Abstract
Deep vein thrombosis (DVT) is a prevalent clinical condition, which markedly affects patients' quality of life, commonly leading to post-thrombotic syndrome. The present study aimed to elucidate the intricate interplay between murine double minute-2 (MDM2) and phosphatase and tensin homolog (PTEN), thus shedding new light on their role in the pathogenesis of DVT. The results showed that both MDM2 and PTEN were upregulated in venous blood samples obtained from patients with DVT. However, MDM2 or PTEN knockdown markedly increased the proliferation, migration, invasion, apoptosis and angiogenesis of oxidized low-density lipoprotein-treated human umbilical vein endothelial cells (HUVECs). Furthermore, MDM2 silencing downregulated PTEN. The association between MDM2 and PTEN was verified through comprehensive analyses, including Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) analysis and co-immunoprecipitation assays. The effect of PTEN on DVT was evaluated by Kyoto Encyclopedia of Genes and Genomes and STRING analysis, which demonstrated that PTEN displayed an inhibitory role in the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) signaling pathway. Notably, treatment with AG-490, an inhibitor of JAK/STAT signaling, reversed the protective effect of PTEN knockdown on the behavior of HUVECs. In summary, the results of the current study indicated that both MDM2 and PTEN were upregulated in patients with DVT. The interaction between MDM2 and PTEN was also verified, thus providing novel insights into their potential collaborative role in the development of DVT. Overall, MDM2 and PTEN may interact to inhibit endothelial cell development and promote the occurrence of DVT via inhibiting the JAK/STAT signaling pathway.
Keywords: murine double minute-2, phosphatase and tensin homolog, deep vein thrombosis, Janus kinase/signal transducer and activator of transcription pathway
Introduction
Deep vein thrombosis (DVT) is a disorder which is characterized by abnormal blood clotting in deep veins, eventually leading to venous return obstruction and luminal occlusion. The incidence of DVT is relatively high in lower limb veins, and particularly in the femoral vein (1). The prevalence of DVT is relative higher in men compared with women and it significantly increases with age (2). It has been reported that 20–50% of patients develop post-thrombotic syndrome (PTS) following the initial onset of DVT (3,4). In severe cases, PTS can be accompanied by persistent symptoms, such as edema, heaviness, pigmentation, pain and even venous ulcers. Furthermore, DVT poses a life-threatening risk when the clot dislodges to promote the development of pulmonary embolism (PE), thus resulting in sudden heart failure with potentially fatal outcomes (4). Catheter-directed thrombolysis is a procedure that involves the use of a catheter to directly deliver drugs into the blood clot in order to dissolve it. This treatment is commonly used to treat DVT and PE (5). Therefore, investigating the molecular mechanisms underlying DVT pathogenesis, searching for novel biological therapies and precise biomarkers for the early diagnosis of DVT and establishing reliable prognostic indicators are of great importance. Such efforts will not only advance the clinical diagnostics and treatment of DVT, but also hold promise for future advancements in this field.
Emerging evidence has suggested that thrombosis is commonly affected by three major factors, namely vascular wall injury, alterations in blood flow and abnormalities in blood composition (6). Among these, vascular wall injury primarily involves the injury of endothelial cells lining the blood vessels. Vascular endothelial cell damage encompasses various processes, including apoptosis, which is recognized as a critical contributor of venous thrombosis. Extensive research has substantiated the close association between vascular endothelial cell injury and the onset and progression of DVT (7). A previous study indicated that the apoptosis of vascular endothelial cells could result in reduced levels of vasoactive substances, and impaired defense mechanisms and stability of endothelial cells (8). These multifaceted consequences could markedly affect the pathogenesis of DVT.
Murine double minute-2 (MDM2), an E3 ubiquitin ligase, plays a crucial role in regulating the stability and activity of p53 (9). In addition to its role as an oncogene, emerging evidence has indicated that MDM2 is also involved in the development of cardiovascular diseases (10). A previous study demonstrated that MDM2 upregulation promotes susceptibility to ischemia-induced neuronal apoptosis (11). Furthermore, MDM2 is upregulated in human atherosclerotic tissue and is associated with vascular cell proliferation and inflammation (12). Notably, other studies revealed that MDM2 not only promotes the induction of vascular calcification via ubiquitinating histone deacetylases, but it can also enhance mitochondrial injury in endothelial cells and trigger inflammatory responses elicited by oxidized low-density lipoproteins (ox-LDL) (13,14). Furthermore, MDM2 can promote vascular endothelial growth factor expression, thus enhancing cancer cell survival and angiogenesis in human neuroblastoma cell lines (15). In terms of DVT, a previous study revealed that MDM2 is upregulated in patients with DVT and mouse models, while MDM2 downregulation can inhibit thrombosis by promoting the migration, invasion and angiogenesis of endothelial progenitor cells (EPCs) (16). The aforementioned findings underscore the crucial role of MDM2 in DVT pathogenesis via modulating the functions of EPCs.
Phosphatase and tensin homolog (PTEN), a tumor suppressor gene located on chromosome 10q23, acts as a phosphatase and is involved in regulating several cellular processes, including cell proliferation, invasion, migration and apoptosis via dephosphorylation (17). PTEN can modulate the migration and proliferation of vascular smooth muscle cells to aggravate atherosclerosis (18). Another study indicates that PTEN can play a significant role in integrin b3-mediated amelioration of cardiomyocyte damage due to hypoxia (19). In addition, the expression levels of PTEN in the blood of patients who have undergone coronary artery bypass grafting surgery can serve as a significant marker of coronary heart disease (20). Additionally, PTEN is found to modulate the activation of several signaling pathways involved in disease progression, such as the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), Janus kinase (JAK)/signal transducer and activator of transcription (STAT), focal adhesion kinase (FAK) and, more recently, extracellular signal-regulated kinase (ERK)1/2 pathways (21,22). The JAK/STAT signaling pathway plays a crucial role in regulating cell development, differentiation, proliferation and apoptosis (23). Notably, the abnormal expression of molecules involved in the JAK/STAT signaling pathway is also involved in the formation of venous thrombosis (24).
The JAK/STAT pathway is a classic intracellular signaling pathway, which is involved in regulating several biological processes (25). This pathway plays a significant role in numerous diseases. In terms of cardiovascular diseases, the JAK/STAT pathway serves a significant regulatory role (26). More specifically, a study demonstrates that small molecule active substances extracted from Buyang Huanwu Decoction can inhibit atherosclerotic inflammation through JAK/STAT signaling (27). In addition, baicalin can regulate macrophage polarization and ameliorate myocardial ischemia-reperfusion injury via the JAK/STAT pathway (28). The JAK/STAT pathway is involved in the onset of coronary artery diseases (29). Ma et al (30) demonstrate that diosmin can affect the JAK/STAT pathway to inhibit vascular endothelial cell apoptosis, thus improving DVT. The aforementioned studies verified that the JAK/STAT pathway is one of the most significant pathways involved in regulating the course of cardiovascular diseases, including DVT.
