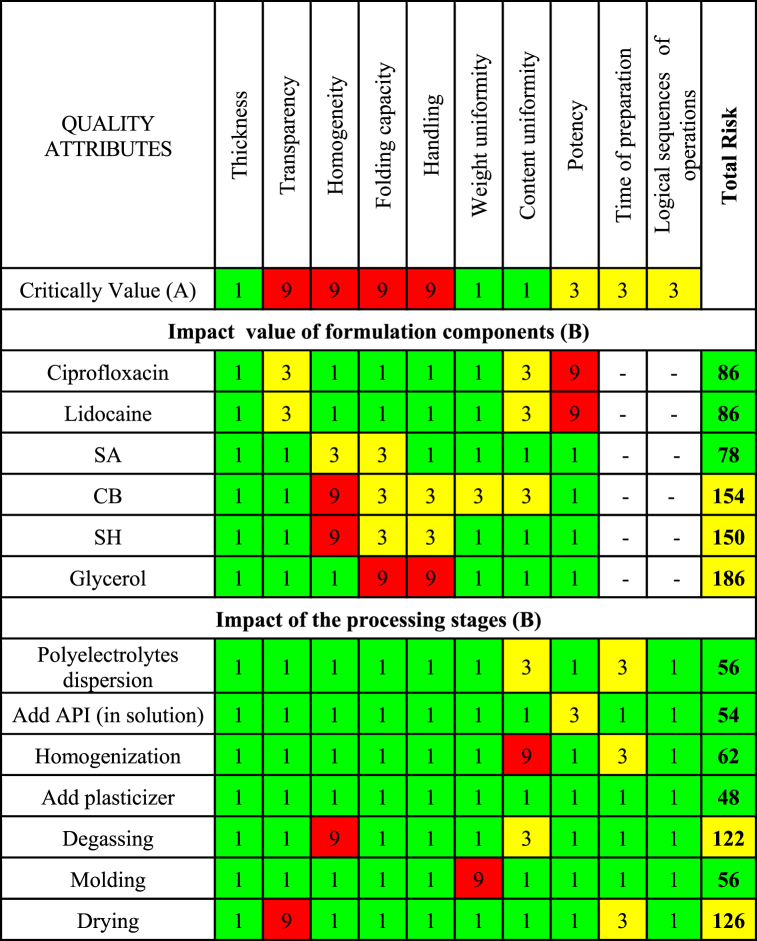
Following this, a risk assessment matrix was employed to quantitatively evaluate each identified risk factor, as the CMA or CPP, based on its likelihood of occurrence and potential severity of impact on the CQAs. Then, a Criticality Value was assigned for each CQA indicating the level of risk: high-risk (9, red), medium-risk (3, yellow) or low-risk level (1, green). For each CMA or CPP was assigned an Impact Value, which indicates the degree of risk that these attributes could affect the CQA (1, 3 or 9, according to the degree of risk). To determine the level of risk associated with a particular CMA or CPP the Total Risk value was used (equation (1)), which was ranked in terms of its potential impact on product quality [24,25] as high risk if the value exceeded 200 (red), medium risk if the values were between 100 and 200 (yellow) and low risk if the values were below 100 (green).
| Equation (1) |
Where A is the Criticality Value assigned to each Quality Attribute (1, 3 or 9, according to the degree of criticality) and B is the Impact Value, which indicates the degree of risk that a CMA (or CPP) could affect the CQA (1, 3 or 9, according to the degree of risk).
2.3. Design of experiments (DoE), films elaboration and quality analysis
A design of experiments (DoE) was used to determine the influence of CMA and CPP on the formulation's performance. A fractional mixed-level factorial design was selected for its efficiency in combining screening and optimization into a single step, making it more effective than the traditional two-step method [26].
A total of 24 laboratory-scale films were prepared using the solvent casting technique and evaluated for CQA. The concentrations of ciprofloxacin and lidocaine (both as hydrochloride salts), were kept constant in the hydrogel, based on available topical formulations, as was the sodium alginate (SA) content. However, the quantity of SH (X1), Cb (X2) or glycerol (X3) were systematically varied as shown in Table 1. It is worth clarifying that the use of coded values has been a strategy to protect intellectual properties during publication. The selection of factors (X1, X2 and X3), as well as their levels, was based on preliminary studies.
Table 1.
Design matrix summarizing the 24 runs in a factorial design.
| N (Runs) | SH (X1) | Cb (X2) | Glycerol (X3) |
|---|---|---|---|
| 1 | −1 | −1 | −1 |
| 2 | −1 | −1 | −0.6 |
| 3 | −1 | −1 | −0.2 |
| 4 | −1 | −1 | 0.2 |
| 5 | −1 | −1 | 0.6 |
| 6 | −1 | −1 | 1 |
| 7 | −0.5 | −1 | −1 |
| 8 | 0 | −1 | −1 |
| 9 | 1 | −1 | −1 |
| 10 | −0.5 | −1 | −0.2 |
| 11 | 0 | −1 | −0.2 |
| 12 | 1.0 | −1 | −0.2 |
| 13 | −0.5 | −1 | 0.2 |
| 14 | 0 | −1 | 0.2 |
| 15 | 1 | −1 | 0.2 |
| 16 | −0.5 | −1 | 0.6 |
| 17 | 0 | −1 | 0.6 |
| 18 | 1 | −1 | 0.6 |
| 19 | −1 | 0 | −0.2 |
| 20 | −1 | 1 | −0.2 |
| 21 | −1 | 0 | 0.2 |
| 22 | −1 | 1 | 0.2 |
| 23 | −1 | 0 | 0.6 |
| 24 | −1 | 1 | 0.6 |
Note: The coded values for X1, X2 and X3 represent different levels of sodium hyaluronate (SH), carbomer 934P NF (CB) and glycerol, respectively. The actual composition details are protected as an invention patent [20].
The hydrogels for casting were prepared by dispersing SA in water under constant magnetic stirring, followed by the addition of ciprofloxacin and lidocaine. Subsequently, the varying amounts of SH (X1), Cb (X2) or glycerol (X3) were added to achieve a translucent hydrogel, which was then stored in a refrigerator overnight. The prepared hydrogels were degassed using a vacuum pump (BOECCO® R-300, Germany), transferred to square silicone mold (20 cm2) and dried at 40 °C in an oven (Dalvo® UpF 464, Argentina) until a constant weight was achieved. The theoretical final amounts of ciprofloxacin and lidocaine (as a base) were 1.0 and 2.2 mg/cm2, respectively.
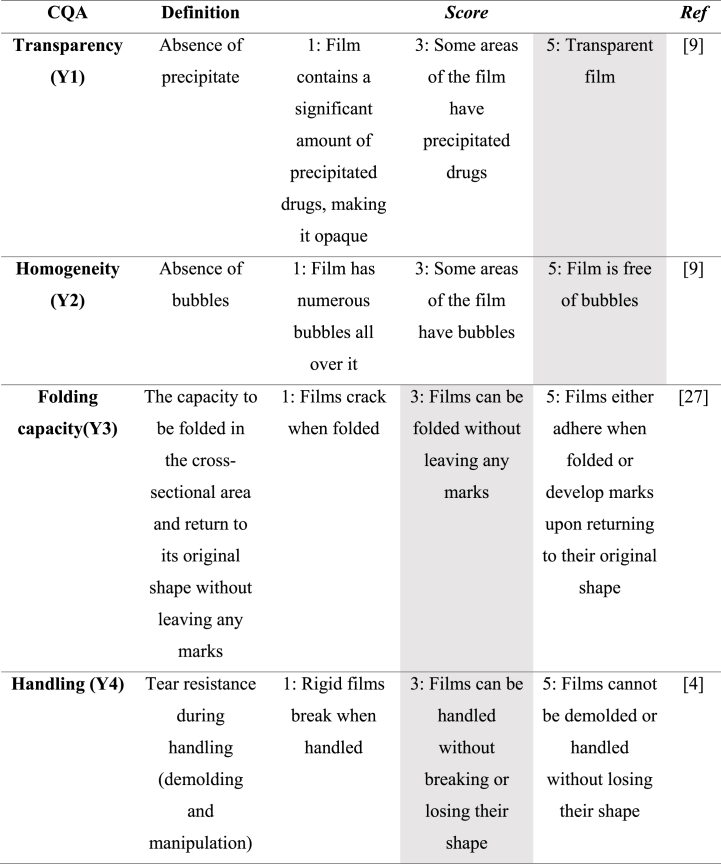
For the evaluation of CQA, a macroscopic categorical score was defined with a Criticality Value of 9 for the following dependent variables: transparency (Y1), homogeneity (Y2), folding capacity (Y3) and handling (Y4) (Table 2). These macroscopic scores facilitated a thorough quality analysis of the 24 laboratory-scale films, ensuring that the DoE findings were effectively translated into high-quality product outcomes.
Table 2.
Shaded cells indicate the values selected for formulation optimization.
2.4. Data analysis, statistical optimization and model validation
Mathematical models were developed using the Design Expert software (Stat-Easy®, Minneapolis, USA) to explain the interactions between the independent variables (X1, X2, and X3) and dependent variables or responses (Y1, Y2, Y3, and Y4). Various statistical parameters, including p-value, R2 value, F value and lack of fit F value, were utilized to assess the significance and validity of the model. Additionally, quadratic polynomial response equations incorporating key and interaction factors were constructed to analyze further the relationship between critical quality attributes (CQAs) and material attributes. The suitability and validity of the presented model were evaluated through ANOVA (p < 0.05).
Then, the optimal values of each response were selected from Table 2 (shaded cells) and a mathematical optimization of the formulation was carried out adjusting to a desirability function. The desirability function ranges from 0 to 1, with 1 representing the optimal value where all the responses meet the predefined objectives simultaneously. The location of the optimized formulation was marked in the overlay plot design space.
