Abstract
Background
SARS-CoV-2 variants have distinct features of transmissibility, infectivity, and aggressiveness that may cause different clinical manifestations. A better understanding of the disease presentation and progression could help to outline more precise preventive and treatment frameworks. This study describes the differences in COVID-19 presentation and outcomes across five variant waves.
Methods
This prospective cohort was conducted in Serrana, São Paulo State, Brazil. Clinical and demographic data was obtained from June 2020 to December 2022 as part of an enhanced health surveillance system for COVID-19, based on increasing access to diagnostic testing for SARS-CoV-2 and patient follow-up. Individuals were assessed for COVID-19 symptoms and comorbidities. Mild cases were followed up for at least 14 days, and severe cases until discharge or death. Samples were genetically sequenced, and variant waves were determined based on global SARS-CoV-2 variant predominance (>90 % sequenced samples), being as follows: Ancestral, Delta, Gamma, Omicron BA.1, and Omicron BA.2 waves. The relationship between clinical data and disease outcomes was analyzed in each variant wave.
Results
Patients infected during the Delta wave were the youngest (36.1 ± 18.2 years, p < 0.001). The proportion of female patients was higher across all waves. Positivity rate, disease severity, and COVID-19-related deaths varied among them. Ageusia and anosmia were related to SARS-CoV-2 positivity during the Ancestral, Gamma, and Delta waves but not in Omicron BA.1 and Omicron BA.2 waves. Diarrhea presented a lower chance of positivity only in Omicron BA.1 and Omicron BA.2. Dyspnea was the most consistent risk factor for severity across all waves.
Conclusions
Although patients with COVID-19 from different SARS-CoV-2 variants shared some clinical-epidemiological characteristics, each variant presented distinguishable features related to positivity and severity. This could help to understand the dynamics of COVID-19 variants and update recommendations for clinical practice.
Keywords: COVID-19, SARS-CoV-2, Symptoms, Severity
1. Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has caused more than 7 million deaths worldwide as of March 2024 [1], and brought into focus its remarkable ability to adapt and evolve [2,3]. The World Health Organization (WHO) classified SARS-CoV-2 variants into variants of interest (VOIs) and variants of concern (VOCs) based on their transmissibility and/or aggressiveness [4]. The emergence of variants has presented a significant challenge in the ongoing battle against the disease, as new variants could overcome vaccine protection [5,6].
In Brazil, more than 37 million COVID-19 cases were reported by December 2023, with more than 700 thousand deaths confirmed [7]. The spread of the disease in the country was critical not only for the number of COVID-19-related deaths and hospitalizations, but also for its economic burden [8].
Several surveillance strategies have been developed worldwide to track and control the disease [9]. Despite a Unified Health System (SUS) in Brazil, there was significant heterogeneity in access to testing across different regions, delays in test results and reporting, and changes in notification procedures. Surveillance systems also varied among regions and nearby cities [[10], [11], [12], [13], [14], [15]]. In 2020, an enhanced health surveillance system was implemented in Serrana, a city in the southeast of Brazil, based on four key components: increasing access to free SARS-CoV-2 diagnostic testing; improving swab collection procedures and providing personal protective equipment training; testing even patients with mild symptoms; and COVID-19 patients' follow-up [10]. Additionally, positive SARS-CoV-2 samples were sequenced [10,16]. Since SARS-CoV-2 diagnostic testing was free and available even for individuals with mild symptoms, and results were released the next working day, these strategies allowed us to monitor the dynamics of the pandemic over several waves.
A better understanding of the characteristics of VOCs and VOIs and their influence on disease manifestations are crucial in devising effective strategies to control the spread and impact of SARS-CoV-2. Therefore, the aim of this study is, based on real-world data of an enhanced health surveillance system, to characterize the clinical manifestations and outcomes of COVID-19 from June 2020 to December 2022 and provide insights on the disease evolution.
2. Methods
2.1. Study design
This is a prospective observational cohort study conducted in Serrana, São Paulo State, Brazil, a town with a population of 43,909 inhabitants in 2022, according to an official and compulsory national census. Approximately a quarter of Serrana’s population commutes daily to work in nearby cities, often using crowded transportation, which facilitates the transmission of infectious diseases.
2.2. Study population and period
Data were collected from June 1, 2020, to December 31, 2022. During this period, symptomatic individuals were assessed for COVID-19 infection as part of the enhanced health surveillance system implemented in Serrana. Briefly, the enhanced surveillance system consisted of increasing access to SARS-CoV-2 testing for all patients, even those with mild symptoms, following up on all positive cases, and conducting SARS-CoV-2 genome sequencing [16]. Therefore, this study is based on a convenience sample of patients.
On the first visit to the healthcare service, guided by a structured questionnaire, patients were thoroughly examined regarding COVID-19-related symptoms. Additionally, they were asked to provide information about comorbidities (diabetes, chronic cardiac diseases, chronic lung diseases, chronic kidney diseases, pregnancy, hypertension, immunosuppression, obesity) and demographic characteristics.
The COVID-19-related symptoms assessed were: ageusia, anosmia, arthralgia, cough, diarrhea, dizziness, dyspnea, fatigue, fever, gastric discomfort, headache, loss of appetite, low oxygen saturation, mental confusion, myalgia, nasal congestion, nausea, respiratory discomfort, runny nose, and sore throat.
If COVID-19 was confirmed, disease severity was defined according to the WHO Clinical Progression Scale (WHO-CPS) at the first visit and during follow-up contacts. Follow-up was performed by phone calls on days 5, 10, and 14 after symptom onset for mild cases (WHO-CPS score 0–3), or, for those who required hospitalization, every other day until discharge or death [10]. For all SARS-CoV-2 positive cases, symptom duration, the highest WHO-CPS score, and death by SARS-CoV-2 infection were recorded at the end of the follow-up. We have analyzed all COVID-19 positive cases, and since an individual could have experienced multiple infections, we will refer to each visit to the healthcare facility as an encounter. Data were grouped and presented according to epidemiological weeks.
