Abstract
C57BL/6 mice were orally immunized with five weekly doses of 2 mg, 200 μg, or 2 μg of Helicobacter pylori (Sydney strain) whole-cell sonicate combined with cholera toxin. One week after the last vaccination, mice were challenged with 5 × 107 CFU of live H. pylori three times at 2-day intervals. At 6 or 18 weeks after the challenge, mice were sacrificed and bacterial cultures and histological studies of the stomach were performed. Vaccination with 2 mg/session or 200 μg/session inhibited H. pylori colonization by 90 and 100%, respectively. These mice were considered protected. Lower levels of H. pylori-specific immunoglobulin A (IgA) were detected in fecal and saliva samples before challenge. However, a significant increase in IgA secretion in mucosal tissue and a higher labeling index for IgA-positive lumina of pyloric glands were noted in these mice in response to challenge and in a vaccine dose-dependent manner. In protected mice, however, severe gastritis characterized by marked infiltration of inflammation mononuclear cells was noted at 6 weeks after challenge, compared with the gastritis seen in unprotected mice or nonvaccinated, ordinarily infected mice. Marked expression of gamma interferon mRNA was detected in the stomach of all protected mice, and 50% of these mice expressed interleukin 4 (IL-4) or IL-5 mRNA. Our findings suggest that local secretory IgA antibody and severe postimmunization gastritis correlate well with protection of mice against H. pylori infection.
Chronic infection caused by Helicobacter pylori is thought to be associated with chronic active gastritis, peptic ulcer, and gastric malignancies, such as mucosa-associated B-cell lymphoma and adenocarcinoma (2, 3, 21, 22). Furthermore, this organism was recently categorized as a class I carcinogen by the World Health Organization (6), and direct evidence of carcinogenesis was recently demonstrated in an animal model (19). Although serological studies have demonstrated H. pylori infection in approximately half of the world’s population, it is still not clear how H. pylori can induce such long-term infection.
Eradication of chronic H. pylori infection with antibiotics markedly alters the natural history of gastroduodenal diseases and reduces clinical symptoms. However, there are several problems with antimicrobial therapy, such as side effects related to the use of a high dose of antibiotics and the emergence of resistant strains of H. pylori (11). Therefore, the development of a prophylactic vaccine might be an attractive strategy against H. pylori infection, especially in young children.
Using a variety of animal models, several investigators have reported that the use of a prophylactic vaccine of crude or purified H. pylori antigen induces protective immune responses against infection with Helicobacter species (1, 8, 10, 13, 16). The Helicobacter felis model in mice has provided several important data. First, the use of a whole-cell sonicate or recombinant protein as an oral immunogen prevents H. felis colonization (10, 16). Second, infection with H. felis induces antigen-specific cellular immune responses manifested by type 1 helper T (Th1) cells, although a type 2 helper T (Th2)-cell response is also involved in protection against H. felis challenge (13).
In the present study, we attempted to delineate the possible mechanisms of protection induced against H. pylori by oral vaccination with a mouse model of H. pylori infection.
MATERIALS AND METHODS
Animals.
Specific-pathogen-free 6-week-old female C57BL/6 mice were obtained from Seac Yoshitomi (Fukuoka, Japan). Mice were housed in a specific-pathogen-free environment and were provided with free access to food and water. Experiments were performed according to the guidelines of the Ethical Committee for Animal Experiments at Oita Medical University, Oita, Japan.
Bacterial strain and preparation of H. pylori antigen.
The Sydney strain of H. pylori, which was kindly provided by A. Lee (School of Microbiology and Immunology, University of New South Wales, Sydney, New South Wales, Australia) (9), was grown in brucella broth containing 10% horse serum under microaerobic conditions (5% O2, 10% CO2, 85% N2) at 37°C. H. pylori sonicated antigen was prepared as described previously (16). Briefly, cultures were centrifuged at 1,000 × g for 10 min. The pellet was washed in phosphate-buffered saline (PBS), and cells were disrupted by sonication. After centrifugation at 1,000 × g for 10 min, the supernatant was collected, the protein concentration was determined, and the supernatant was frozen at −80°C until use.
Vaccination and challenge of mice.
Mice were divided into four groups: groups 1 to 3, oral vaccination with cholera toxin (5 μg) as an adjuvant and H. pylori whole-cell sonicate at a dose of 2 mg (group 1, n = 20), 200 μg (group 2, n = 20), or 2 μg (group 3, n = 20), respectively (vaccinated/challenged groups); and group 4 (n = 20), ordinary infection without vaccination (nonvaccinated/infected group). Vaccination was repeated at weekly intervals for 5 weeks with the same doses of H. pylori as those listed above. One week after the last vaccination, blood, fecal, and saliva samples were collected to monitor the immune response and were stored at −80°C until use. One week after the last vaccination, all mice were challenged with 0.5 ml of live H. pylori (5 × 107 CFU/ml) three times at 2-day intervals.
Assessment of H. pylori in gastric tissue.
At 6 or 18 weeks after the last challenge, all mice were sacrificed and the stomach was isolated for examination for H. pylori, histological examination, and determination of cytokine expression by reverse transcription (RT)-PCR. The stomach was washed in sterile 0.8% NaCl and cut longitudinally into two pieces. One half was used for bacterial examination, while the other was used for histological examination and determination of cytokine expression.
Examination for H. pylori.