Validation of QbD methodology was conducted by preparing the optimized formulation and subsequently comparing the predicted responses with the observed results. A relative error of 5 % was considered acceptable, with a confidence interval of 95 %.
After that, the optimized formulation was deeply characterized by its mechanical properties, thickness and weight variation. Additionally, scanning electron microscopy with energy dispersive X-ray spectroscopy was employed for detailed analysis. Furthermore, in vitro drug release studies were conducted to provide a comprehensive understanding of the formulation's performance.
2.5. Characterization of optimized formulation
2.5.1. Mechanical properties
The mechanical properties of the AA-films were evaluated using an Instron series 3342 (Instron®, EEUU) load frame with a load cell of 500 N. Test specimens were cut into 20 × 100 mm strips and fixed to both clamps of the texturometer at 10 mm, which left an effective surface of 20 × 80 mm2. The clamps were subsequently moved away from each other with a crosshead speed of 0,50 mm/s until breakage of the AA-films (n = 5). Tensile strength at break (MPa) and elongation at break (%) were calculated from Equations Equation (2), Equation (3), respectively.
| Equation (2) |
| Equation (3) |
In addition, the Young's modulus (MPa) was calculated by the tangent to the initial linear portion of the tensile stress versus tensile strain curve [28,29].
2.5.2. Thickness and weight variation
The thickness of the AA-films was measured at the center and in the four corners, using a micrometer (Wemble, Taiwan) with 5 μm sensitivity. The average thickness was calculated and expressed as the mean thickness ± standard deviation (SD) based on six samples.
To assess weight variation, twenty randomly selected AA-films were individually weighed using an Adventurer® AR2140 analytical balance (OHAUS, USA). The mean weight ± SD was then calculated to determine the average weight of the films [29].
2.5.3. Optical microscopy
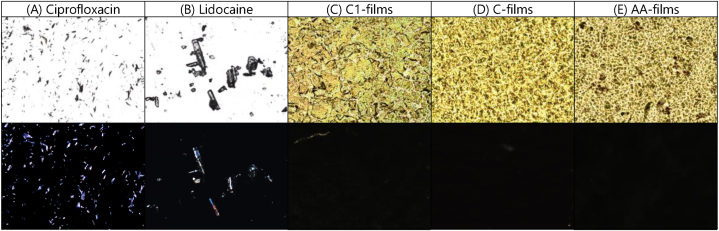
Photomicrographs of AA-films were taken at 100X magnification using an Olympus BX41 microscope (Olympus®, USA) equipped with an Infinity 1 digital camera. To evaluate the amorphous or crystalline nature of the components, polarized light microscopy was performed using the same microscope with an interference filter for monochromatic light (λ = 546 nm) and a Sénarmont compensator introducing an optical retardation of λ/4. Image analysis was conducted using Infinity Analyze v.5.0.2 software. For comparison, ciprofloxacin and lidocaine (both as hydrochloride) were analyzed, along with two control hydrogels: one containing glycerol but no drugs (C-films) and another without drugs or glycerol (C1-films).
2.5.4. Scanning electron microscopy (SEM) with energy dispersive X-ray spectroscopy (EDS)
The surface morphology of the AA-films was studied using SEM-EDS with a FE-SEM Carl Zeiss Σigma (Germany). Before analysis, small portions of AA-films were fixed to aluminum supports and coated with gold. SEM images were obtained under high vacuum conditions with an acceleration voltage of 5.0 kV. EDS analysis was carried out on the same SEM using an Oxford AZtec X-Max 80 mm2 Silicon Drift Detector. Measurements utilized a 20 keV electron beam, 10 nA current, 8.5 mm working distance, and a 10-s live-time counting. Oxford AZtec software was employed for X-ray mapping and elemental analysis.
2.5.5. X-Ray Powder Diffraction
The crystalline arrangement of AA-Films was studied using X-Ray Powder Diffraction (XRPD) at room temperature, with a PAnalytical X'Pert PRO® (PAnalytical, Netherlands) equipped with Cu Kα radiation (λ: 1.54 Å) operating at 40 mA and 40 kV. XRPD patterns were obtained in the angular range of 5–35° 2θ with a scan step of 0.02° 2θ and were plotted using GraphPad Prism® v.8.00. For comparison, the starting materials, the physical mixture of all solids of polymers and the two control hydrogels described earlier (C-films and C1-films) were also analyzed.
2.5.6. Thermogravimetric analysis
Thermogravimetric analyses (TGAs) were performed using a Discovery instrument (TA Instruments®, USA). The analysis was conducted over a temperature range of 25–350 °C, with a heating rate of 10 °C/min. Samples (2.0–5.0 mg) were placed in platinum pans (100 μL) and heated under a nitrogen flow (50 mL/min). To gain better insights into the AA-Films thermal behaviour, the starting materials and the control films (C-films and C1-films) were also analyzed. The data were processed using TRIOS software (TA Instruments®, USA). The first derivative of the mass loss for temperature (dTGA) highlighted the different mass loss events in the samples, which were attributed to water activity or decomposition. The onset or recovery of these events was calculated from the point where the thermogram deviated from or returned to the baseline, respectively.
2.5.7. In vitro drug release of ciprofloxacin and lidocaine
The in vitro release kinetics study was carried out using Franz-type diffusion cells. These cells are bicompartmental chambers: the receiver compartment, with capacity of 15–17 mL, was filled with degassed PBS solution (pH = 7.4 ± 0.1) and thermostated at 37 °C. The donor compartment included a cylinder with a synthetic cellulose acetate membrane base (Sigma®, 12,000 D cutoff value) hydrated overnight, having an effective diffusion area of 1.25 cm2. An accurately weighed circular AA-films with a diameter of 12 mm was placed on top of this membrane. At predetermined time points (5, 10, 15, 30, 45 min, 1, 2, 3, 4 and 5 h), aliquots of approximately 0.9 mL were collected from the receiver compartment, and an equal volume of fresh, thermostated (37 °C) medium was added to maintain sink conditions. This aliquot volume was selected based on previous studies, which have demonstrated that, for Franz diffusion cells with a capacity ranging from 5 to 75 mL, sampling volumes up to 2 mL do not disrupt the system's equilibrium [[30], [31], [32]]. Samples were immediately frozen until further analysis by HPLC-UV as described in Sanchez et al. [19]. Briefly, the analysis was performed using a Waters Breeze HPLC system equipped with a C18 reverse-phase column. The mobile phase consisted of an acetic acid solution (pH 3.4) and acetonitrile in a 4:1 ratio. A 50 μL injection volume was used, with a flow rate of 1 mL/min. UV detection was conducted at wavelengths of 254 nm for lidocaine and 277 nm for ciprofloxacin.
After the assay, the pH of the hydrated films was measured using pH indicator paper with a 5.5 to 9.0 range (Neutralit, Merck®). For comparison, the diffusion of aqueous solutions with an equivalent concentration of ciprofloxacin and lidocaine was used as reference. All these experiments were performed in triplicate.
Furthermore, the release profiles were compared using the difference factor (f1) and similarity factor (f2), calculated by Equations Equation (3), Equation (4), respectively.
| Equation (3) |
| Equation (4) |
where Rt and Tt are the percentages released at each time point for reference (R) and test (T) formulations. An f1 value above 15 and f2 value of between 0 and 49 implied a difference between the release profiles [33].
The drug release kinetics were investigated by applying Korsmeyer-Peppas model (log of cumulative percent of drug released vs log of time) up to 60 % of the total drugs release, 0.5 and 2 h for lidocaine and ciprofloxacin, respectively [34] according Equation (5):
| Equation (5) |
where Qt/Q∞ represents the fraction of drug released at time t, k is the release constant and n is the diffusional exponent. The value of n indicates the drug release mechanism, with n < 0.5 indicating Pseudo-Fickian diffusion, n = 0.5 indicating Fickian diffusion or first-order release, 0.5 < n < 1 indicating Anomalous (non-Fickian) transport and n = 1 indicating Case-II transport or zero-order release [35]. Additionally, the determination coefficient (R2) was used as an indicator of the level of fit of the data.
3. Results
3.1. Identification of quality target product profile (QTPP), critical quality attributes (CQA) and risk analysis
An optimal wound dressing should maintain a moist environment at the wound dressing interface, facilitate gaseous exchange, possess antimicrobial properties, exhibit biocompatibility, absorb moisture and exudate, and be easily removable without causing trauma [36]. Additionally, excipients and dosage forms should not interfere with wound healing [4]. The QTPP for the polymeric films, containing ciprofloxacin and lidocaine, intended for topical wound application, encompasses these desired characteristics, as outlined in Table 3. The CQAs are identified based on their potential impact on clinical efficacy and safety.
Table 3.
Quality Target Product Profile (QTPP) of wound dressings.
| QTPP parameters | Target | CQA | CQA justification |
|---|---|---|---|
| Route of administration | Topical (damaged skin, at any stage of the wound healing process) |
|
Physiological compatibility to allow regeneration |
| Dosage form design | Film containing ciprofloxacin and lidocaine |
|
Batch-to-batch reproducibility |
| Release profile | Modified | Rate and magnitude of the release of the APIs that allow the therapeutic purpose | Carrier system capable of releasing drugs and minimizing dressing changes |
| Physical attributes | Appearance and resistance |
|
Patient acceptance, minimally invasive wound application, permanence in wound site. |
The Ishikawa fish-bone diagram (Fig. 1) identified several high-risk factors that could potentially affect the final quality of the AA-films. Subsequently, these factors were further analyzed using a RAM (Fig. 2). Each risk factor was scored for its likelihood and severity, based on prior knowledge [19,37], literature reviews and preliminary hazard analysis studies. This approach aims to enhance safety, efficacy and quality of the pharmaceutical product.
Fig. 1.