Although MDM2 and PTEN exhibit distinct functions and mechanisms, they interact during tumorigenesis and disease progression. MDM2 could enhance tumor cell proliferation and survival via regulating PTEN (31). However, the regulatory association between MDM2 and PTEN in the context of DVT remains to be elucidated. Given the respective roles of MDM2 and PTEN in DVT, it is imperative to explore their regulatory interplay to gain deeper insights into the pathogenesis of DVT. Therefore, the present study aimed to investigate the regulatory association between MDM2 and PTEN and their association with the development of DVT, thus further enhancing our understanding of DVT pathogenesis. The results of the current study could expand the current knowledge in this area and offer a novel research direction for clinical treatment strategies.
Materials and methods
Samples
Peripheral blood samples were obtained from patients with DVT (n=5) and healthy subjects (n=5) during physical examination between January and June 2023, with age ranging from 40 to 60 years. The sex distribution in the DVT group is 3 males and 2 females, while in the healthy control group, the sex ratio is 2 females to 3 males. Briefly, a total of 10 ml of peripheral blood was isolated from subjects from both groups, and following centrifugation at 800 g, 4°C for 10 min, the serum was isolated and stored at −80°C. All patients provided written informed consent for their enrollment in the present study, which was approved by the Ethics Committee of the Fengyang County People's Hospital.
Cell culture
Human umbilical vein endothelial cells (HUVECs, cat. no. CTCC-009-493), as immortalized cells, not primary cells, were purchased from the Cell Bank of the Chinese Academy of Sciences and cultured in Endothelial Cell Medium (ECM; cat. no. 1001; ScienCell Research Laboratories, Inc.) supplemented with 10% FBS (cat. no. 10099; Gibco; Thermo Fisher Scientific, Inc.) and 0.5% penicillin/streptomycin solution (cat. no. 15640055; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in an incubator with 5% CO2. HUVECs were treated with ox-LDL at 37°C for 48 h (40 µg/ml, cat. no. 20605ES10; YEASEN) to establish an in vitro model.
Reverse transcription-quantitative (RT-q) PCR
Total RNA was isolated from 5×105 cells or 1.5 ml peripheral blood samples using a TRIzol® reagent (cat. no. 15596018CN; Invitrogen; Thermo Fisher Scientific, Inc.) according to the protocols. The quality of the isolated RNA was then verified by UV spectrophotometry and formaldehyde denaturing electrophoresis. Subsequently, a total of 1 µg RNA was reverse transcribed into cDNA using AMV reverse transcriptase (cat. no. 10109118001; Roche Diagnostics, Ltd.) according to the manufacturer's protocols. The gene expression levels was amplified by using the SYBR qPCR kit (cat. no. RK21203; ABclonal), and normalized to GAPDH expression, were calculated using the 2−ΔΔCq method (32). The PCR reaction consisted of pre-denaturation at 94°C for 5 min, 40 cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 30 sec and extension at 72°C for 1 min. PCR was carried out using a real-time amplifier (cat. no. ABI PRISM7700, Thermo Fisher). The assays were performed in triplicate. The primer information was: MDM2: 5′-ATGAAAGCCTGGCTCTGTGT-3 (Forward), 5′-CACTCTCCCCTGCCTGATAC-3′ (Reverse); PTEN: 5′-AGTTCCCTCAGCCGTTACCT-3 (Forward), 5′-AGGTTTCCTCTGGTCCTGGT-3′ (Reverse); and GAPDH: 5′-CAGCCTCAAGATCATCAGCA-3 (Forward), 5′-TGTGGTCATGAGTCCTTCCA-3′ (Reverse).
Cell transfection
Prior transfection, cells were seeded into 24-well plates and cultured until they reached ~30% confluence. For short interfering (si)RNA transfection, 0.67 µg (50 pmol) siRNAs (Sangon Biotech Co., Ltd.) were diluted in the appropriate serum-free diluent at a final volume of 25 µl. Subsequently, 1 µl Entranster-R4000 (cat. no. 4000-3; Engreen Biosystem, Co., Ltd.) was mixed with 24 µl serum-free diluent to a final volume of 25 µl. The Entranster-R4000 diluent was left at room temperature for 5 min. Then, the Entranster-R4000 and RNA diluents were thoroughly mixed by oscillating with an oscillator or blowing with a sampler for >10 times. The resulting transfection complex (50 µl) supplemented with 0.45 ml of complete medium was added into each well. The petri dish was gently moved back and forth to ensure even distribution. Cell morphology was observed at 6 h after transfection and when cell viability was unaffected, this indicated that the transfection reagent did not exhibit toxicity to the cells and the medium was not changed. Cells were then cultured for 24–96 h at 37°C to obtain the desired results. The interference sequence were as follows: si-MDM2: SS sequence: GGAACUUGGUAGUAGUCAAUC, AS sequence: UUGACUACUACCAAGUUCCUG; si-PTEN: SS sequence: AGAUGUUAGUGACAAUGAACC, AS sequence: UUCAUUGUCACUAACAUCUGG; si-negative control (NC): SS sequence: UUCUCCGAACGUGUCACGU, AS sequence: ACGUGACACGUUCGGAGAA.
Clone formation assay
Cells were seeded in a 6-well plate at a density of 700 cells per well in complete medium. The cultures were maintained until the majority of single colonies reach a cell count of >50 cells/colony. The medium was changed every three days. Subsequently, cells were washed once with PBS and fixed with 4% paraformaldehyde (1 ml/well; cat. no. P1110; Beijing Solarbio Science & Technology Co., Ltd.) for 30 min at room temperature, followed by staining with crystal violet solution (1 ml/well cat. no. C0121; Beyotime Institute of Biotechnology) for 10 min at room temperature. Following washing with PBS (cat. no. 10010023; Gibco; Thermo Fisher Scientific, Inc.), cells were allowed to air-dry.
Wound healing assay
Cells were inoculated into a 6-well plate at a density of 5×105 cells/well. The following day, a scratch was made using a sterile pipette tip (200 µl, cat. no. BS-200-T; Biosharp). The cells were then washed three times with PBS and supplemented with serum-free ECM. Finally, cells were cultured in an incubator at 37°C with 5% CO2 for 0, 6, 12 and 24 h and observed under an optical light microscope (cat. no. CKX41, Olympus). Observe under 200× magnification and randomly select 3 fields of view for photography and counting.