Immediately after homogenization of the stomach specimens, they were smeared on 7% sheep blood agar (basic medium, Mueller-Hinton agar; BBL Microbiology Systems, Cockeysville, Md.) and Belo-Horizonte medium and incubated at 37°C for 4 days under microaerobic conditions. The presence of H. pylori in gastric tissue sections was also examined after Gram staining.
Histological examination of gastric mucosa.
Longitudinal sections of gastric tissues from the esophageal-cardiac junction to the duodenum were fixed with neutral buffered 10% formalin and embedded in paraffin. Five-micrometer sections were stained with hematoxylin-eosin (HE) and Giemsa stains. Gastric sections were examined in a blinded fashion by two independent examiners, who provided an assessment of the overall grade of inflammation (on a scale of 0 to 7), which was expressed as a sum of the overall intensity and extent of inflammation. The intensity of inflammation was scored on a scale of 0 to 3 based on criteria modified slightly from those described by Mohammadi et al. (13): grade 0, rare inflammatory cells; grade 1, mild; grade 2, moderate; and grade 3, severe. The extent of inflammation was scored on a scale of 0 to 4 based on the percentage of inflammatory cell infiltration of the mucosal surface (13): grade 0, none; grade 1, <25%; grade 2, 25 to 50%; grade 3, 50 to 75%; and grade 4, >75%.
Immunohistochemical staining with secretory IgA antibody in gastric tissue.
For immunohistochemical studies, 5-μm-thick paraffin sections were preincubated with normal goat serum diluted 1:9 for 10 min at room temperature. Sections were then stained with rabbit anti-mouse immunoglobulin A (IgA) antibody (Zymed Laboratories Inc., South San Francisco, Calif.) for 20 min at room temperature, followed by the avidin-biotin complex staining method (5). Sections were reacted with 0.05 mol of Tris-HCl buffer per liter containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride (Wako Pure Chemicals, Osaka, Japan) and 0.005% H2O2, and the nuclei were counterstained with hematoxylin. Control sections incubated with normal rabbit IgG instead of the primary antibody did not show nonspecific staining. The labeling index (LI) for IgA-positive lumina of pyloric glands was determined by examination of 500 glands in sections of pyloric mucosa, and the percentage of lumina labeled with anti-mouse IgA antibody was used for analysis.
Determination of antibody levels against H. pylori antigens in serum, feces, and saliva.
Blood samples were obtained from the tail vein at 7 days after the last immunization or by cardiac puncture at 6 weeks after the last challenge. Secretory IgA antibody in stool specimens was extracted from fecal pellets by incubation of the samples with PBS containing 5% nonfat dry milk to yield approximately a 7% emulsion, 1 μg of aprotinin per ml, and 10 μM leupeptin (Wako) (16). After extensive vortexing, the fecal material was centrifuged at 13,000 × g for 10 min, and the supernatants were used for the determination of IgA antibody. Saliva samples were collected with a micropipette after intraperitoneal injection of 200 μg of pilocarpine (Sigma) in sterile PBS.
Determination of IgG and IgA antibodies in blood, feces, and saliva samples was performed by an enzyme-linked immunosorbent assay (ELISA). Sera were tested for IgG at a dilution of 1:100 and for IgG1 and IgG2a at a dilution of 1:25. Saliva and fecal samples were tested for IgA at a dilution of 1:4 and undiluted, respectively. Microtiter plates (Nunc, Roskilde, Denmark) were first coated with 100 μl of antigen (H. pylori whole sonicated antigen, 1 μg/well) in carbonate buffer (pH 9.6) for 1 h at 37°C. They were then incubated with PBS containing 5% bovine serum albumin (fraction V; Sigma Chemical Co., St. Louis, Mo.) for 1 h at 37°C to block nonspecific binding and washed with PBS-Tween 20. Wells containing 100 μl of each test sample were incubated for 1 h at 37°C. After being washed, the wells were incubated with 100 μl of peroxidase-conjugated goat anti-mouse IgG, goat-anti mouse IgG1, goat-anti mouse IgG2a, or goat-anti mouse IgA antibodies (Cappel, Malvern, Pa.) for 1 h at 37°C. After the wells were washed, conjugated peroxidase was detected with 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) (ABTS) and hydrogen peroxide. The absorbance at 414 nm was measured after 30 min of incubation at room temperature. Each sample was tested in duplicate.
Extraction of RNA and RT-PCR.
To examine the expression of cytokines in the stomach at the mRNA level, total RNA was extracted from the stomach homogenate by the acid guanidium thiocyanate-phenol-chloroform method (Nippon Gene Co., Tokyo, Japan) as described previously (14). For this purpose, 1 μg of total RNA was reverse transcribed in a final volume of 50 μl containing 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM MgCl2, 5 mM dithiothreitol, 500 μM deoxynucleoside triphosphate, 1 μM oligo(dT)15 primer, 20 U of RNase inhibitor (Toyobo Biomedicals, Osaka, Japan), and 500 U of Moloney murine leukemia virus reverse transcriptase (Gibco-BRL, Gaitherburg, Md.). The reaction mixtures were incubated for 2 h at 37°C, and the resultant cDNAs were used for PCRs.