During our study, individuals may have received up to five vaccine doses, according to the Brazilian National Immunization Program (PNI). Furthermore, since Serrana participated in a stepped-wedge randomized trial, 81.4 % of its adult population received two doses of CoronaVac over eight weeks, starting from February 2021 [17].
2.3. Detection of SARS-CoV-2
SARS-CoV-2 infection was assessed by reverse transcriptase-polymerase chain reaction (rt-PCR), as previously described [16]. Patients with inconclusive results were excluded from the analysis.
2.4. Variants of concern
VOCs were defined according to WHO’s guidelines and were based solely on the sequencing data of positive samples from Serrana [16,18]. The period of predominance of each variant (herein called "waves") was defined based on a prevalence of at least 90 % for the respective variant in each epidemiological week [16]. All positive cases from epidemiological weeks lacking a predominant variant were excluded from the analysis.
Therefore, the periods analyzed in this study were as follows:
-
●
Wave #1 (Ancestral): June 1st, 2020 to March 13th, 2021
-
●
Wave #2 (Gamma): March 14th, 2021 to August 21st, 2021
-
●
Wave #3 (Delta): October 3rd, 2021 to December 25th, 2021
-
●
Wave #4 (Omicron BA.1): January 2nd, 2022 to March 12th, 2022
-
●
Wave #5 (Omicron BA.2): April 24th, 2022 to May 7th, 2022
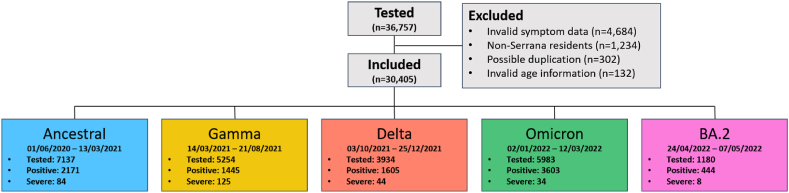
Detailed information about the waves and number of encounters can be found in Fig. 1.
Fig. 1.
Flow diagram of participant selection.
∗Severe cases were defined according to the WHO Clinical Progression Scale (WHO-CPS) as having a score equal or greater than 4 (requiring hospitalization).
2.5. Data handling
Study data were collected and managed using RedCap (Vanderbilt University, Nashville, USA) hosted at Ribeirao Preto Medical School, University of Sao Paulo (FMRP-USP) [19]. All data from the period of study were exported and cleaned in Python using the pandas library [20].
2.6. Statistical analysis
Categorical variables were described as frequencies and percentages, while continuous values were described as medians and interquartile ranges. Group comparisons were made using chi-square tests with Bonferroni corrections for wave to wave comparison. Continuous variables were compared using one-way analysis of variance (ANOVA) with Bonferroni corrections.
Logistic regression models were used to compute odds ratios for outcomes based on each wave, symptoms, and comorbidities. Both univariable and multivariate analyses were performed for each variant wave. The outcomes were SARS-CoV-2 positivity (based on RT-PCR results) and COVID-19 severity (patients with WHO-CPS score ≥4). The logistic regression models were constructed with parameters selected through the following steps: first, we retained symptoms and comorbidities diagnosed by a health professional that exhibited at least 1 % prevalence during any variant wave. Subsequently, for each wave and outcome, we conducted a series of univariable analyses incorporating each significant symptom, adjusted for age, sex, and the most prevalent comorbidities identified in the initial analysis. Ultimately, all significant symptoms from the univariable analyses in each wave were incorporated into the final multivariable model. Additionally, we analyzed all positive cases, dividing them into 10-year age groups and according to each virus variant. Finally, we analyzed the impact of vaccination during the Gamma wave by comparing the demographic and clinical characteristics of individuals aged 18 years and older who were either vaccinated (with two doses) or unvaccinated. Further details can be found in the Supplementary Material.
Results were considered significant when p-value was <0.05. The statistical analysis was performed with STATA 15 software (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC.).
3. Results
3.1. Patient demographic and clinical characteristics
A total of 36,757 encounters were initially obtained from the database. Among these 4684 were excluded due to missing or invalid symptom onset dates, 1234 encounters were not residents of Serrana, 302 cases were possibly duplicates and thus removed, and 132 cases had invalid age information. Following these cleaning steps, 30,405 valid encounters were included in the analysis (Fig. 1).
Demographic and clinical characteristics are presented in Table 1 and Table S1. A total of 9268 (39.5 %) positive cases for COVID-19 were detected among the 23,488 suspected cases. Considering the positive cases, the mean age was 38.5 ± 18 years old, and 57.7 % were female.
Table 1.
Demographic and clinical characteristics of COVID-19 positive cases in Serrana, Brazil, across different variant waves from June 2020 to December 2022.
| Demographic and clinical characteristics of positive cases | ||||||
|---|---|---|---|---|---|---|
| Ancestral (n = 873) | Gamma (n = 1384) | Delta (n = 1558) | Omicron BA.1 (n = 3544) | Omicron BA.2 (n = 437) | p | |
| Age (mean ± SD) | 39.4 ± 17.2 | 39.2 ± 18.1 | 36.1 ± 18.2 | 38.7 ± 18.0 | 38.4 ± 18.7 | <0.001 |
| Female, n (%) | 517 (59.2 %) | 729 (52.7 %) | 864 (55.5 %) | 2127 (60.0 %) | 276 (63.2 %) | <0.001 |
| Comorbidities | ||||||
| No Comorbidities | 689 (78.9 %) | 1061 (76.7 %) | 1255 (80.6 %) | 2884 (81.4 %) | 352 (74.4 %) | <0.001 |
| Hypertension | 115 (13.2 %) | 213 (15.4 %) | 168 (10.8 %) | 445 (12.6 %) | 67 (15.3 %) | 0.002 |
| Diabetes | 45 (5.2 %) | 91 (6.6 %) | 82 (5.3 %) | 203 (5.7 %) | 31 (7.1 %) | 0.357 |
| Asthma/C.O.P.D. | 29 (3.3 %) | 34 (2.5 %) | 40 (2.6 %) | 83 (2.3 %) | 14 (3.2 %) | 0.480 |
| Symptoms, median (IQR) | 6 (4–8) | 6 (4–8) | 7 (5–9) | 6 (4–8) | 4 (3–7) | <0.001 |
| Positivity | 30.4 % | 27.5 % | 40.8 % | 60.2 % | 37.6 % | <0.001 |
| WHO Score >4 | 84 (9.6 %) | 125 (9.0 %) | 44 (2.82 %) | 34 (0.96 %) | 8 (1.83 %) | <0.001 |
| Deaths by COVID19 | 19 (2.2 %) | 31 (2.2 %) | 5 (0.32 %) | 9 (0.25 %) | 4 (0.92 %) | <0.001 |
Patients infected during the Delta wave were significantly younger than patients infected by other variants (39.4, 39.2, 36.1, 38.7, and 38.4 years old for Ancestral, Gamma, Delta, Omicron BA.1, and Omicron BA.2 variants, respectively, p < 0.01). Throughout all waves, the proportion of female patients exceeded that of male individuals, and this difference was statistically significant across SARS-CoV-2 variants, with the highest proportion observed during the Omicron BA.2 wave (59.2 %, 52.7 %, 55.5 %, 60.0 %, and 63.2 % in Ancestral, Gamma, Delta, Omicron BA.1, and Omicron BA.2 waves, respectively).