Transwell assay
A total of 1×105 cells were re-suspended in serum-free ECM and were then added onto the upper chamber of the Transwell insert (cat. no. 14141; Beijing Labselect). Medium containing 10% FBS was supplemented into the lower chamber and cells were cultured for 48 h at 37°C. The cells were subsequently fixed with 70% methanol for 10 min at room temperature (cat. no. 322415; MilliporeSigma) and stained with 1% crystal violet for 5 min at room temperature. The invasive cells were counted at five randomly selected fields under an optical light microscope (cat. no. CKX41, Olympus). Observe under 100× magnification and randomly select 3 fields of view for photography and counting.
Flow cytometry
Cell apoptosis was assessed using an Annexin V-FITC/PI Kit (cat. no. 40302ES20; Shanghai Yeasen Biotechnology Co., Ltd.). Cells were collected, mixed with 5 µl Annexin V for 30 min at 37°C, then incubated with propidium iodide in the dark for 20 min at 37°C. Apoptotic cells were counted using flow cytometry (CytoFLEX LX; Beckman Coulter). Analyze the data using Flowjo software (Version 10.8; flowjo.com/; BD Biosciences). Combine the proportion of early apoptotic cells (Annexin V single positive) and late apoptotic cells were considered apoptotic cell proportion.
Angiogenesis assay
Angiogenesis was evaluated using an Angiogenesis Assay Kit (In Vitro; cat. no. ab204726; Abcam). A 96-well culture plate was coated with an extracellular matrix solution and incubated at 37°C for 1 h to allow gel formation. Subsequently, 70,000 cells were seeded onto the gel and cultured for an additional 18 h. After the incubation medium was removed, the cells/gel were washed. The formation of blood vessels was then evaluated under a light microscope (cat. no. CKX41, Olympus). Observe under 200× magnification and randomly select 3 fields of view for photography and counting.
Co-immunoprecipitation assays
5×105 cells in a petri dish were lysed with the corresponding lysis buffer (cat. no. 20-188; Merck Millipore) with a protease inhibitor (cat. no. P8340; MilliporeSigma) on ice for 30 min. After centrifugation at 2,000 g at 4°C in the maximum rotating speed for 30 min, supernatant was collected. A small portion of the lysate was subjected to western blot analysis, while the remaining lysate was mixed with 2 µg of the corresponding antibody and incubated at 4°C overnight. To prepare the protein A agarose beads (cat. no. P1925; MilliporeSigma), 10 µl of the solution/beads was washed three times with cracking buffer (cat. no. 20-188; Merck Millipore), followed by centrifugation at 1,000 g for 3 min each time at 4°C. A total of 10 µl pre-treated protein A agarose beads was added to the cell lysate, which was incubated with 2 µg MDM2 antibody (cat. no. 33-7100; Thermo Fisher) overnight, and the mixture was gently shaken at 4°C for 2–4 h. The aforementioned procedure allowed the coupling between the antibody and protein A agarose beads. Following immunoprecipitation, the agarose beads were centrifuged at 4°C for 3 min at 1,000 × g. After beads were pelleted, the supernatant was carefully aspirated and the agarose beads were washed with 100 µl Elution buffer (cat. no. P2179S; Beyotime Institute of Biotechnology) for 3–4 times. Lastly, the dilution was then supplemented with 100 µl 2X SDS loading buffer (cat. no. 9172; Takara Biotechnology Co., Ltd.) and the mixture was boiled for 5 min prior western blot analysis.
Western blot analysis
Proteins were extracted from cells using RIPA lysis buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) and protein concentration was quantified using a BCA kit (cat. no. P0011; Beyotime Institute of Biotechnology). A total of 30 µg total protein extracts were separated by 10% SDS-PAGE and were then transferred to PVDF membranes (cat. no. IPVH00010; MilliporeSigma). Subsequently, the membranes were blocked with 5% skimmed milk (cat. no. P0216; Beyotime Institute of Biotechnology) at 4°C for 2 h and then with primary antibodies at 4°C overnight. Following washing with PBS-1% Tween-20 (PBST; cat. no. BL314B; Biosharp Life Sciences) for three times, the membrane was incubated for 2 h with the corresponding secondary antibody. After washing with PBST for three times, the protein bands were visualized using ECL Chemiluminescent buffer (cat. no. 36208ES76; YEASEN) in a dark room. The grayscale values were calculated using ImageJ software (Version 1.8.0; National Institutes of Health). The following primary antibodies were used: Anti-MDM2 (cat. no. ab16895), anti-PTEN (cat. no. ab32199), anti-phosphorylated (p)-JAK1 (cat. no. ab138005), anti-JAK1 (cat. no. ab133666), anti-p-STAT3 (cat. no. ab76315), anti GAPDH (cat. no. ab8245) and anti-STAT (cat. no. ab68153; all from Abcam). In addition, the HRP anti-rabbit IgG antibody (cat. no. ab288151; Abcam) served as a secondary antibody.
Bioinformatics analysis
Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database (string-db.org/) was used to verify the association between MDM2 and PTEN.
Statistical analysis
All statistical analyses were performed using GraphPad prism 6.0 (Dotmatics). The experiments were independently repeated at least three times. The results are expressed as the mean ± standard error of the mean. The differences between two groups were compared using unpaired Student's t-test, while those among multiple groups by ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of MDM2 and PTEN in venous blood samples from patients with DVT
To investigate the involvement of MDM2 and PTEN in DVT, RT-qPCR analysis was performed to detect their expression levels in venous blood samples obtained from patients with DVT. The participants were allocated into two groups, namely the control and DVT groups. The results revealed that MDM2 and PTEN were markedly upregulated in the intravenous blood samples of patients with DVT compared with the control group (Fig. 1A and B). These results strongly supported the potential significance of MDM2 and PTEN in the pathogenesis of DVT.
Figure 1.
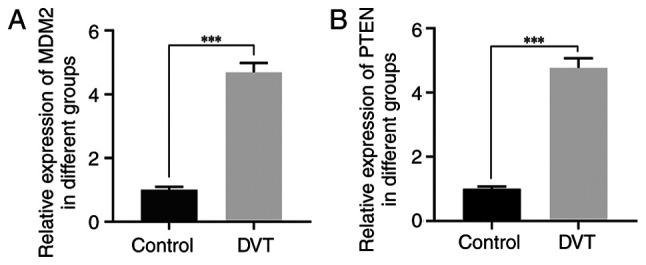
Expression of MDM2 and PTEN in venous blood samples of patients with DVT. (A) Expression of MDM2 in venous blood samples of patients with DVT detected by RT-PCR. (B) The expression of PTEN in venous blood samples of patients with DVT detected by RT-PCR. ***P<0.001. MDM2, murine double minute-2; PTEN, phosphatase and tensin homolog; DVT, deep vein thrombosis; RT-qPCR, reverse transcription-quantitative PCR.