One microliter of cDNA was mixed in a final volume of 50 μl containing 50 mM KCl; 10 mM Tris-HCl (pH 8.8); 1.5 mM MgCl2; 0.1% Triton X-100; 200 μM each dATP, dCTP, dGTP, and dTTP; 20 μM each primer (see below); and 0.2 U of Taq DNA polymerase (Takara Shuzo, Kyoto, Japan). Amplification was performed for 35 cycles in a DNA thermal cycler (ASTEC Co., Fukuoka, Japan) as follows: 94°C for 1 min, 55°C for 2 min, and 72°C for 1 min. The PCR products were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and observed with a UV transilluminator. The primer sequences were as follows (4, 7, 15, 20): gamma interferon (IFN-γ) sense primer, 5′-AACGCTACACACTGCATCT-3′; IFN-γ antisense primer, 5′-TGCTCATTGTAATGCTTGG-3′; interleukin 4 (IL-4) sense primer, 5′-TAGTTGTCATCCTGCTCTT-3′; IL-4 antisense primer, 5′-CTACGAGTAATCCATTTGC-3′; IL-5 sense primer, 5′-AAGATGCTTCTGCACTTGA-3′; IL-5 antisense primer, 5′-ACACCAAGGAACTCTTGCA-3′; β-actin sense primer, 5′-ATGGATGACGATATCGCT-3′; and β-actin antisense primer, 5′-ATGAGGTAGTCTGTCAGGT-3′.
For semiquantitative determination of the expression of cytokine mRNA, we constructed standard recombinant plasmids containing a partial genome of IFN-γ, IL-4, IL-5, or β-actin. The cloning procedures were essentially similar to those described by Sambrook et al. (17). Using cDNA obtained from the thymus gland of newborn mice, we amplified target signals of cytokines by using the specific primers described above. The amplified products were inserted into a pGEM-T vector (Promega Co., Madison, Wis.) and subcloned by transformation with Escherichia coli JM109 competent cells (Toyobo). The concentration of cloned recombinant plasmid containing each cytokine DNA was estimated by measurement of the absorbance at 260 nm. Tested samples were amplified by PCR in parallel with 10-fold serially diluted standard recombinant plasmid. The copy number of each sample was determined by comparing the density with that of standard cDNA by agarose gel electrophoresis and ethidium bromide staining. There were no significant differences among the samples with regard to the expression of β-actin mRNA.
Statistical analysis.
Differences in H. pylori-specific IgG and IgA antibody levels, grade of gastric inflammation, and LI for IgA-positive lumina of pyloric glands among experimental groups were examined for statistical significance by analysis of variance or Student’s t test. Differences in the rate of protection against H. pylori infection were analyzed by Fisher’s exact probability test. A P value of <0.05 was considered statistically significant.
RESULTS
H. pylori-specific antibody levels in feces, saliva, and sera obtained from orally vaccinated mice.
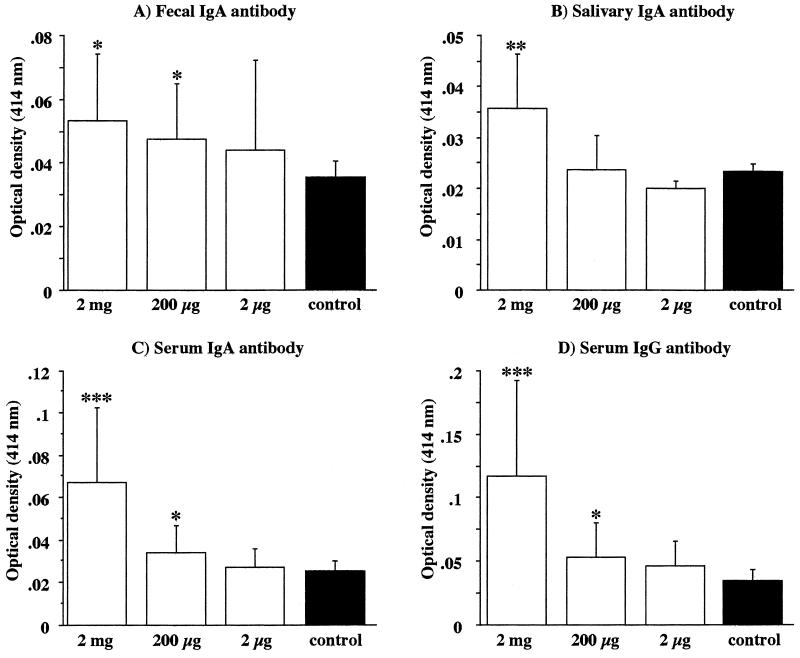
Blood, fecal, and saliva samples were collected, and the antibody responses against H. pylori antigens were examined by an ELISA 1 week after the last vaccination. Although the antibody levels were low and the biologic significance was undefined, increases in fecal and saliva IgA antibody levels were observed in vaccinated (group 1 and 2) (Fig. 1A) mice and in group 1 mice (Fig. 1B), respectively, compared with the nonvaccinated controls (P, <0.05). In the vaccinated groups, the levels of serum H. pylori-specific IgA and IgG antibodies were elevated in proportion to the doses of H. pylori whole-cell sonicate (Fig. 1C and D).
FIG. 1.
Detection of antibody responses in the feces, saliva, and sera of vaccinated (open bars) and nonvaccinated (filled bars) mice at 1 week after the last vaccination. H. pylori-specific fecal (with undiluted extract) IgA (A), salivary (at a 1:4 dilution) IgA (B), and serum (at a 1:100 dilution) IgA (C) and IgG (D) antibodies were measured by an ELISA. Data represent the mean ± standard deviation optical density at 414 nm for each group (n, 17 to 20 mice). P values were <0.05 (∗), <0.01 (∗∗), and <0.005 (∗∗∗) compared with the values for the corresponding nonvaccinated mice (Student’s t test).
Effect of vaccination against H. pylori infection.