The majority (79.6 %) of patients did not report any comorbidity, 15.1 % reported one comorbidity, and 5.3 % reported at least two comorbidities. The most prevalent comorbidities were hypertension (13.1 %), diabetes (6.0 %), chronic lung disease (2.5 %), cardiovascular disease (2.0 %), and chronic renal failure (0.7 %). The distribution of comorbidities had significant variations among waves; participants with two comorbidities were 5.0 %, 6.9 %, 4.1 %, 4.9 %, and 6.2 % in Ancestral, Gamma, Delta, Omicron BA.1, and Omicron BA.2 waves, respectively.
3.2. Impacts of different variants on COVID-19 positivity, severity, and deaths
COVID-19 positivity rates significantly varied among different waves. The highest rate of SARS-CoV-2 positivity was found in the Omicron BA.1 wave, which was also the wave in which most tests were performed (3544 tests, with 60.2 % positive cases). Conversely, during the Gamma wave, the proportion of positive tests was significantly lower (27.5 %, p < 0.001, Table 1).
The highest proportion of patients with severe disease was observed in the Ancestral wave, accounting for 9.6 % of positive cases. Gamma (9.0 %), Delta (2.8 %), Omicron BA.1 (1.0 %), and Omicron BA.2 (1.8 %) variants displayed a significant downward trend in the prevalence of severe cases (p < 0.001, Table 1).
COVID-19-related deaths were higher during the Ancestral and Gamma waves (2.2 % of positive cases in each wave). The proportion of deaths significantly decreased during the Delta and Omicron BA.1 waves (0.32 % and 0.25 %, respectively, p < 0.001). The proportion of deaths, however, slightly increased during the Omicron BA.2 variant wave (0.92 % of positive cases) compared to Delta and Omicron BA.1 waves but was still lower than Ancestral and Gamma waves (Table 1).
3.3. Symptom distribution among different waves
During the Delta variant wave, patients presented more symptoms at sample collection than at any other period (median of 7, IQR 5–9, p < 0.001), while Omicron BA.2 patients had the least number of symptoms (median of 4, IQR 3–7, p < 0.001, Table 1).
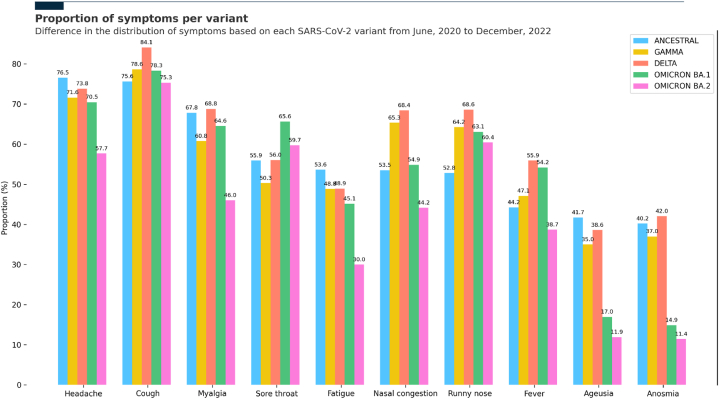
In the Ancestral wave, headache was the most prevalent symptom (72.5 %), followed by cough (70.2 %) and myalgia (65.6 %). Gamma, Delta, and Omicron BA.1 waves presented similar symptoms, with cough (78.3 %, 84.0 %, and 78.1 %, respectively) and headache (71.0 %, 73.6 %, and 70.0 %, respectively) the two most common symptoms. The main difference among Gamma, Delta, and Omicron BA.1 waves was in the third most prevalent symptom: in Gamma was nasal congestion (64.4 %), in Delta was runny nose (68.5 %), and in Omicron BA.1 was sore throat (65.5 %). Patients during the Omicron BA.2 wave exhibited a slightly distinctive symptom manifestation: cough (75.0 %), runny nose (59.9 %), and sore throat (59.5 %) were the most prevalent (Fig. 2 and Table S2).
Fig. 2.
Symptom distribution across SARS-CoV-2 variant waves.
∗Data is shown as the difference in the distribution of the most prevalent symptoms based on each SARS-CoV-2 variant wave from June 2020 to December 2022.
Not only the predominant symptoms were altered across the different pandemic waves, but other distinct symptoms, such anosmia and ageusia, exhibited a substantial decline in the Omicron BA.1 and Omicron BA.2 waves compared to Ancestral, Gamma, and Delta variants (Fig. 2 and Table S2).
3.4. Impact of age and vaccination status on COVID-19 clinical manifestations
The proportion of female patients was similar across all age groups. The number of comorbidities, positivity rate, and severity (percentage of patients with an WHO score equal or greater than 4) were significantly higher in older individuals. The number of symptoms was significantly lower in the age extremes, i.e., in infants and the elderly (Table 2).
Table 2.