Ishikawa fish-bone diagram illustrating risk-contributing factors in the development of AA-films.
Fig. 2.
Risk Assessment Matrix. The Total Risk value was obtaining according to Equation (1) to determine the level of risk associated with a particular CMA or CPP. Total risk was ranked in terms of its potential impact on product quality as high risk if the value exceeded 200 (red), medium risk if the values were between 100 and 200 (yellow) and low risk if the values were below 100 (green).
Weight variation and content uniformity were classified as low-risk due to the good reproducibility reported when films are prepared using solvent casting, supported by preliminary studies confirming drug solubility in the gels [37]. However, it is important to ensure that laboratory equipment, such as balances, is properly calibrated, and that the personnel involved in film preparation are adequately trained.
Drug release properties (magnitude and velocity) were regarded as having moderate-risk criticality. This is because, while they directly influence the therapeutic activity of the drugs, the efficacy of ciprofloxacin and lidocaine in the hydrogels has been previously demonstrated in appropriate in vitro/in vivo models [19,37]. Transparency and homogeneity were identified as high-risk CQAs, as they are critical for ensuring uniform drug distribution and significantly affect patient acceptance and wound assessment [4,38].
Folding capacity and handling were also considered high-risk CQAs due to their importance in the correct application and stability of the film on the wound site. Processing times and the logical sequence of operations were also considered moderate-risk CQAs because they can impact the reproducibility of the developed films.
Based on risk analysis, the CQA parameters that were deemed to have a high-risk impact on the quality of the films (transparency, homogeneity, folding capacity and handling) were selected to be the focus of the DoE. This approach aims to gain a better understanding of the associated risks and identify effective mitigation strategies.
Regarding the materials, the risk analysis indicated that SA, ciprofloxacin and lidocaine have a low-risk impact on the quality of films. In contrast, SH, Cb and glycerol were identified as having a medium-risk impact. Therefore, these materials were selected as the dependent variables (X1, X2 and X3 respectively) in the DoE. Additionally, the analysis showed that selecting ciprofloxacin and lidocaine as hydrochloride salts allows for both drugs to dissolve in a minimal volume of water during the preparation of the casting hydrogel. This choice reduces drying times and simplifies the sequence of operations.
Degassing and drying were identified as having a medium-risk impact on the quality of films. To mitigate these risks, a vacuum pump was used to control degassing parameters, and a calibrated oven with digital controls was employed for drying.
3.2. Design of experiments (DoE), films elaboration and quality analysis
The DoE was selected to further investigate the impact and effects of varying the above-mentioned parameters on the development of antibiotic-anesthetics films. As previously mentioned, the choice of drugs and polyelectrolytes was based on preliminary experiments and its capacity for acid-base interaction [19,37]. The concentration of SA was kept constant in all the formulations because, at this concentration, the drugs neutralize approximately 50 % of the SA equivalents, which likely facilitates the interaction between polyelectrolytes and drugs.
The solvent casting technique was found to be suitable for film development, providing a simple and reproducible process. The 24 hydrogel compositions tested produced continuous films with varying degrees of quality (Table 4).
Table 4.
Experimental runs, independent variables and measured responses of the experimental design (coded values).
| Run | Independent variables (X), (% p/p) |
Responses (Y) |
|||||
|---|---|---|---|---|---|---|---|
| SH (X1) | Cb (X2) | Glycerol (X3) | Transparency (Y1) | Homogeneity (Y2) | Folding capacity (Y3) | Handling (Y4) | |
| 1 | −1 | −1 | −1 | 1 | 5 | 1 | 2 |
| 2 | −1 | −1 | −0.6 | 1 | 5 | 1 | 2 |
| 3 | −1 | −1 | −0.2 | 2 | 5 | 2 | 3 |
| 4 | −1 | −1 | 0.2 | 4 | 5 | 3 | 3 |
| 5 | −1 | −1 | 0.6 | 5 | 5 | 3 | 3 |
| 6 | −1 | −1 | 1 | 5 | 5 | 4 | 4 |
| 7 | −0.5 | −1 | −1 | 1 | 5 | 2 | 2 |
| 8 | 0 | −1 | −1 | 1 | 4 | 2 | 2 |
| 9 | 1 | −1 | −1 | 1 | 2 | 2 | 2 |
| 10 | −0.5 | −1 | −0.2 | 3 | 5 | 2 | 3 |
| 11 | 0 | −1 | −0.2 | 3 | 4 | 2 | 3 |
| 12 | 1.0 | −1 | −0.2 | 3 | 3 | 2 | 3 |
| 13 | −0.5 | −1 | 0.2 | 5 | 5 | 2 | 3 |
| 14 | 0 | −1 | 0.2 | 5 | 4 | 2 | 3 |
| 15 | 1 | −1 | 0.2 | 5 | 3 | 2 | 3 |
| 16 | −0.5 | −1 | 0.6 | 5 | 5 | 3 | 3 |
| 17 | 0 | −1 | 0.6 | 5 | 4 | 3 | 3 |
| 18 | 1 | −1 | 0.6 | 5 | 3 | 3 | 3 |
| 19 | −1 | 0 | −0.2 | 3 | 3 | 5 | 5 |
| 20 | −1 | 1 | −0.2 | 3 | 5 | 5 | 5 |
| 21 | −1 | 0 | 0.2 | 3 | 3 | 5 | 5 |
| 22 | −1 | 1 | 0.2 | 3 | 5 | 5 | 5 |
| 23 | −1 | 0 | 0.6 | 3 | 3 | 5 | 5 |
| 24 | −1 | 1 | 0.6 | 3 | 5 | 5 | 5 |
Data from the DoE were used to build predictive regression models for each response variable, with statistical analysis confirming significant effects of the variables. The response variables were fitted to a three-factor interaction model (3FI) with high correlation, indicating robust explanation of experimental variations. The corresponding equations are presented in Table 5.
Table 5.
Regression and statistic results of the measured responses (coded values).
| Transparency (Y1) | Homogeneity (Y2) | Folding capacity (Y3) | Handling (Y4) | |
|---|---|---|---|---|
| P value | <0.0001 | 0.0072 | <0.0001 | <0.0001 |
| F model | 32.06 | 4.86 | 25.85 | 42.30 |
| Intercept | +3.46 | +3.63 | +3.72 | +4.00 |
| A (SH) | +0.39∗ | −0.97∗∗∗ | −0.13 | −0.09 |
| B (Cb) | −0.11 | −0.32 | +1.36∗∗∗ | +1.14∗∗∗ |
| C (Glycerol) | +1.17∗ | +0.17 | −0.73∗∗ | +0.73∗∗∗ |
| Interaction A-C | 0,21 | +0.39 | −0.46 | |
| Interaction B-C | −1.44∗∗ | −0.82 |
Positive coefficients in the equation indicate a positive influence of the corresponding variable on the predicted response. Conversely, negative coefficients show a negative influence on the predicted response. The significance levels of the coefficients are denoted by asterisks: ∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001.
The analysis revealed that SH positively influenced the transparency response. As expected, SH had a significantly negative impact in terms of the homogeneity response due to high molecular weight, increasing the system viscosity and hindering the removal of air from the hydrogel, compared to hydrogel formulations without SH. The same negative impact on homogeneity was observed for Cb.
On the other hand, glycerol had a positive influence on all the responses evaluated. As a plasticizer, its incorporation enhanced system flexibility [7,39,40], which improved demolding ease and folding capacity compared to formulations without it [40]. Additionally, glycerol increased film transparency, possibly due to residual moisture in the AA-films post-drying. However, glycerol and Cb showed a significant and negative interaction on transparency, which had an impact on the optimization of the final composition.
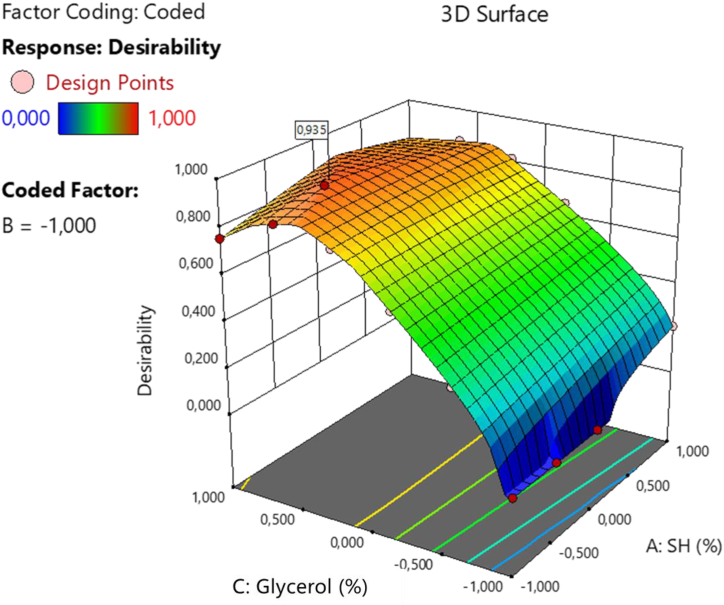
The design space was developed using mathematical models that evaluated correlations between variables and responses, facilitating formulation optimization. Fig. 3 shows the response surface graph generated through the desirability function based on the experimental design. This optimization process suggested 23 potential casting hydrogel compositions. One formulation, with a desirability value of 0.935 (on a scale of 0–1.00), was selected for its superior overall performance. This indicates a 93.5 % probability of meeting the target using the optimized CMAs and CPPs. The optimized formulation is composed of −0.41 SH, −1.00 Cb and 0.63 glycerol (coded values).
Fig. 3.
Desirability response surface plot.