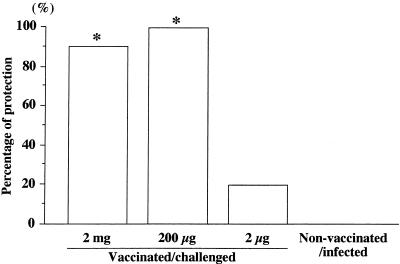
The effect of vaccination was based upon protection against live H. pylori challenge infection. Protection was judged by the absence of H. pylori, determined by both culturing for H. pylori and light microscopic examination of gastric sections stained with Giemsa stain at 6 and 18 weeks after challenge. In nonvaccinated/infected mice, H. pylori had colonized well at 6 and 18 weeks after the challenge. The percentages of protection were 90 and 100% in vaccinated/challenged groups 1 and 2, respectively, at 6 weeks after the challenge. In contrast, in 8 of 10 mice in group 3, H. pylori grew in the stomach, indicating that these mice were not protected. The difference in the levels of H. pylori colonization between vaccinated/challenged mice (groups 1 and 2) and nonvaccinated/infected mice was statistically significant (P, <0.001) (Fig. 2).
FIG. 2.
Effect of oral vaccination with 5 μg of cholera toxin and 2 mg (group 1), 200 μg (group 2), or 2 μg (group 3) of H. pylori whole-cell sonicate on H. pylori infection. Protection against H. pylori infection was based on the absence of H. pylori, determined by both bacterial culturing and light microscopic examination of stomach sections (Giemsa stain) at 6 weeks after the last challenge (n, 10 mice). An asterisk indicates that the P value was <0.001 compared with the value for the corresponding nonvaccinated/infected mice (Fisher’s exact test).
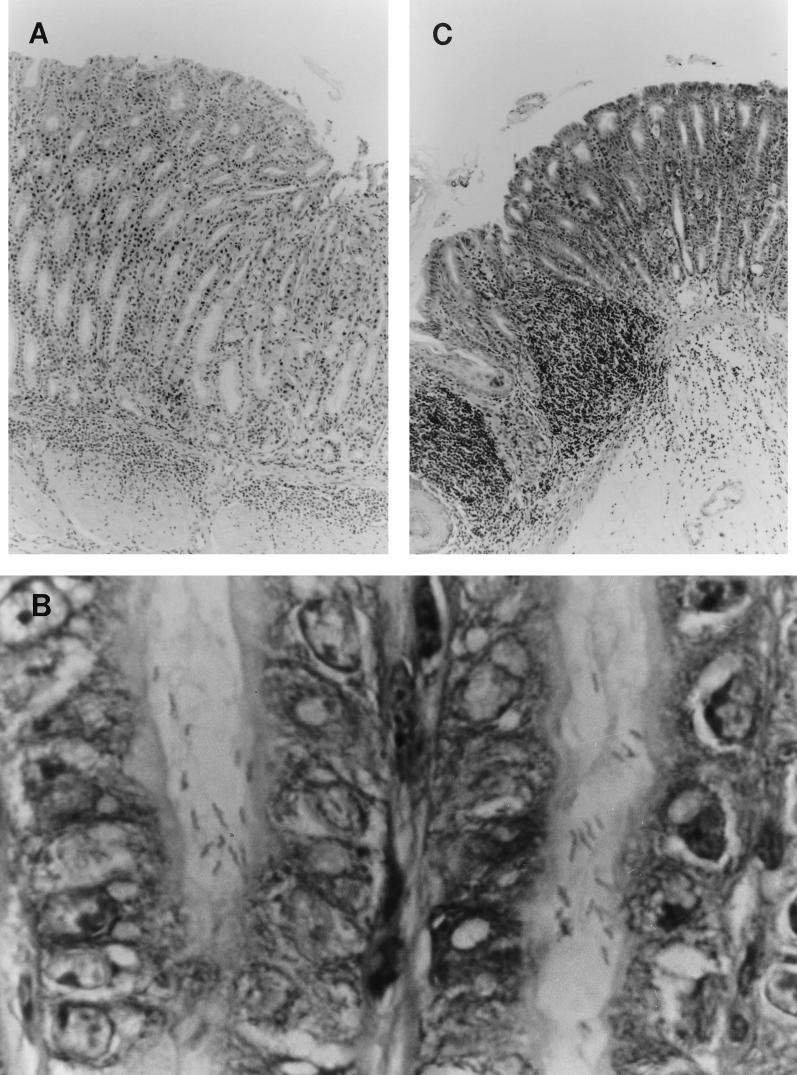
Michetti et al. (12) reported that oral vaccination occasionally induces gastritis without H. pylori challenge. Therefore, we examined whether our oral vaccination alone induced gastric inflammation. Inflammatory cell infiltration was not observed in the stomachs of all vaccinated mice at 6 weeks after the last vaccination (data not shown). In nonvaccinated/infected mice, however, mild inflammation was present in the antral region at 6 weeks after infection (Fig. 3A). In gastric tissue sections of these mice, H. pylori was found in the antral region (Fig. 3B). The cell infiltrates consisted of diffuse aggregates of numerous neutrophils, plasma cells, and a few mononuclear cells. These histopathological inflammatory features were compatible with active chronic gastritis.
FIG. 3.