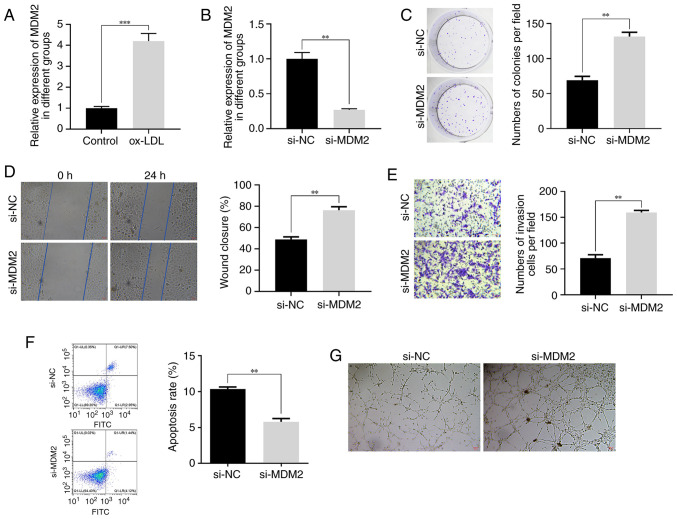
Effect of MDM2 silencing on ox-LDL-treated HUVECs
To investigate the role of MDM2 in the development of DVT, an in vitro model was established. The results demonstrated that HUVECs treatment with ox-LDL significantly upregulated MDM2 (Fig. 2A). To further elucidate the functional significance of MDM2 in different cellular behaviors, the siRNA-mediated knockdown approach was employed in HUVECs (Fig. 2B). Notably, MDM2 knockdown not only promoted the proliferation of ox-LDL-treated HUVECs (Fig. 2C), but also markedly enhanced their migration and invasion abilities (Fig. 2D and E). The aforementioned findings strongly suggested that MDM2 could play a crucial role in facilitating cell metastasis and invasion during DVT progression. In addition, the results showed that MDM2 silencing suppressed the apoptosis of ox-LDL-treated HUVECs. More particularly, cell apoptosis was significantly reduced in the si-MDM2 group compared with the control group (Fig. 2F). This finding indicated that MDM2 could modulate the delicate balance between cell survival and death throughout DVT development. Furthermore, MDM2 silencing also significantly augmented the angiogenic capacity of ox-LDL-treated HUVECs. Therefore, tube formation assays demonstrated that cells in the si-MDM2 group displayed a more pronounced vascular lumen structure (Fig. 2G). Collectively, these compelling findings underscored the critical involvement of MDM2 in DVT pathogenesis and highlighted its potential therapeutic value in this context.
Figure 2.
Effect of low expression of MDM2 on HUVECs treated with ox-LDL. (A) The expression of MDM2 in HUVECs treated with 40 µg/ml ox-LDL detected by RT-qPCR. (B) The expression of MDM2 in HUVECs knocked down by si-MDM2 detected by RT-qPCR. (C) The effect of MDM2 knockdown on HUVECs proliferation detected by cloning assay. (D) The effect of MDM2 knockdown on HUVECs migration detected by wound healing assay. (E) Effect of MDM2 knockdown on HUVECs invasion detected by Transwell assay. Magnification 200×. (F) The effect of MDM2 knockdown on HUVECs apoptosis detected by flow cytometry. (G) The effect of MDM2 knockdown on HUVECs angiogenic ability detected by tube formation assay. Magnification, 200×. **P<0.01, ***P<0.001. MDM2, murine double minute-2; HUVECs, human umbilical vein endothelial cells; ox-LDL, oxidized low-density lipoproteins; RT-q-PCR, reverse transcription-quantitative PCR; si, short interfering.
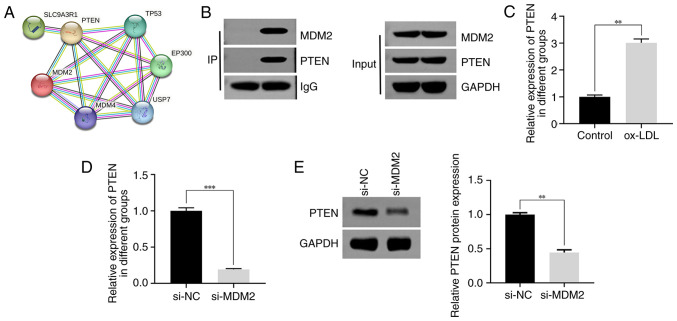
Interaction between MDM2 and PTEN
A previous study revealed that MDM2 interacts with PTEN (33). In the present study, bioinformatics analysis verified the association between MDM2 and PTEN (Fig. 3A). To further validate this finding, a co-immunoprecipitation assay was performed, which verified the binding capacity between MDM2 and PTEN (Fig. 3B). Subsequently, to determine the expression levels of PTEN in different treatment groups, RT-qPCR was employed. Therefore, the results demonstrated that PTEN was notably upregulated in ox-LDL-treated HUVECs compared with the control group (Fig. 3C). To further investigate the effect of MDM2 knockdown on the mRNA and protein expression levels of PTEN, RT-qPCR and western blot analyses were performed, respectively. The results demonstrated a significant reduction in the mRNA expression levels of PTEN in the si-MDM2 group (Fig. 3D). Consistently, western blot analysis further verified that the protein expression levels of PTEN were markedly decreased following MDM2 silencing (Fig. 3E). In summary, the aforementioned findings provided compelling evidence supporting the interaction between MDM2 and PTEN and highlighted the effect of MDM2 knockdown on the mRNA and protein expression levels of PTEN.
Figure 3.
Interaction between MDM2 and PTEN. (A) The interaction between MDM2 and PTEN analyzed by STRING. (B) The binding of MDM2 and PTEN detected by co-immunoprecipitation. (C) The expression of PTEN in HUVECs treated with 40 µg/ml ox-LDL detected by RT-qPCR. (D) The effect of MDM2 knockdown on the transcription expression of PTEN detected by RT-qPCR. (E) The effect of MDM2 knockdown on the protein expression of PTEN detected by western blotting. **P<0.01, ***P<0.001. MDM2, murine double minute-2; PTEN, phosphatase and tensin homolog; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins; ox-LDL, oxidized low-density lipoproteins; IP, immunoprecipitation; RT-q-PCR, reverse transcription-quantitative PCR; si, short interfering; NC, negative control.