To compare predicted and observed values in the preparation of films, three independent batches were fabricated using the optimized formulation. The quality analysis of the films was conducted according to Table 2. The practical values obtained for transparency (5), homogeneity (5), folding capacity (3) and handling (3) were found to be very close to the predicted Y values (Table 6). The mean of the data fell within the predicted interval with a 95 % confidence level (relative error minor than 5 %), demonstrating that the mathematical model effectively predicts the relationship between composition parameters and critical film attributes. This demonstrates the usefulness and reliability of the mathematical model in understanding and predicting the quality attributes of the films.
Table 6.
Confirmation of design model comparing the performance of the optimized formulation respect to predicted responses.
| Solution 1 of 34 Response | Predicted Mean | Predicted Median | Std Dev | n | 95 % PI low | Data Mean | 95 % PI high |
|---|---|---|---|---|---|---|---|
| Transparency | 5.00 | 5.00 | 0.542 | 3 | 4.23 | 5 | 5.77 |
| Homogeneity | 4.36 | 4.36 | 0.756 | 3 | 3.30 | 5 | 5.42 |
| Folding capacity | 3.00 | 3.00 | 0.592 | 3 | 2.17 | 3 | 3.83 |
| Handling | 3.37 | 3.37 | 0.431 | 3 | 2.76 | 3 | 3.97 |
PI: Predicted Interval.
3.3. Characterization of optimized formulation (AA-films)
3.3.1. Mechanical properties
The mechanical properties of film formulations are critical quality attributes not only during production but also for patient handling [41]. Films used for wound dressings must be flexible to adapt and protect the wound surface, preventing tearing, while also being resistant to stress during application, handling or storage. Therefore, these films should combine high strength, ductility and flexibility to accommodate skin movements [4,42,43]. The mechanical properties of the AA-films, including tensile strength, elongation at break and Young's modulus, are presented in Table 7.
Table 7.
AA-films mechanical properties.
| Parameter | Signification | Result | Reference value [29] |
|---|---|---|---|
| Tensile strength at break (MPa) | The maximum force applied to the films until tearing or breakage | 23.7 ± 0.5 | >2 |
| Elongation at break (%) | The maximum elongation of the films when force is applied | 59 ± 7 | >10 |
| Young's modulus (MPa) | The slope of the stress–strain curve in the elastic region, which defines the stiffness of the films | 37 ± 3 | <550 |
AA-films showed adequate flexibility related with elongation at break values. This result was associated to the presence of glycerol in adequate proportion that acted as a plasticizer through changes in glass transition temperature, increasing inter-intra molecular interactions such as hydrogen bonding between polymer and plasticizer and reducing the polymer-polymer interactions [44].
However, the AA-films also exhibited high mechanical strength, which can be attributed to the interactions between drugs and polyelectrolytes, with ciprofloxacin mainly acting as a cross-linker. The dimeric nature of zwitterionic ciprofloxacin results in amine groups at both ends of the dimer, which can contribute to the formation of non-covalent cross-linking points in the anionic polyelectrolytes, thus enhancing the mechanical strength [19].
3.3.2. Thickness and weight variation
The AA-films had a thickness of 181 ± 5 μm. A thickness less than that of human skin (0.5–2.0 mm) is generally acceptable, as it allows adequate fluid absorption while remaining thin enough for water vapor permeation [15]. The films weighed 477 ± 4 mg, and the low variation (CV% = 0.89) suggests that the manufacturing method ensures homogeneous drug distribution.
3.3.3. Optical microscopy
Fig. 4 presents optical microscopy images of the studied systems under both bright and polarized light. Ciprofloxacin and lidocaine, appear as crystalline and birefringent solids due to their anisotropic properties. The control films and AA-films displayed uniform structures with varying lattice arrangements under bright light. Moreover, under polarized light, the AA-films exhibited no birefringence of the drug crystals, indicating an increase in the drug's apparent solubility. This effect is attributed to the drug-polyelectrolyte interactions and the role of glycerol as a compatibilizer. Additionally, this observation is consistent with the transparency of the AA-films.
Fig. 4.
Microphotographs under bright light (top row) and polarized light (bottom row) of A) ciprofloxacin hydrochloride; B) lidocaine hydrochloride; C) C1-films; D) C-films; and E) AA-films. Magnification: 100×.
3.3.4. Scanning electron microscopy (SEM)
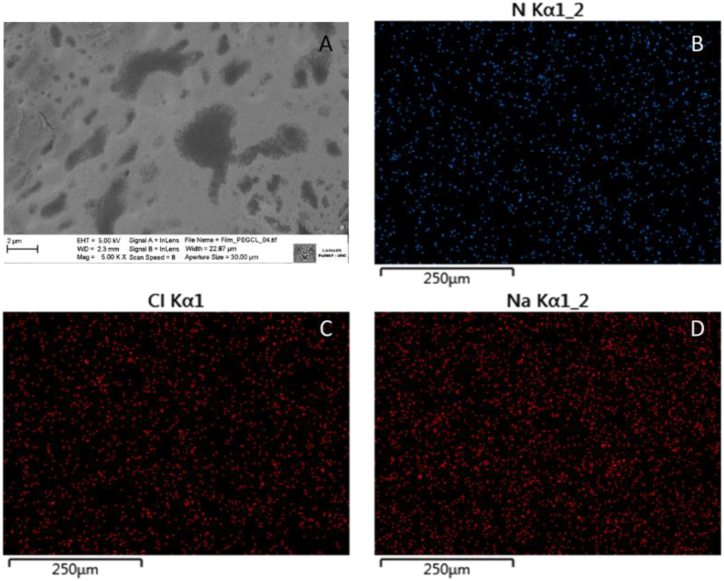
SEM images show that the AA-films have a continuous, rough surface with microregions of varying sizes, and no visible pinholes or cracks. This structural pattern is attributed to the cross-linked nature of the drug-polyelectrolyte complex present in the system [19]. Additionally, no solid drug precipitates were observed on the surface (Fig. 5A), suggesting that the drying process does not compromise the solubility of ciprofloxacin in the AA-film. Furthermore, the microdistribution of components within the films appeared uniform throughout, as evident from the examination of AA-films surfaces using EDS (Fig. 5B–D). This analysis demonstrated consistent relative brightness and colour intensity representing elements such as nitrogen, sodium and chloride present in the sample. It is worth noting that this method had previously been employed to assess content uniformity [45,46].
Fig. 5.
A) SEM of AA-films at 5000X. SEM-EDS of B) Nitrogen, C) Chlorine and D) Sodium atoms in AA-films.
3.3.5. Powder X-ray diffraction studies
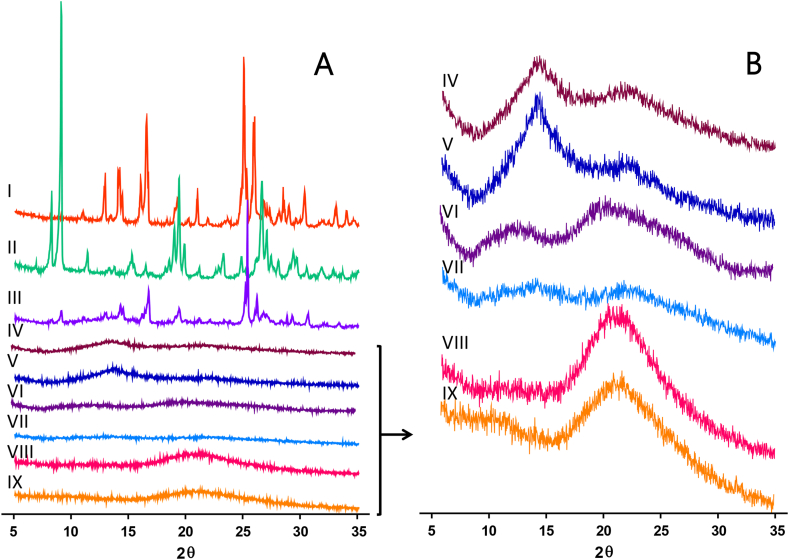
The X-ray diffraction patterns were recorded to understand the structure of the materials. As shown in Fig. 6A, both drugs, ciprofloxacin and lidocaine, exhibited crystalline patterns. The position and intensity of the reflections were comparable to previously published data [47,48]. In contrast, the polyelectrolytes (SA and SH) displayed characteristic amorphous halos, consistent with the literature [39,49]. Specifically, SA showed a well-defined halo around 14° 2θ and a diffuse one around 22° 2θ. SH exhibited two broad halos with intensity peaks at 11° 2θ and 20° 2θ. Moreover, the physical mixture of these two polyelectrolytes resulted in a pattern that was the proportional sum of each individual polyelectrolyte spectrum (Fig. 6B).
Fig. 6.
A) Diffractograms presented on the equal intensity scale for: I) lidocaine hydrochloride; II) ciprofloxacin hydrochloride; III) physical mixture of solids (lidocaine hydrochloride, ciprofloxacin hydrochloride, sodium alginate and sodium hyaluronate); IV) physical mixture of polyelectrolytes (sodium alginate and sodium hyaluronate); V) sodium alginate; VI) sodium hyaluronate; VII) C1-films; VIII) C-films and IX) AA-films. B) Diffractograms for amorphous solids or films are displayed at a higher intensity scale compared to those in part A).
To determine if material processing caused microstructural changes, the C1-films were evaluated. This membrane showed a different pattern from the physical mixture, indicating that during the formation of the macromolecular network—promoted by solvent evaporation during drying—a self-arrangement of the polymer chains occurred [39]. On the other hand, the C-film displayed an amorphous pattern, distinct from all previously observed, with a single halo at 21° 2θ. This suggests that the presence of glycerol appears to induce microstructural modifications by solvating the polyelectrolyte chains, thereby reducing inter-chain interactions and altering the amorphous regions of the polymers. Finally, the AA-films exhibited a pattern very similar to the C-films. However, the reflections typical of the crystalline structures of ciprofloxacin or lidocaine were not observed.