Micrographs of gastric tissues of vaccinated/challenged mice and nonvaccinated/infected mice at 6 weeks after challenge. (A) Antral mucosa of a representative nonvaccinated/infected mouse, showing active chronic inflammation mainly in the submucosal layer (HE stain; original magnification, ×125). (B) Large numbers of spiral bacteria throughout the antral crypts in a representative nonvaccinated/infected mouse (Giemsa stain; original magnification, ×1,000). (C) Fundic mucosa of a representative protected mouse in the vaccinated/challenged group, showing high intensity of infiltration by inflammatory cells composed mainly of mononuclear cells (HE stain; original magnification, ×100).
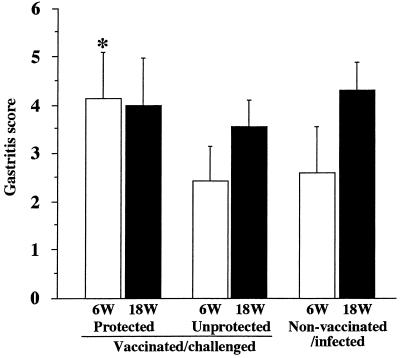
In vaccinated/challenged mice (groups 1 and 2) the grade of gastritis was more severe than that in nonvaccinated/infected mice; marked infiltration of mononuclear cells was noted, mainly in the corpus of the stomach (Fig. 3C). In unprotected mice the gastritis score at 6 weeks after the challenge was significantly lower than that at 18 weeks after the challenge in nonvaccinated/infected mice (Fig. 4). On the other hand, the gastritis score at 6 weeks after the challenge in protected mice was significantly higher than that in unprotected mice (P, <0.0005). In addition, gastritis observed in protected mice lasted for 18 weeks, and the severity at 6 weeks after the challenge had reached levels almost equal to those observed at 18 weeks for unprotected or nonvaccinated/infected mice.
FIG. 4.
Gastritis scores in vaccinated/challenged and nonvaccinated/infected mice at 6 (open bars) and 18 (filled bars) weeks (W) after the challenge. HE-stained gastric sections were scored based on the overall grade of inflammation (on a scale of 0 to 7), which included the intensity and extent of inflammation (see Materials and Methods). Data represent the mean ± standard deviation gastritis scores for each group (n, 5 to 10 mice). The asterisk indicates that the P value was <0.0005 compared with the value for the corresponding nonvaccinated/infected mice at 6 weeks after the challenge (Student’s t test).
Immunohistopathological examination of gastric tissue.
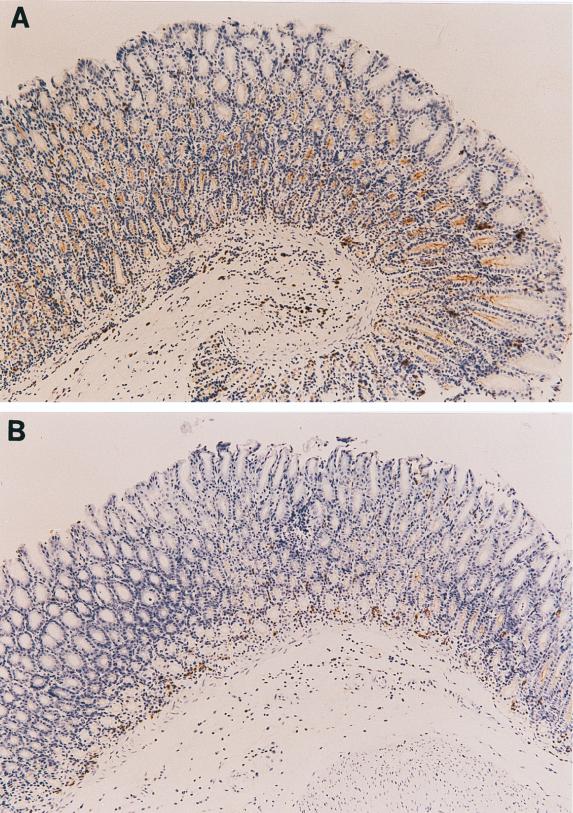
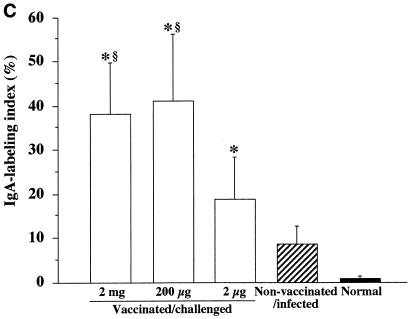
To examine whether secretory IgA is induced and actively secreted in gastric mucosa following oral vaccination, immunohistochemical staining for IgA antibody in gastric mucosa was performed at 6 weeks after the challenge. The number of gastric gland lumina positively stained with IgA antibody was significantly higher in vaccinated/challenged mice (Fig. 5A) than in nonvaccinated/infected mice (Fig. 5B) (P, <0.05). In vaccinated/challenged mice, the LI for IgA-producing glands in groups 1 and 2 was significantly higher than that in group 3 (P, <0.001) (Fig. 5C).
FIG. 5.
Immunohistochemical analysis of gastric tissues from vaccinated/challenged mice (magnification, ×100) (A) and nonvaccinated/infected mice (magnification, ×100) (B) at 6 weeks after the challenge. (C) IgA LI for the gastric mucosa in vaccinated/challenged, nonvaccinated/infected, and normal control mice at 6 weeks after the challenge. The LI represent the percentage of anti-mouse IgA antibody-labeled lumina among 500 pyloric glands of the gastric mucosa. Data represent the mean ± standard deviation LI for each group (n, 10 mice). An asterisk indicates that the P value was <0.05 compared with the value for the corresponding nonvaccinated/infected mice at 6 weeks after the challenge (Student’s t test). A section sign indicates that the P value was <0.001 compared with the value for the corresponding vaccinated/challenged mice (2 μg) at 6 weeks after the challenge (Student’s t test).