Demographic and clinical characteristics of COVID-19 positive cases in Serrana, Brazil, per age group, from June 2020 to December 2022.
| Clinical and demographic characteristics per age group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0-10 (n = 404) | 11-20 (n = 774) | 21-30 (n = 1697) | 31-40 (n = 1647) | 41-50 (n = 1317) | 51-60 (n = 1007) | 61-70 (n = 559) | 71+ (n = 391) | Total (n = 7796) | p | |
| Female, n (%) | 201 (49.8 %) | 432 (55.8 %) | 994 (58.6 %) | 965 (58.6 %) | 769 (58.4 %) | 601 (59.7 %) | 327 (58.5 %) | 224 (57.3 %) | 4513 (57.9 %) | 0.041 |
| Comorbidities | ||||||||||
| No comorbidities | 385 (95.3 %) | 722 (93.3 %) | 1592 (93.8 %) | 1467 (89.1 %) | 995 (75.6 %) | 623 (61.9 %) | 273 (48.8 %) | 157 (40.2 %) | 6214 (79.7 %) | 0.000 |
| Hypertension | 0 (0.0 %) | 3 (0.4 %) | 31 (1.8 %) | 98 (5.9 %) | 199 (15.1 %) | 291 (28.9 %) | 212 (37.9 %) | 174 (44.5 %) | 1008 (12.9 %) | 0.000 |
| Diabetes | 1 (0.3 %) | 1 (0.1 %) | 16 (0.9 %) | 27 (1.6 %) | 96 (7.3 %) | 112 (11.1 %) | 112 (20.0 %) | 87 (22.25 %) | 452 (5.8 %) | 0.000 |
| Asthma/C.O.P.D. | 9 (2.2 %) | 34 (4.4 %) | 32 (1.9 %) | 34 (2.1 %) | 31 (2.4 %) | 25 (2.5 %) | 19 (3.4 %) | 16 (4.1 %) | 200 (2.6 %) | 0.004 |
| Symptoms, median (IQR) | 5 (2–8) | 6 (2–10) | 7 (3–11) | 7 (4–10) | 6 (2–10) | 6 (2–10) | 5 (1–9) | 4 (0–8) | 6 (2–10) | 0.000 |
| Positivity (%) | 26.9 % | 35.1 % | 38.4 % | 39.2 % | 42.0 % | 43.9 % | 45.4 % | 48.0 % | 39.5 % | 0.000 |
| WHO Score≥4, n (%) | 3 (0.74 %) | 1 (0.13 %) | 10 (0.59 %) | 43 (2.61 %) | 38 (2.89 %) | 51 (5.06 %) | 59 (10.55 %) | 90 (23.02 %) | 295 (3.78 %) | 0.000 |
| Deaths, n (%) | 0 (0 %) | 0 (0 %) | 1 (0.06 %) | 3 (0.18 %) | 9 (0.68 %) | 7 (0.7 %) | 13 (2.33 %) | 35 (8.95 %) | 68 (0.87 %) | 0.000 |
During the Gamma wave, age, gender, and number of comorbidities were similar between vaccinated and unvaccinated populations (Table 3). However, patients who had received two doses of CoronaVac presented significantly less headache and fever, but more nasal congestion and runny nose (Table S9).
Table 3.
Demographic and clinical characteristics of COVID-19 positive cases according to vaccination status during the Gamma wave in individuals aged 18 years and older.
| Clinical and demographic characteristics per vaccination status - Gamma wave | |||
|---|---|---|---|
| Vaccinated (n = 474) | Unvaccinated (n = 202) | p | |
| Age (mean ± SD) | 43.3 ± 15.9 | 42.1 ± 14.5 | 0.196 |
| Female, n (%) | 242 (51.1 %) | 105 (52.0 %) | 0.826 |
| Comorbidities | |||
| No comorbidities | 345 (72.8 %) | 151 (74.8 %) | 0.843 |
| Hypertension | 92 (19.4 %) | 40 (19.8 %) | 0.906 |
| Diabetes | 42 (8.9 %) | 17 (8.4 %) | 0.851 |
| Asthma/C.O.P.D. | 9 (1.9 %) | 7 (3.5 %) | 0.220 |
| Symptoms, median (IQR) | 6 (2–10) | 6 (3–9) | 0.557 |
| Positivity (%) | 39.5 % | 40.9 % | 0.757 |
| WHO Score≥4, n (%) | 28 (5.9 %) | 17 (8.4 %) | 0.231 |
| Deaths, n (%) | 8 (1.7 %) | 1 (0.5 %) | 0.216 |
3.5. Multivariate model for symptoms and COVID-19 outcomes
The results of multivariate analyses are presented in Table 4, Table 5. During the Ancestral wave, patients with a runny nose and sore throat had lower chances of being positive for COVID-19 (OR 0.66, p < 0.01 and OR 0.77, p < 0.01, respectively) or developing severe disease (OR 0.46, p = 0.01 and OR 0.50, p = 0.02, respectively). Patients with dyspnea had a lower chance of being positive for SARS-CoV-2 (OR 0.47, p < 0.01) but those positive had a higher chance of developing a severe disease (OR 7.54, p < 0.01). Finally, patients with fever had higher chances of being positive for SARS-CoV-2 (OR 1.57, p < 0.01) and having a more severe disease (OR 1.96, p = 0.02).
Table 4.