3.3.6. Thermogravimetric studies
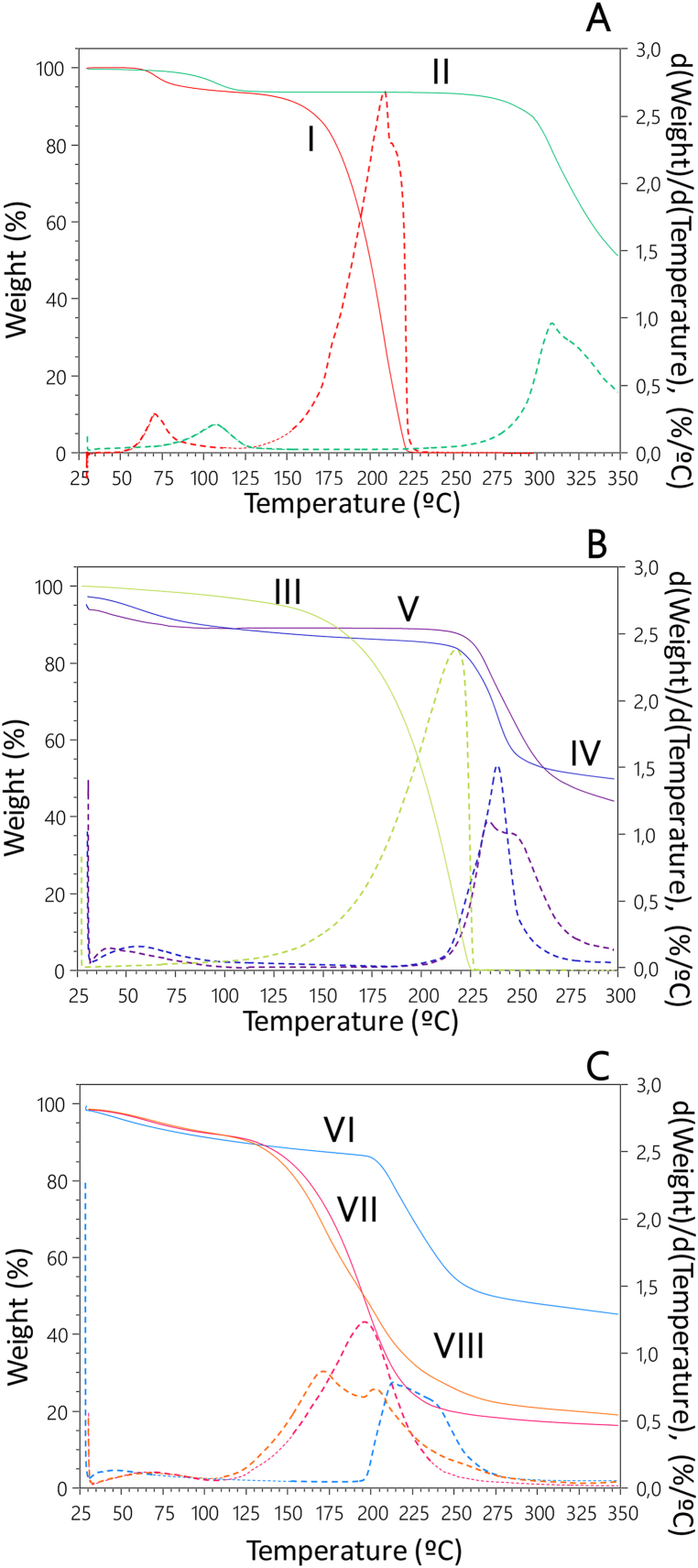
Fig. 7 shows the thermograms representing the weight loss of the materials during heating, along with the corresponding derivative thermogravimetry (DTG) curves as a function of temperature.
Fig. 7.
The thermograms depicting mass loss (%) as a function of temperature (°C) are shown with solid lines, while the first derivative of mass loss with respect to temperature is represented by dotted lines for A) drugs: I) lidocaine hydrochloride; II) ciprofloxacin hydrochloride; B) excipients: III) glycerol; IV) sodium alginate; V) sodium hyaluronate; and C) the films: VI) C1-films; VII) C-films and VIII) AA-films.
Ciprofloxacin exhibited an initial weight loss (4.7 %), associated with the release of crystallization water, corresponding to the monohydrate crystal form (starting at 88 °C and ending at 132 °C). A second weight loss (91.7 %) corresponded to the melting/decomposition temperature reported in the literature, with a maximum at 309 °C [50]. Similarly, lidocaine showed a weight loss (5.3 %) equivalent to one water molecule from the crystal (starting at 54 °C and ending at 95 °C). The final weight loss event (92 %, with a maximum at 208 °C) was due to its decomposition [51] (Fig. 7A).
The TGA profiles of SA and SH showed similar behavior. Both polyelectrolytes experienced an initial weight loss below 100 °C, mainly due to water evaporation (9.5 % and 10.8 %, respectively). A second stage of weight loss started at 215 °C for both polyelectrolytes; for SA, the maximum occurred at 238 °C, while for SH, a sharp peak at 234 °C was followed by a shoulder peak in the DTG curve. These second-stage weight losses were incomplete (48.2 % for SA and 53.1 % for SH) and corresponded to the first stage of thermal decomposition of the polyelectrolytes [43,52] (Fig. 7B).
Glycerol showed a gradual weight loss starting at 125 °C, corresponding to its decomposition process, with a maximum at 218 °C [39] (Fig. 7B).
Fig. 7C shows the thermal profiles of the studied membranes. The thermogram of C1-films was similar to that of the polyelectrolytes, with an initial weight loss (8.5 %) at temperatures below 100 °C. A second weight loss (51.7 %, starting at 195 °C) corresponded to the first stage of membrane decomposition, resulting from the combined degradation events of SA and SH.
On the other hand, the curve of the C-films showed an initial weight loss (8.3 %) related to membrane dehydration and a second weight loss (80.7 %) with a maximum at 196 °C, corresponding to the first stage of decomposition.
Finally, the thermal profile of AA-films did not significantly change compared to C-films after the addition of the drugs (8.3 % for the first weight loss and 78.5 % for the second).
The second stage of degradation of AA-films and C-films occurred at much lower temperatures than that of C1-films (starting around 110 °C). This difference was attributed to the presence of glycerol. Similar results were found by Gao et al. in membranes of SA and glycerin compared to membranes made solely from SA [39].
Moreover, all the membranes, like the polyelectrolytes, exhibited two stages of weight loss. However, the rate of weight loss in the first stage (water) was slower in the membranes compared to the starting materials. These differences might be due to the structural organization of the membrane components and the way water interacted with them [43].
3.3.7. In vitro drug release of ciprofloxacin and lidocaine
Franz diffusion cells were used to study the release properties of ciprofloxacin and lidocaine from AA-films, based on their proven reliability in simulating topical drug delivery. Although this method was initially used for gels, it has been extensively described for evaluating topical and transdermal films [[53], [54], [55]]. In these systems, the release process begins with the hydration and swelling of the polymer to form the gel, which then allows the drug to diffuse [32]. The receptor media was PBS solution (pH = 7.4), which allows simulate the properties of initial stages of wound [56]. Solutions of free drugs were used as references (Fig. 8).
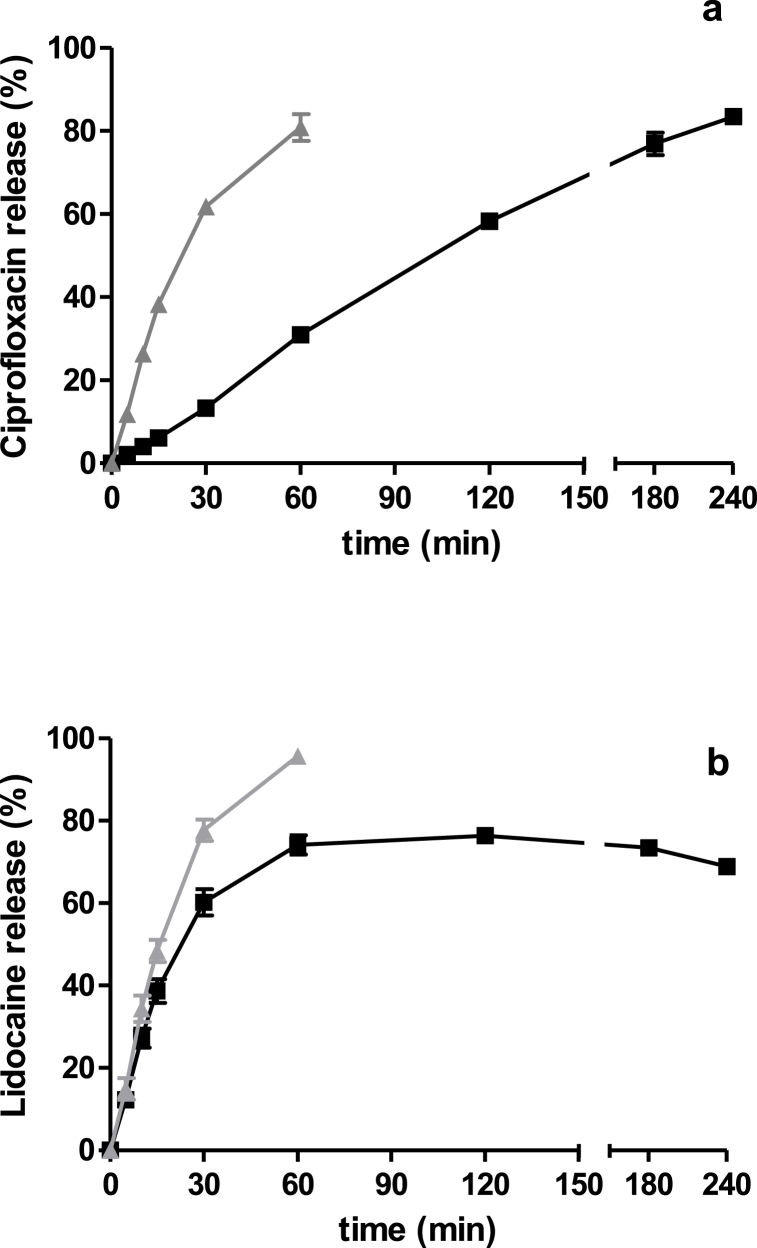
Fig. 8.