H. pylori-specific serum IgG antibody subclasses before and after challenge.
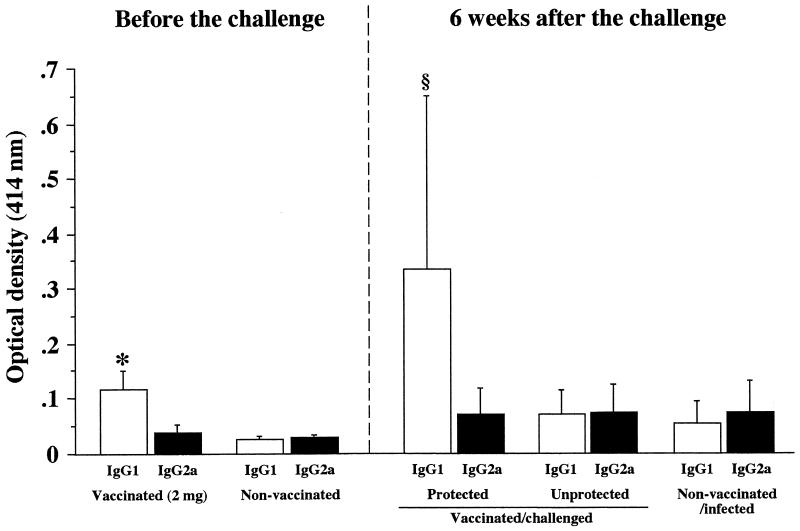
To determine the phenotypes of Th cells induced by oral vaccination, the subclasses of H. pylori-specific IgG were determined for vaccinated and/or infected mice by an ELISA before and at 6 weeks after challenge. At 1 week after the last vaccination, group 1 produced serum IgG1 antibody (P, <0.05 [versus control mice]). At 6 weeks after the challenge, the titer of serum IgG1 in protected mice was significantly higher than that in unprotected mice (P, <0.05) (Fig. 6). Although these findings do not reflect the local immune responses in the stomach, they suggest that Th2 cell-mediated immune responses are operational at least in the whole body.
FIG. 6.
Secondary responses of IgG subclasses in vaccinated mice at 6 weeks after challenge infection. H. pylori-specific serum (at a 1:25 dilution) IgG1 and IgG2a antibodies were quantitated by an ELISA. Data represent the mean ± standard deviation optical density at 414 nm for each group (n, 10 mice). The asterisk indicates that the P value was <0.05 compared with the value for the corresponding nonvaccinated mice at 1 week after the last vaccination (Student’s t test). The section sign indicates that the P value was <0.05 compared with the value for the corresponding unprotected mice in the vaccinated/challenged group and for the nonvaccinated/infected mice at 6 weeks after the challenge (Student’s t test).
Local expression of cytokine mRNAs after challenge.
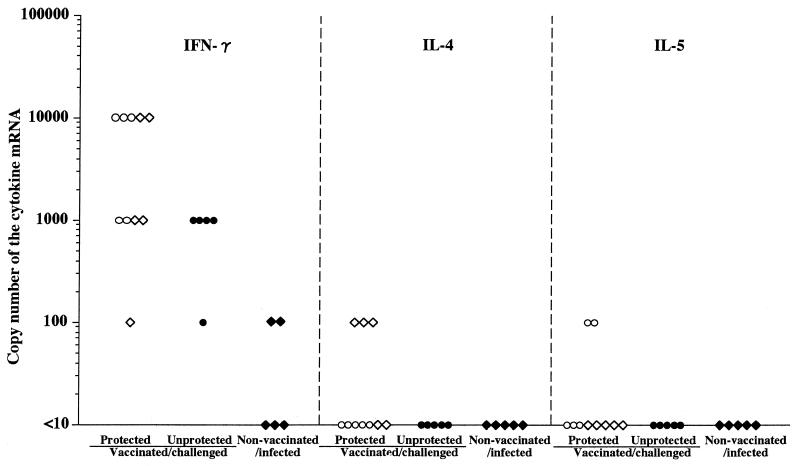
To further determine the local immune responses in the stomach, we compared the expression of mRNAs of IFN-γ, IL-4, and IL-5 by RT-PCR using stomach homogenates of vaccinated/challenged and nonvaccinated/infected mice (Fig. 7). Interestingly, the expression of IFN-γ mRNA in the stomach was significantly higher in protected than in unprotected mice. In nonvaccinated/infected mice the level of IFN-γ mRNA was lower than 102 copies and was lower than that in vaccinated/challenged mice (Fig. 7). The level of IFN-γ mRNA in vaccinated mice without challenge was undetectable at 6 weeks after the last vaccination (data not shown). On the other hand, IL-4 mRNA was detected in 3 of 10 protected mice and IL-5 mRNA was detected in another 2 of 10 protected mice. In other words, 5 of 10 protected mice expressed the mRNA of either IL-4 or IL-5, although the levels were lower than 102 copies. The same mRNAs were not detected in stomach homogenates of unprotected mice and nonvaccinated/infected mice (Fig. 7).
FIG. 7.