Multivariate analysis for COVID-19 positivity across different variant waves, from June 2020 to December 2022.
| Multivariate model for SARS-CoV-2 positivity | |||||
|---|---|---|---|---|---|
| Ancestral | Gamma | Delta | Omicron BA.1 | Omicron BA.2 | |
| Anosmia | 2.47 [2.09–2.92] | 2.57 [2.01–3.27] | 3.76 [2.89–4.90] | N/A | N/A |
| Ageusia | 1.56 [1.32–1.85] | 1.35 [1.06–1.72] | NS | N/A | N/A |
| Headache | NS | NS | NS | N/A | N/A |
| Nasal congestion | NS | N/A | 1.26 [1.06–1.49] | NS | N/A |
| Runny nose | 0.66 [0.59–0.74] | 0.64 [0.56–0.74] | 0.84 [0.71–0.99] | NS | N/A |
| Diarrhea | N/A | N/A | N/A | 0.62 [0.54–0.72] | 0.59 [0.40–0.86] |
| Dyspnea | 0.47 [0.35–0.65] | N/A | N/A | N/A | 0.62 [0.43–0.89] |
| Sore throat | 0.77 [0.69–0.86] | 0.66 [0.58–0.76] | 0.56 [0.48–0.66] | 1.36 [1.21–1.52] | N/A |
| Myalgia | 1.37 [1.21–1.54] | NS | 1.50 [1.27–1.76] | 1.17 [1.04–1.32] | N/A |
| Fatigue | NS | N/A | 0.74 [0.64–0.87] | N/A | N/A |
| Fever | 1.57 [1.40–1.76] | 1.63 [1.42–1.87] | 1.78 [1.53–2.06] | 1.16 [1.03–1.30] | N/A |
| Nausea | N/A | 0.67 [0.56–0.80] | N/A | 0.74 [0.65–0.85] | N/A |
| Cough | 1.40 [1.24–1.58] | 1.39 [1.18–1.62] | 1.77 [1.47–2.13] | 1.33 [1.16–1.52] | N/A |
∗NS (Not Significant) - parameter analyzed in a given wave, but with no statistically significant results. N/A (Not applicable) - parameter not analyzed in this model in a given wave, as it was not significant in the previous model.
Table 5.
Multivariate analysis for COVID-19 severity across different variant waves, from June 2020 to December 2022.
| Multivariate model for COVID-19 severity | |||||
|---|---|---|---|---|---|
| Ancestral | Gamma | Delta | Omicron BA.1 | Omicron BA.2 | |
| Headache | N/A | N/A | NS | NS | N/A |
| Nasal congestion | NS | 0.39 [0.25–0.62] | NS | N/A | N/A |
| Runny nose | 0.46 [0.25–0.81] | 0.49 [0.31–0.77] | 0.22 [0.10–0.52] | NS | N/A |
| Diarrhea | N/A | NS | N/A | N/A | N/A |
| Dyspnoea | 7.54 [3.49–16.28] | 3.46 [2.16–5.54] | 5.98 [2.73–13.09] | 5.55 [2.19–14.06] | 10.56 [1.72–64.78] |
| Sore throat | 0.50 [0.29–0.88] | N/A | 0.43 [0.19–0.98] | 0.01 [0.00–0.12] | N/A |
| Myalgia | N/A | N/A | NS | NS | N/A |
| Fatigue | N/A | NS | N/A | N/A | N/A |
| Fever | 1.96 [1.12–3.42] | NS | N/A | NS | N/A |
| Cough | N/A | N/A | N/A | NS | N/A |
∗NS (Not Significant) - parameter analyzed in a given wave, but with no statistically significant results. N/A (Not applicable) - parameter not analyzed in this model in a given wave, as it was not significant in the previous model.
Change or loss of taste and smell, hallmarks of COVID-19 infection, were significantly associated with a higher probability of SARS-CoV-2 positivity during the Ancestral wave (ORs 2.47, p < 0.01 and OR 1.56, p < 0.01 respectively). A similar pattern was also found in patients with cough (OR 1.40, p < 0.01) and myalgia (OR 1.37, p < 0.01). However, none of these symptoms were significantly associated with COVID-19 severity during this wave.
In the Gamma variant wave, runny nose and sore throat kept having lower chances of being positive for SARS-CoV-2 (OR 0.64, p < 0.01; 0.66, p < 0.01; respectively), but only runny nose maintained a lower chance of developing severe COVID-19 (OR 0.49, p < 0.01). Fever, ageusia, and anosmia also had the same pattern, i.e., higher chances of having a positive RT-PCR result (OR 1.63, p < 0.01; 1.39, p < 0.01; 1.35, p = 0.02; respectively). Similarly, patients with dyspnea had a significantly higher chance for worse progression (OR 3.46, p < 0.01). Differently from the Ancestral wave, nausea appeared as a symptom negatively associated with SARS-CoV-2 positivity (0.67, p < 0.01, respectively) and nasal congestion with a lower risk of developing severe COVID-19 (OR 0.39, p < 0.01).
During the Delta wave, similarly, anosmia, fever, cough, and nasal congestion maintained their association with SARS-CoV-2 positivity (OR 3.76, p < 0.01; 1.78, p < 0.01; 1.77, p < 0.01; and 1.26, p = 0.01; respectively), and dyspnea with a higher chance of developing severe COVID-19 (OR 5.98, p < 0.01). Of note, runny nose and sore throat presented a different pattern, being associated with both positivity (OR 0.84, p = 0.04 and OR 0.56, p < 0.01, respectively) and severity (OR 0.22, p < 0.01 and OR 0.43, p = 0.05, respectively).
In the Omicron BA.1 and Omicron BA.2 waves, we noted that the symptom pattern changed even more. With the Omicron BA.1 variant, patients with diarrhea and nausea had lower chances of being positive for SARS-CoV-2 (OR 0.62, p < 0.01 and 0.74, p < 0.01, respectively). Sore throat increased the risk of SARS-CoV-2 positivity (OR 1.36, p < 0.01) but strikingly lowered the chance of developing severe COVID-19 (OR 0.01, p < 0.01). Similarly, dyspnea figured as the only symptom to be associated with an increase in the risk of severe COVID-19 development (OR 5.55, p < 0.01). In the Omicron BA.2 wave, only diarrhea was associated with lower risks of SARS-CoV-2 positivity (OR 0.59).
4. Discussion
We showed that although patients with COVID-19 from different SARS-CoV-2 variants shared some clinical characteristics, each variant presented distinguishable features related to positivity and severity. Patients infected during the Delta wave were the youngest, and during the Gamma wave presented the highest mortality rate. Ageusia and anosmia, related to SARS-CoV-2 positivity during the Ancestral, Gamma, and Delta waves, lost this significant relation during the Omicron BA.1 and Omicron BA.2 waves. In contrast, diarrhea presented a lower chance of positivity only in the last two waves. Dyspnea was the most consistent risk factor for severity across all waves.