Amount released versus time of a) ciprofloxacin and b) lidocaine from the AA-films in PBS solution as receptor media (■). For comparison, the release profiles of lidocaine or ciprofloxacin references are shown (▲). The values are expressed as mean ± SD (n = 3).
Ciprofloxacin was slowly released from the AA-films compared to the reference aqueous solution (f1 = 74; f2 = 22), with a zero-order kinetic, since n was 1.070 (Table 8). After 4 h, over 80 % of ciprofloxacin was released, indicating the reversible interaction and its capability to play a therapeutic role when AA-films were administered on the surface of the skin wound.
Table 8.
Mathematical modeling of AA-films release parameters using Korsmeyer-Peppas equation.
| Drug | Drug released (240 min,%) | Korsmeyer-Peppas parameters |
||
|---|---|---|---|---|
| K | N | r2 | ||
| Ciprofloxacin | 83.5 | 1.454 | 1.070 | 0.998 |
| Lidocaine | 68.9 | 2.085 | 0.886 | 0.9767 |
Lidocaine exhibited significantly lower release from AA-films compared to its aqueous reference (f1 = 22; f2 = 43) and followed a non-Fickian transport (n was 0.886, Table 8). Additionally, the determination coefficient (R2) was used as an indicator of the level of fit of the data kinetics. However, lidocaine released much faster than ciprofloxacin, reaching 60.2 % of its initial concentration at 30 min.
4. Discussion
The DoE approach was efficient in demonstrating the appropriate correlation between variables and the significance of their interactions. The fractional mixed-level factorial design simplifies the experimental process and reduces the number of trials required compared to the traditional two-step approach. Moreover, it has been reported that this design offers greater parameter density, allowing for the capture of non-linearities, enhancing the robustness of regression models against variability and outliers, and enabling a more comprehensive exploration of the experimental space [26].
The outcomes obtained through the DoE highlight the significant influence of formulation variables on the properties of the films. Glycerol was identified as the primary factor responsible for increasing transparency and significantly impacting folding and handling properties. High glycerol concentrations result in sticky films, whereas low concentrations produce fragile films. Indeed, an antiplasticization effect using a plasticizer at low concentrations has been described [4]. These phenomena, linked to glycerol's ability to reduce polymer-polymer interactions and increase molecular mobility, have been extensively studied [[57], [58], [59], [60]]. Conversely, the presence of SH and Cb adversely affected the homogeneity of the films, potentially due to increased viscosity and difficulty in removing air bubbles [4]. In particular, Cb was found to be an incompatible polymer, as films containing it were opaque and exhibited poor mechanical properties. This finding is noteworthy because other studies have reported the successful development of hydrophilic films of Cb combined with egg white, where Cb improved the mechanical strength and crystallinity of the films [61].
The selection of acidic polyelectrolytes in their salt forms was based on their ability to interact with the basic groups of ciprofloxacin and lidocaine. Furthermore, the presence of drugs and polyelectrolytes in solution facilitates their spontaneous acid-base interaction (polyelectrolytes-drugs complexation) [19]. Similar phenomena have been described for other drugs-polyelectrolytes complexes [37,62]. This interaction is crucial for enhancing drug solubility and maintaining a stable amorphous system, positively impacting preparation time, operational sequence, and product quality. This makes the process more robust, straightforward, and cost-effective, which is particularly advantageous during scaling or transfer stages of formulation.
The mechanical properties of the optimized AA-films demonstrate adequate flexibility and high mechanical strength, attributable to non-covalent cross-linking facilitated by ciprofloxacin within the polyelectrolyte matrix. This characteristic is essential for the durability and effectiveness of the film during application. The mechanical properties align with the standards proposed for wound dressings, which should match the mechanical properties of human skin, which range from 5 to 30 MPa depending on the body region [15]. Further research suggests variability in skin tensile strength and Young's modulus due to factors like body region, collagen fiber orientation, age, and physio-pathological condition, with ranges for tensile strength between 1.74 and 42.5 MPa and Young's modulus between 0.01 and 57 MPa [63].
These findings underscore the significance of employing a macroscopic categorical score for assessing folding capacity and handling in the context of film quality analysis (CQA). This approach could be valuable in evaluating the mechanical properties of films intended for wound application, which are not yet standardized by the USP or the European Pharmacopoeia. Currently, only oromucosal films have established standards, emphasizing the importance of adequate mechanical strength to withstand handling without damage. This requirement ensures that films maintain their integrity during manufacturing, packaging, and patient use—criteria that should also apply to wound dressing films. In this context, Visser et al. [29] assessed the tensile strength, elongation at break, and Young's modulus of polymeric orodispersible films, including 3 commercially available ones, establishing threshold values aligning with European Pharmacopoeia requirements. The mechanical properties of AA-films fall within these ranges, as shown in Table 7.
Various factors influence the mechanical properties of films as components, including the type and amount of film-forming material, plasticizers, drugs, and solvents. Additionally, the manufacturing process and storage conditions play a significant role, as they impact the microstructure of the film network and the intermolecular forces between their components [41].
Ciprofloxacin's cross-linking role in the films helps maintain mechanical resistance even when hydrated, offering advantages over non-cross-linked systems and preventing drug precipitation or crystallization, ensuring film transparency for wound inspection [4,33].
Controlled ciprofloxacin release enhances therapeutic efficacy [19], but it is crucial to consider antibiotic resistance risks due to overuse and misuse. Further research is needed to ensure the safety and effectiveness of antibiotic films in wound healing process [4].
Lidocaine, a smaller molecule, demonstrated rapid release. Considering that the doses used are commonly employed for topical applications, this performance could offer a rapid pain relief, which is crucial because dressing changes can be extremely painful for patients [64]. Indeed, recent studies on a hydrogel containing ciprofloxacin, lidocaine, and Cb, with a similar lidocaine release profile, demonstrated an anesthetic effect of lidocaine within 5 min, highlighting its efficacy even with controlled release [37].
It is known that AA-films drug delivery can be sensitive to various external factors, as pH, temperature, ionic strength, among others [65]. In the case of diffusion towards injured skin, it can be triggered by the presence of wound exudate. Thus, a moist wound could play a critical role in facilitating drug release.
An important aspect to highlight is that the stability and sterility of the AA-films must be ensured during their shelf life. In this regard, the photosensitivity of ciprofloxacin in the system should be studied. Additionally, the required dose of gamma radiation to exert its sterilizing effect without causing damage to the material should be investigated. Therefore, it is crucial for the primary packaging to be hermetic and light-resistant.
Furthermore, validating the efficacy of AA-films through in vivo wound healing models, which should include an assessment of their antimicrobial effect in infected wounds, and conducting clinical studies holds significant value. These steps are essential to ensure the quality and safety of these films for broader clinical applications.
The lack of standardized methods and regulatory guidelines for characterizing films, a promising dosage form with considerable clinical potential, poses a significant challenge. Without specific standards or pharmacopeial methods, the quality control of wound dressings, which are an essential application of films, becomes a complex and uncertain process [13]. This issue highlights the pressing need to explore, define, and establish clear specifications and quality attributes for these innovative medical products.
The safety and efficacy of films have been evaluated by various research groups through different quality attributes, focusing on factors such as strength, flexibility, and appearance [4,13,15]. While this is an important step in assessing their viability, it is crucial to define acceptable quality parameters that can be consistently met, even as the production of these films scales up to large manufacturing processes. Further research particularly clinical studies, is necessary to confirm their effectiveness in real-world settings and ensure their safety and usefulness across diverse patient groups [4].
5. Conclusions
The findings of this research provide a valuable foundation for establishing the quality criteria and standards that should guide the use of biopolymeric films as wound dressings. Qualitatively, the CQAs of transparency, homogeneity, folding capacity and ease of handling were identified as the most significant characteristics of these films. The composition of the AA-films was observed to have a substantial impact on these defined CQAs, with SH positively influencing transparency while negatively affecting homogeneity due to its high molecular weight. Glycerol contributed to flexibility and ease of demoulding, and Cb improved folding capacity and handling, although a negative interaction with glycerol.
Furthermore, the strong predictive capabilities of the mathematical model developed during the experimentation phase demonstrated the effective relationship between various factors and evaluation indexes. The optimized AA-films formulation confirmed that the films were transparent, featured a homogeneously distributed content, had an appropriate thickness for topical application and displayed uniform weight. Moreover, the sustained in vitro drug release could be appropriated for topical treatment of wounds.
This study lays the foundation for quality standards, enhancing the effectiveness and reliability of these films in wound care, benefiting healthcare professionals and patients. The knowledge acquired here is essential for advancing the field further.
CRediT authorship contribution statement
María Florencia Sanchez: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Laura Carolina Luciani-Giacobbe: Writing – review & editing, Writing – original draft, Software, Methodology, Formal analysis, Data curation. Fiamma Barbieri: Investigation, Methodology, Writing – original draft. María Eugenia Olivera: Writing – review & editing, Visualization, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Data availability statement
Most of the results and data are provided within the publication. However, certain additional data are protected due to a pending patent application.
Declaration of generative AI in scientific writing
During the preparation of this work the authors used ChatGTP in order to improve readability and language. After using this tool, the authors reviewed and edited the content as needed and takes full responsibility for the content of the publication.