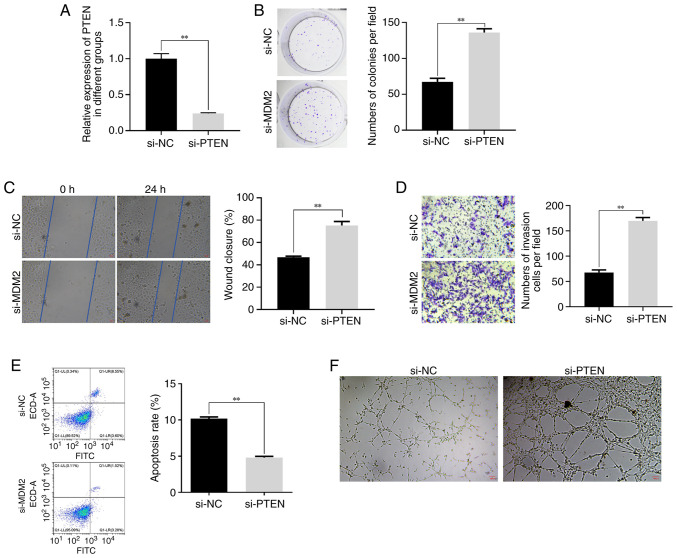
Effect of PTEN silencing on ox-LDL-treated HUVECs
Subsequently, the current study aimed to investigate the role of PTEN in DVT. Therefore, PTEN was knocked down in HUVECs (Fig. 4A). Clone formation assay revealed that PTEN silencing promoted the proliferation of ox-LDL-treated HUVECs (Fig. 4B). Furthermore, the wound healing and Transwell assays further substantiated the promoting effect of PTEN knockdown on HUVECs migration and invasion (Fig. 4C and D). More particularly, the migration and invasion abilities of HUVECs were markedly enhanced in the PTEN silencing group compared with the control group. Additionally, flow cytometry demonstrated that PTEN silencing suppressed the apoptosis of ox-LDL-induced HUVECs (Fig. 4E). Lastly, tube formation assays showed that the angiogenic capability of ox-LDL-treated HUVECs was increased in PTEN-depleted cells (Fig. 4F). Overall, the aforementioned results supported the pleiotropic effects of PTEN silencing on the behavior of HUVECs, including cell proliferation, migration, invasion, apoptosis and angiogenesis. These findings, combined with the effect of MDM2 silencing on PTEN expression, further supported the strong association between PTEN and MDM2.
Figure 4.
Effect of low expression of PTEN on HUVECs treated with ox-LDL. (A) The expression of PTEN in HUVECs knocked down by si-PTEN detected by RT-qPCR. (B) The effect of PTEN knockdown on HUVECs proliferation detected by cloning assay. (C) The effect of PTEN knockdown on HUVECs migration detected by wound healing assay. (D) The effect of PTEN knockdown on HUVECs invasion detected by Transwell assay. (E) The effect of PTEN knockdown on HUVECs apoptosis detected by Flow cytometry. (F) The effect of PTEN knockdown on HUVECs angiogenic ability detected by tube formation assay. Magnification, 200×. **P<0.01. PTEN, phosphatase and tensin homolog; HUVECs, human umbilical vein endothelial cells; ox-LDL, oxidized low-density lipoproteins; si, short interfering; RT-q-PCR, reverse transcription-quantitative PCR; NC, negative control.
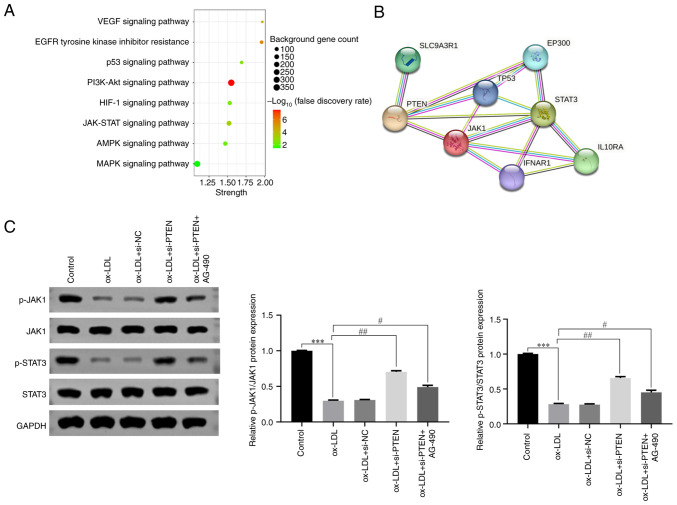
PTEN is involved in the pathogenesis of DVT via inhibiting JAK/STAT signaling
Based on previous studies, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis was performed to identify the signaling pathways associated with PTEN expression (34). The analysis showed that PTEN could regulate several crucial signaling pathways, including the JAK/STAT signaling pathway (Fig. 5A). This finding was further supported by STRING analysis, which further revealed the interactions between PTEN and proteins involved in the JAK/STAT signaling pathway (Fig. 5B). Furthermore, to elucidate the functional involvement of PTEN in the JAK/STAT pathway, western blot analysis was conducted to detect the expression levels of key proteins (p-JAK1, JAK1, p-STAT3, STAT3) in different treatment groups. The results unveiled that HUVECs treatment with ox-LDL significantly attenuated the phosphorylation levels of JAK1 and STAT (Fig. 5C), thus suppressing the activity of JAK/STAT signaling. Intriguingly, the JAK/STAT signaling pathway was activated following PTEN silencing (Fig. 5C). To further validate the aforementioned finding, cells were also treated with AG-490, a specific inhibitor of the JAK/STAT pathway. As expected, HUVECs treatment with AG-490 effectively inhibited the activation of JAK/STAT signaling (Fig. 5C). Collectively, these findings suggested that PTEN could serve a significant role in DVT via modulating the JAK/STAT signaling pathway.
Figure 5.
PTEN participates in DVT by inhibiting JAK/STAT signaling pathway. (A) PTEN gene-related signaling pathways analyzed by KEGG. (B) The interaction between PTEN and JAK/STAT pathways analyzed by STRING. (C) The expression level of JAK/STAT signaling pathway associated proteins in HUVECs treated with ox-LDL (40 µg/ml) detected by western blotting. ***P<0.001, #P<0.05, ##P<0.01. PTEN, phosphatase and tensin homolog; DVT, deep vein thrombosis; JAK, Janus kinase; STAT, signal transducer and activator of transcription; STRING, Search Tool for the Retrieval of Interacting Genes/Proteins; KEGG, Kyoto Encyclopedia of Genes and Genomes; HUVECs, human umbilical vein endothelial cells; ox-LDL, oxidized low-density lipoproteins; p-, phosphorylated; NC, negative control.