In contrast to other studies in Brazil in which men had a higher positivity for SARS-CoV-2, the proportion of women with COVID-19 in our study was higher than men [21]. In Serrana, with the enhanced health surveillance system, we facilitated access to diagnostic tests for all suspected cases [10]. As there is a higher frequency of women attending medical care, this might be one explanation for the difference we observed [22].
The positivity rates varied among the different VOCs, reaching a peak during the Omicron BA.1 wave (60.2 % of suspected cases were positive for SARS-CoV-2). The high positivity rate during Omicron BA.1 could be attributed to the variant’s transmissibility, socioeconomic factors affecting the Serrana population, and the enhanced health surveillance system. Although positivity rates as high as 60 % were not common, other regions have also reported such rates. According to an official report from the Brazilian Health System, the positivity rate in 2022 reached up to 78.8 % in Sergipe and 56.32 % in São Paulo city [23]. Additionally, a report from the Chinese Center for Disease Control and Prevention indicated that some regions experienced a positivity rate of approximately 60 % [24]. In a cross-sectional study at a walk-up community COVID-19 testing site in San Francisco during the Omicron BA.1 wave, symptomatic participants had a positivity rate of 41.6 % [25]. Positivity rates were also higher in older individuals. Previous studies have shown that infants and school-age children tend to have more respiratory infections, in addition to COVID-19, compared to the older population [26,27]. Consequently, as we found, the positivity rate in suspected cases was lower in the younger age group.
The severity of cases decreased over time, ranging from 9.6 % in the Ancestral wave to 1.83 % in the Omicron BA.2 wave. Our findings align with other studies regarding fewer severe cases during Omicron BA.1 and Omicron BA.2 compared to previous waves [[28], [29], [30]]. We also found that the severity of COVID-19 was significantly higher in older individuals, which is consistent with previous studies showing that the disease is more severe in the elderly [31,32].
Symptoms across waves changed significantly, and several of them are similar to those of other endemic viruses. We did not find any single symptom/pattern for each variant that could make the COVID-19 diagnosis without testing. For example, although ageusia and anosmia were predictors of SARS-CoV-2 positivity during the first three waves, they had a frequency of approximately 40 % among positive cases. Additionally, their frequency decreased even more with the Omicron BA.1 and Omicron BA.2 waves and were not any longer significantly associated with positivity. Thus, keeping updated about the clinical manifestation and risk factors for positivity and severity is essential for clinicians, especially in areas with scarcities of diagnostic tests, isolated rooms, and ICU beds. Additionally, it is important to update institutional screening questions/protocols based on the circulating virus to reflect the most prevalent symptoms, while removing those that are less effective for screening.
During the Ancestral wave, patients presenting fever, cough, anosmia, and ageusia were more prone to be positive for SARS-CoV-2 infection [33]. Although individuals with dyspnea at the moment of sample collection had a lower positivity rate, when present, they had a considerably higher chance of developing a severe disease. This pattern of dyspnea was observed across all variants in this study and could be considered an alert signal for severe COVID-19 cases. Dyspnea has also been related to long COVID [34]. Therefore, some symptoms may help during the patient triage, while others are more useful to indicate the severity and, consequently, the need for hospitalizations. Such information is relevant especially during a pandemic with limited ICU beds [35].
Noticeable alterations in clinical manifestations were observed mainly when the rapid-spreading Omicron BA.1 variant became the most prevalent among sequenced samples. Symptoms such as anosmia and ageusia remarkably associated with COVID-19 in the first waves sharply decreased during the Omicron BA.1 and Omicron BA.2 variants, similar to what was observed in other studies [28,36,37]. An intriguing finding was the prominence of respiratory symptoms, as patients with sore throats had increased risks of being positive for SARS-CoV-2 (OR 1.36) during the Omicron BA.1 wave, in contrast to the preceding waves. Indeed, these symptoms were suggested to be a hallmark of the Omicron BA.1 variant [30,38,39]. In our model, however, sore throat had a strikingly low correlation with severe COVID-19 development. Another interesting shift of patterns observed during the Omicron BA.1 and Omicron BA.2 wave was the appearance of diarrhea as a significant symptom associated with lower positivity during this period [28].
Despite variations in clinical manifestations across different variant waves, symptoms such as cough and fever displayed minor fluctuations throughout the pandemic's progression, maintaining a strong correlation with COVID-19 positivity irrespective of the predominant VOC [39]. Of note, vaccination also influences COVID-19 clinical manifestation, shifting it towards a less systemic disease. Vaccinated adult patients infected during the Gamma wave presented significantly less headache and fever, but more nasal congestion and runny nose. Similarly, Nakakubo et al. have also demonstrated that vaccination and previous infection reduced the prevalence of systemic symptoms in Omicron BA.2 and BA.5 variants, but increased upper respiratory symptoms [28].
Several measures during the pandemic, such as lockdowns and vaccination [40,41], may have impacted the dynamics of COVID-19 positivity and severity across waves and not only the VOCs themselves. Of note, from February to April 2021, Serrana became part of the 'Projeto S′, a stepped-wedge clinical trial to assess the efficacy of the CoronaVac vaccine, resulting in approximately 81.4 % of adults e 60.9 % of the entire population receiving two doses of the vaccine in 8 weeks [17]. This immunization coverage in a short period may have impacted disease severity in the following months.
This study has limitations. First, as comorbidities were self-reported, some of their proportions may have been underreported. In our study, among individuals over 18 years, the prevalence of hypertension was 19 % and diabetes was 8 %, which were close to the community prevalence rates. However, obesity prevalence was much lower than expected. In a cross section study using data from the National Health Survey (PNS) with 88,531 adults in Brazil, the self-reported prevalence of hypertension in individuals aged 18 years or older was 23.9 % (95%CI: 23.5–24.4), and the prevalence of diabetes was 7.7 % (95%CI: 7.4 %–8.0 %) [16,42]. Second, genetic sequencing was performed on a sampling basis, therefore the determined period of each wave may contain patients infected by other variants. Third, during the study, individuals may have received up to five vaccine doses and experienced several reinfections. Consequently, we could not account for all these variables in the analysis of COVID-19 clinical manifestations between vaccinated and unvaccinated populations across all waves. However, since 81.4 % of Serrana's adult population received two doses of CoronaVac within a short period (eight weeks starting in February 2021), we were able to analyze the impact of vaccination during the Gamma wave. Finally, the high positivity rate, particularly during the Omicron BA.1 wave, has not been observed across several regions and counties, which makes these results less generalizable.