Funding information
This work was supported by the Fondo para la Investigación Científica y Tecnológica (FonCyT, grant number PICT 2015-3331 and PICT 2020-1309), and the Universidad Nacional de Córdoba (Consolidar III 2018–2022 SECYT-UNC, grant number 411/2018).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Authors wish to acknowledge the assistance of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Universidad Nacional de Córdoba both of which provided support and facilities for this investigation. The authors extend special thanks to Paul Hobson, a native English speaker, for his assistance in reviewing the manuscript.
References
- 1.World Health Organization (WHO) 2018. Burns, Fact Sheet N°365. [Google Scholar]
- 2.Devalliere J., Dooley K., Hu Y., Kelangi S.S., Uygun B.E., Yarmush M.L. Co-delivery of a growth factor and a tissue-protective molecule using elastin biopolymers accelerates wound healing in diabetic mice. Biomaterials. 2017;141:149–160. doi: 10.1016/j.biomaterials.2017.06.043. [DOI] [PubMed] [Google Scholar]
- 3.Chantre C.O., Campbell P.H., Golecki H.M., Buganza A.T., Capulli A.K., Deravi L.F., Dauth S., Sheehy S.P., Paten J.A., Gledhill K., Doucet Y.S., Abaci H.E., Ahn S., Pope B.D., Ruberti J.W., Hoerstrup S.P., Christiano A.M., Parker K.K. Production-scale fibronectin nanofibers promote wound closure and tissue repair in a dermal mouse model. Biomaterials. 2018;166:96–108. doi: 10.1016/j.biomaterials.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borbolla-Jiménez F.V., Peña-Corona S.I., Farah S.J., Jiménez-Valdés M.T., Pineda-Pérez E., Romero-Montero A., Del Prado-Audelo M.L., Bernal-Chávez S.A., Magaña J.J., Leyva-Gómez G. Films for wound healing fabricated using a solvent casting technique. Pharmaceutics. 2023;15:1–27. doi: 10.3390/pharmaceutics15071914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pita-López M.L., Fletes-Vargas G., Espinosa-Andrews H., Rodríguez-Rodríguez R. Physically cross-linked chitosan-based hydrogels for tissue engineering applications: a state-of-the-art review. Eur. Polym. J. 2021;145 doi: 10.1016/j.eurpolymj.2020.110176. [DOI] [Google Scholar]
- 6.Wang Q., Zuo Z., Cheung C.K.C., Leung S.S.Y. Updates on thermosensitive hydrogel for nasal, ocular and cutaneous delivery. Int. J. Pharm. 2019;559:86–101. doi: 10.1016/j.ijpharm.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Karki S., Kim H., Na S.-J.J., Shin D., Jo K., Lee J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016;11:559–574. doi: 10.1016/j.ajps.2016.05.004. [DOI] [Google Scholar]
- 8.Bassi P., Kaur G. Polymeric films as a promising carrier for bioadhesive drug delivery: development, characterization and optimization. Saudi Pharm. J. 2017;25:32–43. doi: 10.1016/J.JSPS.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colobatiu L., Gavan A., Mocan A., Bogdan C., Mirel S., Tomuta I. Development of bioactive compounds-loaded chitosan films by using a QbD approach – a novel and potential wound dressing material. React. Funct. Polym. 2019;138:46–54. doi: 10.1016/j.reactfunctpolym.2019.02.013. [DOI] [Google Scholar]
- 10.Musazzi U.M., Khalid G.M., Selmin F., Minghetti P., Cilurzo F. Trends in the production methods of orodispersible films. Int. J. Pharm. 2020;576 doi: 10.1016/j.ijpharm.2019.118963. [DOI] [PubMed] [Google Scholar]
- 11.Visser J.C., Woerdenbag H.J., Crediet S., Gerrits E., Lesschen M.A., Hinrichs W.L.J., Breitkreutz J., Frijlink H.W. Orodispersible films in individualized pharmacotherapy: the development of a formulation for pharmacy preparations. Int. J. Pharm. 2015;478:155–163. doi: 10.1016/J.IJPHARM.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Sanjarnia P., Picchio M.L., Polegre A.N., Schuhladen K., Fliss P.M., Politakos N., Metterhausen L., Calder M., Osorio-blanco E.R. Bringing innovative wound care polymer materials to the market: challenges, developments, and new trends. Adv. Drug Deliv. Rev. 2024;207 doi: 10.1016/j.addr.2024.115217. [DOI] [PubMed] [Google Scholar]
- 13.Savencu I., Iurian S., Porfire A., Bogdan C., Tomuță I. Review of advances in polymeric wound dressing films. React. Funct. Polym. 2021;168 doi: 10.1016/j.reactfunctpolym.2021.105059. [DOI] [Google Scholar]
- 14.Hodge J.G., Zamierowski D.S., Robinson J.L., Mellott A.J. Evaluating polymeric biomaterials to improve next generation wound dressing design. BioMed Central. 2022 doi: 10.1186/s40824-022-00291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bombaldi de Souza R.F., Bombaldi de Souza F.C., Bierhalz A.C.K., Pires A.L.R., Moraes Â.M. Biopolymer-based films and membranes as wound dressings. Biopolym. Membr. Film. Heal. Food, Environ. Energy Appl. 2020:165–194. doi: 10.1016/B978-0-12-818134-8.00007-9. [DOI] [Google Scholar]
- 16.Silva B.M.A., Vicente S., Cunha S., Coelho J.F.J., Silva C., Reis M.S., Simões S. Retrospective Quality by Design (rQbD) applied to the optimization of orodispersible films. Int. J. Pharm. 2017;528:655–663. doi: 10.1016/j.ijpharm.2017.06.054. [DOI] [PubMed] [Google Scholar]
- 17.ICH (International Conference on Harmonization) 2009. Pharmaceutical Development Q8(R2), ICH Harmon. Tripart. Guidel; pp. 1–28. [Google Scholar]
- 18.Csóka I., Pallagi E., Paál T.L. Extension of quality-by-design concept to the early development phase of pharmaceutical R&D processes. Drug Discov. Today. 2018;23:1340–1343. doi: 10.1016/J.DRUDIS.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Sanchez M.F., Guzman M.L., Apas A.L., Alovero F. del L., Olivera M.E. Sustained dual release of ciprofloxacin and lidocaine from ionic exchange responding film based on alginate and hyaluronate for wound healing. Eur. J. Pharm. Sci. 2021 doi: 10.1016/j.ejps.2021.105789. [DOI] [PubMed] [Google Scholar]
- 20.Olivera M.E., Sanchez M.F. Membrana hidrocoloide cicatrizante, transparente, biocompatible de uso terapéutico y su proceso de obtención. Solicitud de Patente Argentina. Presentación: 08/19. Examen preliminar técnico aprobado (09/20) Nro de acta 20190102232. 2019 https://link.lens.org/pNP4qiqz6Tk Publicado en Boletín 1135 (02/2021), The patent number is AR115740A1. [Google Scholar]
- 21.Sanchez M.F., Guzman M.L., Flores-Martín J., Cruz Del Puerto M., Laino C., Soria E.A., Donadio A.C., Genti-Raimondi S., Olivera M.E. Ionic complexation improves wound healing in deep second-degree burns and reduces in-vitro ciprofloxacin cytotoxicity in fibroblasts. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19969-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ICH Expert Working Group, Q8(R2) vol. 8. 2009. pp. 1–29. (Pharmaceutical Development). [Google Scholar]
- 23.ICH Expert Working Group Q9 Quality Risk Management. 2005:1–23. doi: 10.1007/s11095-007-9511-1. [DOI] [Google Scholar]
- 24.Saleme H.R., Cuadro Julio E.J., Mora Huertas C.E. Aplicación de la calidad basada en el diseño (QbD) en la reformulación de tabletas masticables. Rev. Colomb. Cienc. Quím. Farm. 2013;42:190–214. [Google Scholar]
- 25.Brindle A., Davy S., Tiffany D., Watts C. Risk analysis and mitigation matrix (ramm) – a risk tool for quality management. Pharm. Eng. 2012;32:1–19. [Google Scholar]
- 26.Grangeia H.B., Silva C., Simões S.P., Reis M.S. Quality by design in pharmaceutical manufacturing: a systematic review of current status, challenges and future perspectives. Eur. J. Pharm. Biopharm. 2020;147:19–37. doi: 10.1016/j.ejpb.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi Y., Ikeda N., Tahara K., Takeuchi H. Mechanical characteristics of orally disintegrating films: comparison of folding endurance and tensile properties. Int. J. Pharm. 2020;589 doi: 10.1016/j.ijpharm.2020.119876. [DOI] [PubMed] [Google Scholar]
- 28.American Society of Testing Materials (ASTM) Annu. B. ASTM Stand. 2002. D 882: standard test method for tensile properties of thin plastic sheeting. Philadelphia, USA. [Google Scholar]
- 29.Visser J.C., Dohmen W.M.C., Hinrichs W.L.J., Breitkreutz J., Frijlink H.W., Woerdenbag H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015;485:70–76. doi: 10.1016/j.ijpharm.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Baena Y., Dallos L.J., Manzo R.H., Ponce D’León L.F. Estandarización de celdas de Franz para la realización de ensayos de liberación de fármacos a partir de complejos con polielectrolitos Resumen Standardization of Franz cells to evaluate drugs release from drug- Introducción. Rev. Colomb. Cienc. Quím. Farm. 2011;40:174–188. [Google Scholar]
- 31.Ng S., Rouse J.J., Sanderson F.D., Meidan V., Eccleston G.M. Validation of a Static Franz Diffusion Cell System for In Vitro Permeation Studies. 2010;11:1432–1441. doi: 10.1208/s12249-010-9522-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maver T., Maver U. Importance of Franz diffusion cell volume for in vitro drug release testing of wound dressings. ACTA MEDICO-BIOTECHNICA. 2022;15:41–48. [Google Scholar]