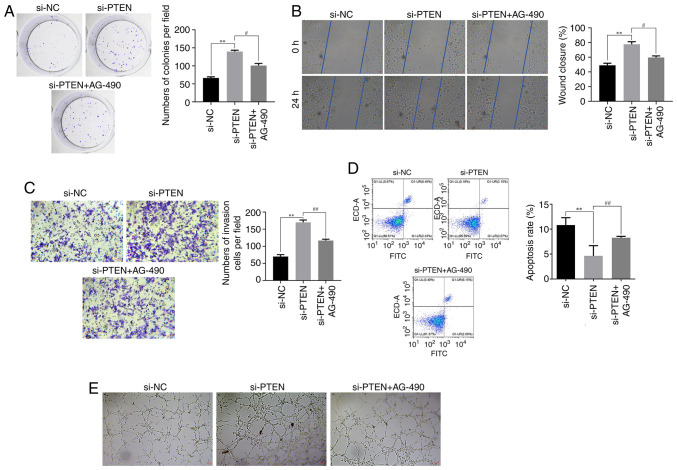
PTEN acts on ox-LDL-treated HUVECs via by inhibiting the JAK/STAT signaling pathway
The aforementioned findings indicated that PTEN could inhibit the JAK/STAT signaling pathway, thus affecting HUVECs proliferation, migration, invasion, apoptosis and angiogenesis. The results obtained from the clone formation assays revealed that PTEN silencing could promote proliferation of ox-LDL-treated HUVECs. However, the stimulatory effect of PTEN silencing was reversed following cell treatment with AG-490 (Fig. 6A). Furthermore, the results demonstrated that PTEN silencing enhanced the migratory and invasive capabilities of HUVECs, as evidenced by the wound healing and Transwell assays, respectively. Conversely, these effects were notably diminished in AG-490-treated HUVECs (Fig. 6B and C). Additionally, flow cytometry analysis further elucidated that PTEN knockdown reduced the apoptosis of ox-LDL-treated HUVECs, which was also reversed by AG-490 (Fig. 6D). In addition, the tube formation assays revealed that PTEN silencing promoted the vasculogenesis of HUVECs, which was counteracted by the presence of AG-490 (Fig. 6E). These findings further supported the role of PTEN in modulating the JAK/STAT signaling pathway and its subsequent effects on the behavior of HUVECs.
Figure 6.
Effect of PTEN on HUVECs treated with ox-LDL mediated by inhibiting JAK/STAT signaling pathway. (A) Effect of AG-490 on HUVEC proliferation detected by cloning assay. (B) Effect of AG-490 on HUVEC migration detected by wound healing assay. Magnification 200×. (C) The effect of AG-490 on HUVECs invasion detected by Transwell assay. Magnification 200×. (D) The effect of AG-490 on HUVECs apoptosis detected by flow cytometry. (E) The effect of AG-490 on HUVECs angiogenic ability detected by tube formation assay. Magnification 200×. **P<0.01, #P<0.05, ##P<0.01. PTEN, phosphatase and tensin homolog; HUVECs, human umbilical vein endothelial cells; ox-LDL, oxidized low-density lipoproteins; JAK, Janus kinase; STAT, signal transducer and activator of transcription; NC, negative control; si, short interfering.
Discussion
DVT is a serious medical condition that occurs when a blood clot is formed in one of the deep veins, typically in those of the leg. If left untreated, DVT can lead to potentially life-threatening complications (4). Markedly, the treatment approach for patients with DVT may vary depending on individual factors, including the extent and location of the clot, the presence of any underlying genetic factors and the overall health of the patient. Therefore, consulting with a healthcare professional for an accurate diagnosis and individualized treatment plan for DVT is of great importance.
Previous studies reveal that the expression levels of MDM2 (16) and PTEN (35) were both increased in patients with DVT. Consistent with this, in the present study, MDM2 and PTEN were also upregulated in intravenous blood samples of patients with DVT. HUVECs are commonly used to study the pathogenesis and treatment of DVT. In the present study, ox-LDL-treated HUVECs were used as an in vitro model of DVT to explore the effects of MDM2 knockdown on the development of DVT. Therefore, following HUVECs treatment with ox-LDL, the expression levels of MDM2 were increased, thus suggesting that the DVT in vitro model was successfully established. Additionally, MDM2 silencing promoted the proliferation, migration, invasion and angiogenic capacity of HUVECs, and reduced apoptosis. These results indicated that MDM2 could display a critical role in the development of DVT via regulating the delicate balance between cell survival and death during DVT progression (16).
The STRING database is widely used to assess protein-protein interactions (PPIs) (36). Therefore, this database is commonly used to explore and analyze PPI networks, identify potential interaction partners for a given protein and gain insights into protein function and cellular processes (37). In the current study, bioinformatics analysis using the STRING database showed that MDM2 could interact with PTEN. Furthermore, the expression levels of PTEN were reduced in MDM2-depleted HUVECs, thus supporting the positive regulatory effect of MDM2 on PTEN expression. A study also demonstrates that PTEN, a dual specificity phosphatase that antagonizes PI3K/AKT signaling, can inhibit the nuclear translocation and destabilize MDM2 (38).
Subsequently, the role of PTEN in DVT was investigated. Suppressing PTEN could enhance HUVECs proliferation, migration, invasion and angiogenic capacity, while inhibiting apoptosis. These findings suggested that PTEN could serve a crucial role in maintaining the intricate equilibrium between cell survival and death during the progression of DVT (39). Furthermore, KEGG pathway analysis revealed that the JAK/STAT signaling pathway was significantly associated with PTEN. Additionally, PPI analysis also verified that both JAK1 and STAT3, which are involved in the JAK/STAT signaling pathway, could interact with PTEN. A previous study also showed that JAK/STAT signaling can play a key role in the development of DVT (40). In the present study, the results demonstrated that JAK1 and STAT3 were downregulated in ox-LDL-treated HUVECs. However, their expression levels were reversed following PTEN silencing. These findings indicated that PTEN could exert a negative regulatory effect on JAK/STAT signaling. However, HUVECs treatment with AG-490, an inhibitor of the JAK/STAT signaling pathway, abrogated the effect of PTEN knockdown on expression levels of JAK1 and STAT3. Consistently, the effects of PTEN silencing on cell proliferation, migration, invasion, angiogenic capacity and apoptosis were also recovered by AG-490. Altogether, these findings indicated that PTEN could play a key role in DVT via modulating the JAK/STAT signaling pathway. However, the aforementioned results are not consistent with those reported in previous studies: A previous review details how non-coding RNAs regulate the plasticity of endothelial cells by modulating the PTEN and Hippo pathways, establishing a new perspective on intercellular communication in physiological and pathological angiogenesis processes (41). Non-coding RNAs play a regulatory role in the process of DVT. A study indicates that miR-513c-5p reduction-induced VEC pyroptosis is involved in the progression of DVT and suggests a potential therapeutic strategy targeting the miR-513c-5p/caspase-1/GSDMD signaling axis for DVT management (42).