Expression of IFN-γ, IL-4, and IL-5 mRNAs in the stomachs of nonvaccinated/infected and vaccinated/challenged mice at 6 weeks after the challenge. mRNAs were detected by RT-PCR and expressed as the copy number determined semiquantitatively (see Materials and Methods). Mice were vaccinated with 2 mg (○), 200 μg (◊), and 2 μg (●) of whole-cell sonicate; ⧫, nonvaccinated/infected mice. The ordinate is the logarithmic copy number of each cytokine mRNA.
DISCUSSION
Several investigators have recently suggested that oral vaccination with either H. pylori whole-cell antigen or purified antigen and appropriate adjuvants can prevent infection by Helicobacter species (1, 8, 10, 12, 13, 16). In the present study, we showed that oral vaccination with a dose of greater than 200 μg of H. pylori whole-cell sonicate per session for 5 weeks was effective in protecting C57BL/6 mice against H. pylori infection.
We first noted a severe inflammatory cell infiltration in the corpus at 6 weeks after the challenge, especially in protected mice. We also demonstrated that the development of more severe inflammation at this stage might lead to protection. A number of investigators (1, 13, 16) have reported that gastric inflammation occurs in immunized mice after challenge; this effect has been referred to as postimmunization gastritis. However, the details of postimmunization gastritis differ among studies. For example, Pappo et al. (16) showed that oral immunization with recombinant urease and cholera toxin resulted in protection against H. felis infection and that the gastritis in recombinant urease-immunized mice subsequently challenged with H. felis exhibited Thy1.2+ T cells in mucosal and submucosal layers. On the other hand, Ferrero et al. (1) demonstrated that orogastric immunization of mice with recombinant H. pylori GroES-like and urease B-subunit proteins protected against H. felis challenge and that mild gastric inflammation may be a necessary prerequisite for successful immunization. In that study, the severity of postimmunization gastritis was milder than that of ordinary H. felis infection. Mohammadi et al. (13) showed that the grade of postimmunization gastritis was more severe than that of ordinary H. felis infection when H. felis whole-cell sonicate was used as the oral immunogen, similar to our findings. These results suggest that postimmunization gastritis might correlate with protection against Helicobacter challenge. However, the extent of postimmunization gastritis is different in each experimental design. The different natures of oral immunogens with regard to immunogenicity or cross-reactivity against host proteins, differences in mouse strains used, and the strain diversity of Helicobacter species might be the factors responsible for the differences in the extent of postimmunization gastritis. The same can be said of the discrepant finding that gastric inflammation was not observed in all vaccinated mice without H. pylori challenge in the present study, in contrast to the occasional induction of gastritis by vaccination only demonstrated by Michetti et al. (12).
Measurement of mRNAs for a group of cytokines in the stomach in the present study showed that IFN-γ mRNA was strongly expressed in protected mice. In contrast, the expression was weaker (less than 103 copies) in unprotected mice as well as in nonvaccinated/infected mice. We also showed that the expression of the mRNA for either IL-4 or IL-5 was detected in 50% of protected mice. These findings suggest that postimmunization gastritis represents a reactive inflammation of the gastric mucosa induced primarily by Th1 immune responses together with secondary Th2 responses induced by the challenge. In support of the involvement of Th1 immune responses or IFN-γ in postimmunization gastritis, Mohammadi and coworkers (13) showed that treatment of nonimmunized/infected and immunized/challenged mice with anti-IFN-γ antibody led to a significant reduction in the severity of gastric inflammation.
With regard to mucosal immunity, it is clear that local IgA antibody plays a primary role in protection against foreign organisms. However, there are no reports directly indicating that gastric tissue functions as an effector organ of mucosal immunity, similar to gut-associated lymphoid tissue. In the present study, we observed protection against H. pylori in vaccinated mice (mainly groups 1 and 2), in which salivary and fecal H. pylori-specific IgA was detected. In these mice, high levels of IgA were also secreted in the pyloric mucosa, and these levels were vaccine dose dependent (Fig. 5). However, this IgA is not necessarily specific for H. pylori, since it has been demonstrated that IFN-γ and IL-4 up-regulate the expression of the polymetric immunoglobulin receptor, which transports IgA in human intestinal epithelium (18). In support of the involvement of Th2 cell-mediated immune responses in the stomach, we demonstrated the expression of mRNAs for IL-4 and IL-5 in the stomachs of mice protected against challenge infection (Fig. 7). To our knowledge, this is the first report of mRNAs for these cytokines in situ, although a previous study demonstrated the secretion of these cytokines in the supernatants of lymphocyte cultures from the stomach after antigenic stimulation (13). Taken together, these findings suggest that local secretory IgA antibody in gastric mucosal tissue correlates well with protection against H. pylori infection. However, whether the stomach functions as a primary priming site for inducing the protective IgA response remains to be elucidated in future studies.
Cellular immune responses to H. pylori have been studied for humans, but the nature of such responses, including the possible involvement of protective immunity, is not well defined. In the present study, we demonstrated that Th1 cell-mediated immune responses occur in the stomach, as indicated by the marked expression of IFN-γ mRNA. However, the mechanism(s) through which a challenge infection of vaccinated mice induces the strong expression of IFN-γ is still not clear, particularly in protected mice. The expression of IFN-γ might be associated with the appearance of postimmunization gastritis (13). Furthermore, the development of severe postimmunization gastritis during the early postchallenge infection period is probably an essential prerequisite for protection, as demonstrated in the present study and previous reports (1, 13). However, the mechanism of protection after the appearance of postimmunization gastritis remains unidentified.