- 33.Guzman M.L., Romañuk C.B., Sanchez M.F., Giacobbe L.C.L. Urinary excretion of ciprofloxacin after administration of extended release tablets in healthy volunteers . Swellable drug-polyelectrolyte matrix versus bilayer tablets. Drug Deliv. Transl. Res. 2017;8:123–131. doi: 10.1007/s13346-017-0442-z. [DOI] [PubMed] [Google Scholar]
- 34.Dash S., Murthy P.N., Nath L., Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. 2010;67:217–223. [PubMed] [Google Scholar]
- 35.Vigata M., Meinert C., Hutmacher D.W., Bock N. Hydrogels as drug delivery systems: a review of current characterization and evaluation techniques. Pharmaceutics. 2020;12 doi: 10.3390/pharmaceutics12121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Colobatiu L., Gavan A., Potarniche A.-V., Rus V., Diaconeasa Z., Mocan A., Tomuta I., Mirel S., Mihaiu M. Evaluation of bioactive compounds-loaded chitosan films as a novel and potential diabetic wound dressing material. React. Funct. Polym. 2019;145 doi: 10.1016/j.reactfunctpolym.2019.104369. [DOI] [Google Scholar]
- 37.Sanchez M.F., Breda S.A., Soria E.A., Tártara L.I., Manzo R.H., Olivera M.E. Ciprofloxacin-lidocaine-based hydrogel: development, characterization, and in vivo evaluation in a second-degree burn model. Drug Deliv. Transl. Res. 2018;8:1000–1013. doi: 10.1007/s13346-018-0523-7. [DOI] [PubMed] [Google Scholar]
- 38.Kathe K., Kathpalia H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017;12:487–497. doi: 10.1016/j.ajps.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gao C., Pollet E., Avérous L. Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll. 2017;63:414–420. doi: 10.1016/j.foodhyd.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Mazumder S., Pavurala N., Manda P., Xu X., Cruz C.N., Krishnaiah Y.S.R. Quality by Design approach for studying the impact of formulation and process variables on product quality of oral disintegrating films. Int. J. Pharm. 2017;527:151–160. doi: 10.1016/j.ijpharm.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 41.Preis M., Knop K., Breitkreutz J. Mechanical strength test for orodispersible and buccal films. Int. J. Pharm. 2014;461:22–29. doi: 10.1016/j.ijpharm.2013.11.033. [DOI] [PubMed] [Google Scholar]
- 42.Singh B., Sharma S., Dhiman A. Design of antibiotic containing hydrogel wound dressings: biomedical properties and histological study of wound healing. Int. J. Pharm. 2013;457:82–91. doi: 10.1016/J.IJPHARM.2013.09.028. [DOI] [PubMed] [Google Scholar]
- 43.Rezvanian M., Ahmad N., Mohd Amin M.C.I., Ng S., Cairul M., Mohd I., Ng S. Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int. J. Biol. Macromol. 2017;97:131–140. doi: 10.1016/j.ijbiomac.2016.12.079. [DOI] [PubMed] [Google Scholar]
- 44.Vuddanda P.R., Montenegro-Nicolini M., Morales J.O., Velaga S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017;96:290–298. doi: 10.1016/j.ejps.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Yassue C., Ordeiro P.H., Zandonai H., Genesi B.P. Development of chitosan/silver sulfadiazine/zeolite composite films for wound dressing. Pharmaceutics. 2019;11:1–23. doi: 10.3390/pharmaceutics11100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambrogi V., Pietrella D., Donnadio A., Latterini L., Di Michele A., Luffarelli I., Ricci M. Biocompatible alginate silica supported silver nanoparticles composite films for wound dressing with antibiofilm activity. Mater. Sci. Eng. C. 2020;112 doi: 10.1016/j.msec.2020.110863. [DOI] [PubMed] [Google Scholar]
- 47.Wang Q., Zhang N., Hu X., Yang J., Du Y. Chitosan/polyethylene glycol blend fibers and their properties for drug controlled release. J. Biomed. Mater. Res. Part A. 2007;85A:881–887. doi: 10.1002/jbm.a.31544. [DOI] [PubMed] [Google Scholar]
- 48.Tomoda K., Asahiyama M., Ohtsuki E., Nakajima T., Terada H., Kanebako M., Inagi T., Makino K. Preparation and properties of carrageenan microspheres containing allopurinol and local anesthetic agents for the treatment of oral mucositis. Colloids Surfaces B Biointerfaces. 2009;71:27–35. doi: 10.1016/J.COLSURFB.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Trastullo R., Abruzzo A., Saladini B., Gallucci M.C., Cerchiara T., Luppi B., Bigucci F. Design and evaluation of buccal films as paediatric dosage form for transmucosal delivery of ondansetron. Eur. J. Pharm. Biopharm. 2016;105:115–121. doi: 10.1016/J.EJPB.2016.05.026. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y., Wang J., Yin Q. The crystal habit of ciprofloxacin hydrochloride monohydrate crystal. J. Cryst. Growth. 2005;276:237–242. doi: 10.1016/j.jcrysgro.2004.11.323. [DOI] [Google Scholar]
- 51.Kevadiya B.D., Joshi G.V., Mody H.M., Bajaj H.C. Biopolymer-clay hydrogel composites as drug carrier: host-guest intercalation and in vitro release study of lidocaine hydrochloride. Appl. Clay Sci. 2011;52:364–367. doi: 10.1016/j.clay.2011.03.017. [DOI] [Google Scholar]
- 52.Coimbra P., Alves P., Valente T.A.M., Santos R., Correia I.J., Ferreira P. Sodium hyaluronate/chitosan polyelectrolyte complex scaffolds for dental pulp regeneration: synthesis and characterization. Int. J. Biol. Macromol. 2011;49:573–579. doi: 10.1016/j.ijbiomac.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 53.Kumar M., Sharma A., Mahmood S., Thakur A., Mirza M.A., Bhatia A. Franz diffusion cell and its implication in skin permeation studies. J. Dispers. Sci. Technol. 2024;45:943–956. doi: 10.1080/01932691.2023.2188923. [DOI] [Google Scholar]
- 54.Pleguezuelos-Villa M., Nácher A., Hernández M.J., Busó M.A.O.V., Barrachina M., Peñalver N., Díez-Sales O. A novel lidocaine hydrochloride mucoadhesive films for periodontal diseases. J. Mater. Sci. Mater. Med. 2019;30 doi: 10.1007/s10856-018-6213-7. [DOI] [PubMed] [Google Scholar]
- 55.Lieb S., Szeimies R.M., Lee G. Self-adhesive thin films for topical delivery of 5-aminolevulinic acid. Eur. J. Pharm. Biopharm. 2002;53:99–106. doi: 10.1016/S0939-6411(01)00193-X. [DOI] [PubMed] [Google Scholar]
- 56.Gethin G. The significance of surface pH in chronic wounds. Wounds U. K. 2007;3:52–56. [Google Scholar]
- 57.Giz A.S., Berberoglu M., Bener S., Aydelik-Ayazoglu S., Bayraktar H., Alaca B.E., Catalgil-Giz H. A detailed investigation of the effect of calcium crosslinking and glycerol plasticizing on the physical properties of alginate films. Int. J. Biol. Macromol. 2020;148:49–55. doi: 10.1016/j.ijbiomac.2020.01.103. [DOI] [PubMed] [Google Scholar]
- 58.Verčimáková K., Karbowniczek J., Sedlář M., Stachewicz U., Vojtová L. The role of glycerol in manufacturing freeze-dried chitosan and cellulose foams for mechanically stable scaffolds in skin tissue engineering. Int. J. Biol. Macromol. 2024;275 doi: 10.1016/j.ijbiomac.2024.133602. [DOI] [PubMed] [Google Scholar]
- 59.Visser J.C., Dohmen W.M.C., Hinrichs W.L.J., Breitkreutz J., Frijlink H.W., Woerdenbag H.J. Quality by design approach for optimizing the formulation and physical properties of extemporaneously prepared orodispersible films. Int. J. Pharm. 2015;485:70–76. doi: 10.1016/j.ijpharm.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 60.Ben Z.Y., Samsudin H., Yhaya M.F. Glycerol: its properties, polymer synthesis, and applications in starch based films. Eur. Polym. J. 2022;175 doi: 10.1016/j.eurpolymj.2022.111377. [DOI] [Google Scholar]
- 61.Rezaei S., Imani R. Highly absorbent egg white carbomer‐940 hydrofilm as a potential diabetic wound dressing. Macromol. Biosci. 2024 doi: 10.1002/mabi.202300353. [DOI] [PubMed] [Google Scholar]
- 62.Olivera M.E., Manzo R.H., Alovero F., Jimenez-Kairuz A.F., V Ramírez-Rigo M. In: Nanostructures Oral Med. Andronescu E., Grumezescu A.M., editors. Elsevier; 2017. Chapter 13 - polyelectrolyte-drug ionic complexes as nanostructured drug carriers to design solid and liquid oral delivery systems; pp. 365–408. [DOI] [Google Scholar]
- 63.Joodaki H., Panzer M.B. Skin mechanical properties and modeling: a review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018;232:323–343. doi: 10.1177/0954411918759801. [DOI] [PubMed] [Google Scholar]
- 64.Day R.M. In: Adv. Wound Repair Ther. Farrar D., editor. Woodhead Publishing; 2011. 6 - functional requirements of wound repair biomaterials; pp. 155–173. [DOI] [Google Scholar]
- 65.Rani Raju N., Silina E., Stupin V., Manturova N., Chidambaram S.B., Achar R.R. Multifunctional and smart wound dressings-A review on recent research advancements in skin regenerative medicine. Pharmaceutics. 2022;14 doi: 10.3390/pharmaceutics14081574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Most of the results and data are provided within the publication. However, certain additional data are protected due to a pending patent application.