In conclusion, the present study demonstrated that both MDM2 and PTEN were upregulated in patients with DVT, thus providing novel insights into their potential collaborative role in the development of DVT. More specifically, the results indicated that MDM2 and PTEN can work together to inhibit endothelial cell growth and promote the occurrence of DVT via suppressing the JAK/STAT signaling pathway. However, the present study also has certain limitations. The present research was limited to in vitro cell levels and did not use animal models. At the same time, experiments on overexpression systems were not performed, that is, the present results indicated that inhibiting the JAK/STAT pathway affected the proliferation of endothelial cells and promoted DVT. However, the present study did not observe the effects of activating the JAK/STAT pathway on the effect of PTEN and MDM2 on DVT. In addition, ox-LDL mainly promotes thrombosis by upregulating genes involved in the coagulation process and enhancing the recruitment and adhesion of white blood cells to endothelial cells (43). However, these mechanisms were not investigated in the present study, which is also one of its limitations. Furthermore, blood contains various types of cells and the present study did not detect the expression levels of MDM2 and PTEN after sorting out endothelial cells, but rather directly measured the expression levels of MDM2 and PTEN in the blood, which has certain limitations. Despite that, the results were sufficient to verify the diagnostic significance of MDM2 and PTEN in the management of DVT. The aforementioned results could provide the theoretical basis for the future development of drugs targeting both PTEN and MDM2 to significantly improve the condition of thrombotic patients in the clinic.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- DVT
deep vein thrombosis
- PTS
post-thrombotic syndrome
- PE
pulmonary embolism
- RT-q-PCR
reverse transcription-quantitative PCR
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
Funding Statement
The present study was supported by the Project of Chuzhou County Science and Technology Bureau (grant no. 2022ZN009).
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
JJ conceived and designed the present study and drafted the manuscript. DZ and JP performed the experiments and data extraction. YL analyzed the data. All authors read and approved the final manuscript. JJ, DZ, JP and YL confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The experimental protocols were approved by the Ethics Committee of Fengyang County People's Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bartholomew JR. Update on the management of venous thromboembolism. Cleve Clin J Med. 2017;84((Suppl 3)):S39–S46. doi: 10.3949/ccjm.84.s3.04. [DOI] [PubMed] [Google Scholar]
- 2.Lutsey PL, Zakai NA. Epidemiology and prevention of venous thromboembolism. Nat Rev Cardiol. 2023;20:248–262. doi: 10.1038/s41569-022-00787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giordano NJ, Jansson PS, Young MN, Hagan KA, Kabrhel C. Epidemiology, pathophysiology, stratification, and natural history of pulmonary embolism. Tech Vasc Interv Radiol. 2017;20:135–140. doi: 10.1053/j.tvir.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Boon G, Van Dam LF, Klok FA, Huisman MV. Management and treatment of deep vein thrombosis in special populations. Exp Rev Hematol. 2018;11:685–695. doi: 10.1080/17474086.2018.1502082. [DOI] [PubMed] [Google Scholar]
- 5.Kim KA, Choi SY, Kim R. Endovascular treatment for lower extremity deep vein thrombosis: An overview. Korean J Radiol. 2021;22:931–943. doi: 10.3348/kjr.2020.0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 7.Ghosh N, Garg I, Srivastava S, Kumar B. Influence of integrins on thrombus formation: A road leading to the unravelling of DVT. Mol Cell Biochem. 2021;476:1489–1504. doi: 10.1007/s11010-020-03961-x. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, Wang C, Ouyang Y, Zhang D. Elevated miR-195-5p expression in deep vein thrombosis and mechanism of action in the regulation of vascular endothelial cell physiology. Exp Ther Med. 2019;18:4617–4624. doi: 10.3892/etm.2019.8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Qin JJ, Rajaei M, Li X, Yu X, Hunt C, Zhang R. Targeting MDM2 for novel molecular therapy: Beyond oncology. Med Res Rev. 2020;40:856–880. doi: 10.1002/med.21637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lam B, Roudier E. Considering the role of murine double minute 2 in the cardiovascular system? Front Cell Dev Biol. 2019;7:320. doi: 10.3389/fcell.2019.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez C, Ramos-Araque ME, Domínguez-Martínez M, Sobrino T, Sánchez-Morán I, Agulla J, Delgado-Esteban M, Gómez-Sánchez JC, Bolaños JP, Castillo J, Almeida A. Single-Nucleotide polymorphism 309T>G in the MDM2 promoter determines functional outcome after stroke. Stroke. 2018;49:2437–2444. doi: 10.1161/STROKEAHA.118.022529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hashimoto T, Ichiki T, Ikeda J, Narabayashi E, Matsuura H, Miyazaki R, Inanaga K, Takeda K, Sunagawa K. Inhibition of MDM2 attenuates neointimal hyperplasia via suppression of vascular proliferation and inflammation. Cardiovasc Res. 2011;91:711–719. doi: 10.1093/cvr/cvr108. [DOI] [PubMed] [Google Scholar]
- 13.Kwon DH, Eom GH, Ko JH, Shin S, Joung H, Choe N, Nam YS, Min HK, Kook T, Yoon S, et al. MDM2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat Commun. 2016;7:10492. doi: 10.1038/ncomms10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Y, Xu J, Hua YQ, Peng Y, Xu XL. MDM2 contributes to oxidized low-density lipoprotein-induced inflammation through modulation of mitochondrial damage in endothelial cells. Atherosclerosis. 2020;305:1–9. doi: 10.1016/j.atherosclerosis.2020.05.020. [DOI] [PubMed] [Google Scholar]
- 15.Zhou S, Gu L, He J, Zhang H, Zhou M. MDM2 regulates vascular endothelial growth factor mRNA stabilization in hypoxia. Mol Cell Biol. 2011;31:4928–4937. doi: 10.1128/MCB.06085-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lou Z, Ma H, Li X, Zhang F, Du K, Wang B. Hsa_circ_0001020 accelerates the lower extremity deep vein thrombosis via sponging miR-29c-3p to promote MDM2 expression. Thromb Res. 2022;211:38–48. doi: 10.1016/j.thromres.2021.12.022. [DOI] [PubMed] [Google Scholar]
- 17.Hu WN, Duan ZY, Wang Q, Zhou DH. The suppression of ox-LDL-induced inflammatory response and apoptosis of HUVECs by lncRNA XIAT knockdown via regulating miR-30c-5p/PTEN axis. Eur Rev Med Pharmacol Sci. 2019;23:7628–7638. doi: 10.26355/eurrev_201909_18886. [DOI] [PubMed] [Google Scholar]