The main strength of our study is that the Serrana population had facilitated access to diagnostic tests for COVID-19, and we followed nearly all suspected cases during the pandemic. In addition, the comprehensive questionnaire at sample collection and case follow-up of all positive individuals provided insightful information on disease development among the different waves in the general population, not only in hospitalized patients.
Examining alterations in demographic characteristics and symptom manifestations and understanding their influence on both the positivity and severity of COVID-19 enhances our comprehension of the disease's pathophysiology. As clinicians rely on symptoms when they are seeing patients and new SARS-CoV-2 variants are still emerging, our findings highlight the importance of studying SARS-CoV-2 clinical manifestations to update guidelines and recommendations.
CRediT authorship contribution statement
Bruno Belmonte Martinelli Gomes: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Natasha Nicos Ferreira: Writing – review & editing, Methodology, Investigation, Formal analysis, Data curation. Pedro Manoel Marques Garibaldi: Writing – review & editing, Supervision, Methodology, Conceptualization. Cassia Fernanda Sales de Lima Dias: Writing – review & editing, Methodology, Data curation, Conceptualization. Letícia Nakamura Silva: Writing – review & editing, Supervision, Methodology. Maria Aparecida Alves Leite dos Santos Almeida: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Glenda Renata de Moraes: Writing – review & editing, Supervision, Resources. Dimas Tadeu Covas: Writing – review & editing, Resources, Project administration, Funding acquisition. Simone Kashima: Writing – review & editing, Supervision, Methodology. Rodrigo Tocantins Calado: Writing – review & editing, Supervision, Resources, Funding acquisition. Benedito Antônio Lopes Fonseca: Writing – review & editing, Supervision, Resources, Funding acquisition. Gustavo Jardim Volpe: Writing – review & editing, Supervision, Methodology, Formal analysis, Data curation, Conceptualization. Marcos de Carvalho Borges: Writing – review & editing, Supervision, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Ethical considerations
The local research ethics committee approved this analysis as a public health investigation and surveillance and waived the requirement for informed consent (CAAE 51760221.2.0000.5440).
Data availability
Data will be made available on request.
Funding source
This work was supported by Fundação de Apoio à Pesquisa do Estado de São Paulo (FAPESP).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e40113.
Contributor Information
Bruno Belmonte Martinelli Gomes, Email: bbmgomes@cpcs.faepa.br.
Natasha Nicos Ferreira, Email: natashanicos@heserrana.faepa.br.
Pedro Manoel Marques Garibaldi, Email: pmmgaribaldi@cpcs.faepa.br.
Cassia Fernanda Sales de Lima Dias, Email: cferdias@gmail.com.
Letícia Nakamura Silva, Email: lnakamura@cpcs.faepa.br.
Maria Aparecida Alves Leite dos Santos Almeida, Email: maria.aalmeida@fundacaobutantan.org.br.
Glenda Renata de Moraes, Email: glendamoraes66@gmail.com.
Dimas Tadeu Covas, Email: dimas@fmrp.usp.br.
Simone Kashima, Email: skashima@hemocentro.fmrp.usp.br.
Rodrigo Tocantins Calado, Email: rtcalado@fmrp.usp.br.
Benedito Antônio Lopes Fonseca, Email: baldfons@fmrp.usp.br.
Gustavo Jardim Volpe, Email: gjvolpe@hcrp.usp.br.
Marcos de Carvalho Borges, Email: marcosborges@fmrp.usp.br.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.COVID-19 deaths | WHO COVID-19 dashboard. datadot http://data.who.int/dashboards/covid19/cases.
- 2.Naqvi A.A.T., et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866:165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troyano-Hernáez P., Reinosa R., Holguín Á. Evolution of SARS-CoV-2 envelope, membrane, nucleocapsid, and spike structural proteins from the beginning of the pandemic to september 2020: a global and regional approach by epidemiological week. Viruses. 2021;13:243. doi: 10.3390/v13020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi J.Y., Smith D.M. SARS-CoV-2 variants of concern. Yonsei Med. J. 2021;62:961. doi: 10.3349/ymj.2021.62.11.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tregoning J.S., Flight K.E., Higham S.L., Wang Z., Pierce B.F. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat. Rev. Immunol. 2021;21:626–636. doi: 10.1038/s41577-021-00592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emmelot M.E., et al. Omicron BA.1 mutations in SARS-CoV-2 spike lead to reduced T-cell response in vaccinated and convalescent individuals. Viruses. 2022;14:1570. doi: 10.3390/v14071570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brazil Country Overview | World Health Organization. https://www.who.int/countries/bra.