Further studies are necessary to clarify these issues and identify the antigen(s) that induces protective immunity following oral vaccination, with the hope of preventing postimmunization gastritis.
ACKNOWLEDGMENTS
We thank Adrian Lee for donating the Sydney strain and for careful reading of the manuscript and Kiyomi Ohno and Mami Kimoto for technical assistance. We also thank F. G. Issa (Word-Medex, Sydney, Australia) for careful reading and editing of the manuscript.
REFERENCES
- 1.Ferrero R L, Thiberge J M, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499–6503. doi: 10.1073/pnas.92.14.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujioka T, Kodama R, Honda S, Hua G G, Nishizono A, Nasu M. Long-term sequelae of experimental gastritis with Helicobacter pylori: a 5-year follow-up study. J Clin Gastroenterol. 1997;25(Suppl. 1):S8–S12. doi: 10.1097/00004836-199700001-00004. [DOI] [PubMed] [Google Scholar]
- 3.Graham D Y, Lew G M, Klein P D, Evans D G, Evans D J, Jr, Saeed Z A, Malaty H M. Effect of treatment of Helicobacter pylori infection on the long term recurrence of gastric and duodenal ulcers: a randomized controlled study. Ann Intern Med. 1992;116:705–708. doi: 10.7326/0003-4819-116-9-705. [DOI] [PubMed] [Google Scholar]
- 4.Gray P W, Goeddel D V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci USA. 1983;80:5842. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guesdon J, Ternynck T, Avrameas S. The use of avidin-biotin interaction in immunoenzymatic techniques. J Histochem Cytochem. 1979;8:1131–1139. doi: 10.1177/27.8.90074. [DOI] [PubMed] [Google Scholar]
- 6.International Agency for Research on Cancer, World Health Organization. Schistosomes, liver flukes and Helicobacter pylori. IARC working group on the evaluation of carcinogenic risks to humans. Monogr Eval Carcinog Risks Hum. 1994;61:218–220. [PMC free article] [PubMed] [Google Scholar]
- 7.Kinashi T, Harada N, Severinson E, Tanabe T, Sideras P, Konishi M, Azuma C, Tominaga A, Bergstedt-Lindquist S, Takahashi M. Cloning of complementary DNA encoding T-cell replacing factor and identity with B-cell growth factor II. Nature (London) 1986;324:70. doi: 10.1038/324070a0. [DOI] [PubMed] [Google Scholar]
- 8.Lee A, Chen M. Successful immunization against gastric infection with Helicobacter species: use of a cholera toxin B-subunit–whole-cell vaccine. Infect Immun. 1994;62:3594–3597. doi: 10.1128/iai.62.8.3594-3597.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee A, O’Rourke J, Ungria M C D, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee C K, Weltzin R, Thomas W D, Jr, Kleanthous H, Ermak T H, Soman G, Hill J E, Ackerman S K, Monath T P. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161–171. doi: 10.1093/infdis/172.1.161. [DOI] [PubMed] [Google Scholar]
- 11.Megraud F. H. pylori resistance to antibiotics. In: Hunt R H, Tytgat G N J, editors. Helicobacter pylori—basic mechanism to clinical cure. Dordecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 570–583. [Google Scholar]
- 12.Michetti P, Corthesy-Theulaz I, Davin C, Haas R, Vaney A C, Heitz M, Bille J, Kraehenbuhl J P, Saraga E, Blum A L. Immunization of BALB/c mice against Helicobacter pylori urease. Gastroenterology. 1994;107:1002–1011. doi: 10.1016/0016-5085(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 13.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 14.Montgomery R A, Dallman M J. Analysis of cytokine gene expression during fetal thymic ontogeny using the polymerase chain reaction. J Immunol. 1991;147:554–560. [PubMed] [Google Scholar]
- 15.Otsuka T, Villaret D, Yokota T. Structural analysis of the mouse chromosomal gene encoding interleukin 4 which expresses B cell, T cell and mast cell stimulating activities. Nucleic Acids Res. 1987;15:333–344. doi: 10.1093/nar/15.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pappo J, Thomas W D, Jr, Kabok Z, Taylor N S, Murphy J C, Fox J G. Effect of oral immunization with recombinant urease on murine Helicobacter felis gastritis. Infect Immun. 1995;63:1246–1252. doi: 10.1128/iai.63.4.1246-1252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. pp. 53–72. [Google Scholar]
- 18.Sarkar J, Gangopadhyay N N, Moldoveanu Z, Mestecky J, Stephesen C B. Vitamin A is required for regulation of polymeric immunoglobulin receptor (pIgR) expression by interleukin-4 and interferon-gamma in a human intestinal epithelial cell line. J Nutr. 1998;128:1063–1069. doi: 10.1093/jn/128.7.1063. [DOI] [PubMed] [Google Scholar]
- 19.Sugiyama A, Maruta F, Ikeno T, Ishida K, Kawasaki S, Katsuyama T, Shimizu N, Tatematsu M. Helicobacter pylori infection enhances N-methyl-N-nitrosourea-induced stomach carcinogenesis in the Mongolian gerbil. Cancer Res. 1998;58:2067–2069. [PubMed] [Google Scholar]
- 20.Tokunaga K, Taniguchi H, Yoda K, Shimizu M, Sakiyama S. Nucleotide sequence of a full-length cDNA of mouse cytoskeletal β-actin mRNA. Nucleic Acids Res. 1986;14:2829–2839. doi: 10.1093/nar/14.6.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639–642. [PubMed] [Google Scholar]
- 22.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Monschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]