- 18.Zhu J, Liu B, Wang Z, Wang D, Ni H, Zhang L, Wang Y. Exosomes from nicotine-stimulated macrophages accelerate atherosclerosis through miR-21-3p/PTEN-mediated VSMC migration and proliferation. Theranostics. 2019;9:6901–6919. doi: 10.7150/thno.37357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei L, Zhou Q, Tian H, Su Y, Fu GH, Sun T. Integrin b3 promotes cardiomyocyte proliferation and attenuates hypoxia-induced apoptosis via regulating the PTEN/Akt/mTOR and ERK1/2 pathways. Int J Biol Sci. 2020;16:644–654. doi: 10.7150/ijbs.39414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tahtasakal R, Sener EF, Delibasi N, Hamurcu Z, Mehmetbeyoglu E, Bayram KK, Gunes I, Goksuluk D, Emirogullari ON. Overexpression of the PTEN gene in myocardial tissues of coronary bypass surgery patients. Arq Bras Cardiol. 2023;120:e20220169. doi: 10.36660/abc.20220169. (In English, Portuguese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chetram MA, Hinton CV. PTEN regulation of ERK1/2 signaling in cancer. J Recept Signal Transduct Res. 2012;32:190–195. doi: 10.3109/10799893.2012.695798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J, Feng Q, Liang D, Shi J. MiRNA-26a inhibits myocardial infarction-induced apoptosis by targeting PTEN via JAK/STAT pathways. Cells Dev. 2021;165:203661. doi: 10.1016/j.cdev.2021.203661. [DOI] [PubMed] [Google Scholar]
- 23.Roger I, Milara J, Montero P, Cortijo J. The role of JAK/STAT molecular pathway in vascular remodeling associated with pulmonary hypertension. Int J Mol Sci. 2021;22:4980. doi: 10.3390/ijms22094980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu Y, Shen Y, Li J, Liu J, Liu S, Song H. Viral infection related venous thromboembolism: Potential mechanism and therapeutic targets. Ann Palliat Med. 2020;9:1257–1263. doi: 10.21037/apm.2020.04.05. [DOI] [PubMed] [Google Scholar]
- 25.Pang Q, You L, Meng X, Li Y, Deng T, Li D, Zhu B. Regulation of the JAK/STAT signaling pathway: The promising targets for cardiovascular disease. Biochem Pharmacol. 2023;213:115587. doi: 10.1016/j.bcp.2023.115587. [DOI] [PubMed] [Google Scholar]
- 26.Baldini C, Moriconi FR, Galimberti S, Libby P, De Caterina R. The JAK-STAT pathway: An emerging target for cardiovascular disease in rheumatoid arthritis and myeloproliferative neoplasms. Eur Heart J. 2021;42:4389–4400. doi: 10.1093/eurheartj/ehab447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu X, Sun Z, Long Q, Tan W, Ding H, Liu X, Wu L, Wang Y, Zhang W. Glycosides from Buyang Huanwu Decoction inhibit atherosclerotic inflammation via JAK/STAT signaling pathway. Phytomedicine. 2022;105:154385. doi: 10.1016/j.phymed.2022.154385. [DOI] [PubMed] [Google Scholar]
- 28.Xu M, Li X, Song L. Baicalin regulates macrophages polarization and alleviates myocardial ischaemia/reperfusion injury via inhibiting JAK/STAT pathway. Pharm Biol. 2020;58:655–663. doi: 10.1080/13880209.2020.1779318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue W, Deng L. EP300 improves endothelial injury and mitochondrial dysfunction in coronary artery disease by regulating histone acetylation of SOCS1 promoter via inhibiting JAK/STAT pathway. Cytokine. 2024;176:156507. doi: 10.1016/j.cyto.2024.156507. [DOI] [PubMed] [Google Scholar]
- 30.Ma Y, Tan F, Yu S. Diosmin inhibits apoptosis of vascular endothelial cells in rats with traumatic deep vein thrombosis through JAK-STAT signaling pathway. Panminerva Med. 2019;24:10.23736/S0031–0808.19.03718-2. doi: 10.23736/S0031-0808.19.03718-2. [DOI] [PubMed] [Google Scholar]
- 31.Ren G, Yang EJ, Tao S, Mou PK, Pu Y, Chen LJ, Shim JS. MDM2 inhibition is synthetic lethal with PTEN loss in colorectal cancer cells via the p53-dependent mechanism. Int J Biol Sci. 2023;19:3544–3557. doi: 10.7150/ijbs.82566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Szklarczyk D, Gable AL, Nastou KC, Lyon D, Kirsch R, Pyysalo S, Doncheva NT, Legeay M, Fang T, Bork P, et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021;49((D1)):D605–D612. doi: 10.1093/nar/gkaa1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen L, Zhang YH, Wang S, Zhang Y, Huang T, Cai YD. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways. PLoS One. 2017;12:e0184129. doi: 10.1371/journal.pone.0184129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun LL, Xiao L, Du XL, Hong L, Li CL, Jiao J, Li WD, Li XQ. MiR-205 promotes endothelial progenitor cell angiogenesis and deep vein thrombosis recanalization and resolution by targeting PTEN to regulate Akt/autophagy pathway and MMP2 expression. J Cell Mol Med. 2019;23:8493–8504. doi: 10.1111/jcmm.14739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Liu J, Jia W, Tian X, Jiang P, Cheng Z, Li J. AGEs/RAGE blockade downregulates Endothenin-1 (ET-1), mitigating Human Umbilical Vein Endothelial Cells (HUVEC) injury in deep vein thrombosis (DVT) Bioengineered. 2021;12:1360–1368. doi: 10.1080/21655979.2021.1917980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athanasios A, Charalampos V, Vasileios T, Ashraf GM. Protein-Protein interaction (PPI) network: Recent advances in drug discovery. Curr Drug Metab. 2017;18:5–10. doi: 10.2174/138920021801170119204832. [DOI] [PubMed] [Google Scholar]
- 38.Chang CJ, Freeman DJ, Wu H. PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem. 2004;279:29841–29848. doi: 10.1074/jbc.M401488200. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Guan R, Xia F, Du J, Xu L. Curcumin promotes venous thrombi resolve process in a mouse deep venous thrombosis model via regulating miR-499. Microvasc Res. 2021;136:104148. doi: 10.1016/j.mvr.2021.104148. [DOI] [PubMed] [Google Scholar]
- 40.Maeshima T, Aisu S, Ohkura N, Watanabe M, Itagaki F. The association between deep vein thrombosis, pulmonary embolism, and janus kinase inhibitors: Reporting status and signal detection in the Japanese adverse drug event report database. Drugs Real World Outcomes. 2024;11:369–375. doi: 10.1007/s40801-024-00447-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orozco-García E, van Meurs DJ, Calderón JC, Narvaez-Sanchez R, Harmsen MC. Endothelial plasticity across PTEN and Hippo pathways: A complex hormetic rheostat modulated by extracellular vesicles. Transl Oncol. 2023;31:101633. doi: 10.1016/j.tranon.2023.101633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu C, Wang B, Zhang Z, Liu W, Sun S, Liang G, Zhang X, An H, Wei R, Zhu X, et al. miR-513c-5p suppression aggravates pyroptosis of endothelial cell in deep venous thrombosis by promoting caspase-1. Front Cell Dev Biol. 2022;10:838785. doi: 10.3389/fcell.2022.838785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Obermayer G, Afonyushkin T, Binder CJ. Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost. 2018;16:418–428. doi: 10.1111/jth.13925. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.