- 8.Sott M.K., Bender M.S., da Silva Baum K. Covid-19 outbreak in Brazil: health, social, political, and economic implications. Int. J. Health serv. Plan. Adm. Eval. 2022;52:442–454. doi: 10.1177/00207314221122658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han E., et al. Lessons learnt from easing COVID-19 restrictions: an analysis of countries and regions in Asia Pacific and Europe. Lancet. 2020;396:1525–1534. doi: 10.1016/S0140-6736(20)32007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferreira N.N., et al. The impact of an enhanced health surveillance system for COVID-19 management in Serrana, Brazil. Public Health Pract. 2022;4:100301. doi: 10.1016/j.puhip.2022.100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Candido D.S., et al. 2020. Evolution and Epidemic Spread of SARS-CoV-2 in Brazil. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croda J.H.R., Garcia L.P. Immediate health surveillance response to COVID-19 epidemic. Epidemiol. E Serviços Saúde. 2020;29 doi: 10.5123/S1679-49742020000100021. [DOI] [PubMed] [Google Scholar]
- 13.Croda J., et al. COVID-19 in Brazil: advantages of a socialized unified health system and preparation to contain cases. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0167-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kameda K., et al. Testing COVID-19 in Brazil: fragmented efforts and challenges to expand diagnostic capacity at the Brazilian Unified National Health System. Cad. Saúde Pública. 2021;37 doi: 10.1590/0102-311X00277420. [DOI] [PubMed] [Google Scholar]
- 15.Covid-19: Lack of testing in Brazil is a “major failure,” says MSF - ProQuest. https://www.proquest.com/openview/6c2049ef287d1f82c84a76b5ace12006/1?pq-origsite=gscholar&cbl=2043523. [DOI] [PubMed]
- 16.Slavov S.N., et al. Dynamics of SARS-CoV-2 variants of concern in vaccination model city in the state of Sao Paulo, Brazil. Viruses. 2022;14:2148. doi: 10.3390/v14102148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borges M.C., et al. Projeto S: a stepped-wedge randomized trial to assess CoronaVac effectiveness in Serrana, Brazil. 2021. Preprint - SSRN Scholarly Paper at. [DOI]
- 18.Zhou, H. et al. Sensitivity to vaccines, therapeutic antibodies, and viral entry inhibitors and advances to counter the SARS-CoV-2 omicron variant. Clin. Microbiol. Rev. 35, e00014-22. [DOI] [PMC free article] [PubMed]
- 19.Harris P.A., et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McKinney W. Data structures for statistical computing in Python. Proc. 9th Python Sci. Conf. 2010:56–61. doi: 10.25080/Majora-92bf1922-00a. [DOI] [Google Scholar]
- 21.De Souza W.M., et al. Epidemiological and clinical characteristics of the COVID-19 epidemic in Brazil. Nat. Hum. Behav. 2020;4:856–865. doi: 10.1038/s41562-020-0928-4. [DOI] [PubMed] [Google Scholar]
- 22.Boccolini C.S., de Souza Junior P.R.B. Inequities in healthcare utilization: results of the Brazilian national health Survey, 2013. Int. J. Equity Health. 2016;15:150. doi: 10.1186/s12939-016-0444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministério da Saúde B. Boletim epidemiológico N° 106 - boletim COE coronavírus. 2022. https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/covid-19/2022/boletim-epidemiologico-no-106-boletim-coe-coronavirus.pdf/view
- 24.Chinese Center for Disease Control and Prevention COVID-19 clinical and surveillance data — december 9, 2022 to january 23, 2023, China. 2023. https://en.chinacdc.cn/news/latest/202301/W020230126558725888448.pdf
- 25.Marquez C., et al. COVID-19 symptoms and duration of rapid antigen test positivity at a community testing and surveillance site during pre-delta, delta, and omicron BA.1 periods. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.35844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avendaño Carvajal L., Perret Pérez C. Epidemiology of respiratory infections. Pediatr. Respir. Dis. 2020;263–272 doi: 10.1007/978-3-030-26961-6_28. [DOI] [Google Scholar]
- 27.Thomas M., Bomar P.A. StatPearls. StatPearls Publishing; Treasure Island (FL): 2024. Upper respiratory tract infection. [PubMed] [Google Scholar]
- 28.Nakakubo S., et al. Associations of COVID-19 symptoms with omicron subvariants BA.2 and BA.5, host status, and clinical outcomes in Japan: a registry-based observational study. Lancet Infect. Dis. 2023;23:1244–1256. doi: 10.1016/S1473-3099(23)00271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu F.-H., et al. Clinical outcomes of the severe acute respiratory syndrome coronavirus 2 Omicron and Delta variant: systematic review and meta-analysis of 33 studies covering 6 037 144 coronavirus disease 2019-positive patients. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023;29:835–844. doi: 10.1016/j.cmi.2023.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee S., Bhattacharya M., Nag S., Dhama K., Chakraborty C. A detailed overview of SARS-CoV-2 omicron: its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses. 2023;15:167. doi: 10.3390/v15010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller A.L., McNamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging. 2020;12:9959–9981. doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartleson J.M., et al. SARS-CoV-2, COVID-19 and the aging immune system. Nat. Aging. 2021;1:769–782. doi: 10.1038/s43587-021-00114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilinska K., Butowt R. Anosmia in COVID-19: a bumpy road to establishing a cellular mechanism. ACS Chem. Neurosci. 2020;11:2152–2155. doi: 10.1021/acschemneuro.0c00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sudre C.H., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikbakht Nasrabadi A., Abbasi S., Mardani A., Maleki M., Vlaisavljevic Z. Experiences of intensive care unit nurses working with COVID-19 patients: a systematic review and meta-synthesis of qualitative studies. Front. Public Health. 2022;10:1034624. doi: 10.3389/fpubh.2022.1034624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aghagoli G., et al. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocrit. Care. 2021;34:1062–1071. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yen Y.-F., et al. Olfactory disorder in patients infected with SARS-CoV-2. J. Microbiol. Immunol. Infect. 2021;54:992–996. doi: 10.1016/j.jmii.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whitaker M., et al. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat. Commun. 2022;13:1957. doi: 10.1038/s41467-022-29521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menni C., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet Lond. Engl. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajkumar E., et al. The psychological impact of quarantine due to COVID-19: a systematic review of risk, protective factors and interventions using socio-ecological model framework. Heliyon. 2022;8 doi: 10.1016/j.heliyon.2022.e09765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moura E.C., et al. Covid-19: temporal evolution and immunization in the three epidemiological waves, Brazil, 2020–2022. Rev. Saude Publica. 2022;56:105. doi: 10.11606/s1518-8787.2022056004907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis R.C.P.D., Duncan B.B., Malta D.C., Iser B.P.M., Schmidt M.I. Evolution of diabetes in Brazil: prevalence data from the 2013 and 2019 Brazilian national health Survey. Cad. Saude Publica 38Suppl. 2022;1 doi: 10.1590/0102-311X00149321. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.