Abstract
Tissue factor (TF) is the major activator of the coagulation protease cascade and contributes to lethality in sepsis. Despite several studies analyzing TF expression in animal models of endotoxemia, there remains debate about the cell types that are induced to express TF in different tissues. In this study, we performed a detailed analysis of the induction of TF mRNA and protein expression in two rabbit models of endotoxemia to better understand the cell types that may contribute to local fibrin deposition and disseminated intravascular coagulation. Northern blot analysis demonstrated that lipopolysaccharide (LPS) increased TF expression in the brain, lung, and kidney. In situ hybridization showed that TF mRNA expression was increased in cells identified morphologically as epithelial cells in the lung and as astrocytes in the brain. In the kidney, in situ hybridization experiments and immunohistochemical analysis showed that TF mRNA and protein expression was increased in renal glomeruli and induced in tubular epithelium. Dual staining for TF and vWF failed to demonstrate TF expression in endothelial cells in LPS-treated animals. These results demonstrate that TF expression is induced in many different cell types in LPS-treated rabbits, which may contribute to local fibrin deposition and tissue injury during endotoxemia.
Tissue factor (TF) is the major in vivo activator of blood coagulation leading to thrombin generation and fibrin deposition (2, 11). TF is constitutively expressed in a variety of cell types, including astrocytes in the brain, cardiomyocytes in the heart, epidermis of the skin, renal glomeruli, adventitia of blood vessels, some mucosa, organ capsules, and placenta (9, 12, 13). Intravascular cells, such as endothelial cells or monocytes, normally do not express TF but can be induced to express it under certain pathological conditions (6, 15, 19, 24, 34).
TF has been demonstrated to play an important role in the pathophysiology of bacterial lipopolysaccharide (LPS)-induced disseminated intravascular coagulation (DIC) and the fatal septic shock syndrome. Baboons, chimpanzees, rabbits, and mice treated with Escherichia coli or LPS are protected against DIC and death by anti-TF antibodies (7, 16, 28, 32). In these models, TF is induced in circulating monocytes (17, 24, 26, 27). In septic baboons, increased TF antigen is observed in lung epithelial cells and renal glomerular epithelial cells (8). TF antigen was also selectively induced in endothelial cells of the splenic microvasculature in vessels of the marginal zone (8). In LPS-treated rabbits, TF functional activity was increased in both renal glomerular and tubular tissue, although the cell type responsible was not identified (4, 30). Similarly, mice administered a single dose of LPS had increased TF mRNA expression in the lung and kidney, with specific expression in lung epithelial cells and in renal tubular cells (19, 21, 33).
In this study, we tested the hypothesis that administration of LPS to rabbits increases TF expression in a variety of cell types. We performed a detailed analysis of the LPS induction of TF expression in two rabbit endotoxemia models: a single-injection model and a three-injection model, which was designed to mimic the multiple exposures to LPS that occur in septic patients. We have previously used this model to demonstrate glomerular fibrin deposits and tubular necrosis in the kidney (22). Administration of LPS to rabbits resulted in inducible TF expression in astrocytes in the brain, renal glomerular cells and tubular epithelial cells, and epithelial cells in the lung, but not in endothelial cells. These studies demonstrate that the pattern of LPS induction of TF expression varies among different species employed as models of endotoxemia, and they highlight the need to be cautious in extrapolating results from these models to humans.
MATERIALS AND METHODS
Animals.
LPS (Salmonella minnesota Re595) was prepared as previously described (31). To study the effects of LPS on TF expression in vivo, we employed two rabbit endotoxemia models: a three-injection model and a single-injection model. For the three-injection model, outbred New Zealand White rabbits (three animals per group) received three intravenous injections (at −24, −5, and 0 h) of LPS (10 μg/dose) and were sacrificed at 3, 6, 12, and 24 h after the last injection. Microscopic examination of hematoxylin and eosin and periodic acid-Schiff staining of sections was used to assess tissue morphology and fibrin deposition. The results were consistent with our previous studies (22) and showed fibrin deposits in glomerular capillaries and focal tubular necrosis, splenic necrosis and fibrin deposition and accumulation of neutrophils, and pulmonary edema and fibrin deposition. For the single-injection model, rabbits (two animals per group) received 10 μg of LPS intravenously and were sacrificed at 2, 7, and 24 h postinjection. The dose of 10 μg of LPS was chosen as the lowest dose to induce maximal TNF production. Tissues were collected from the rabbits and either placed in OCT medium (Sakura Finetek, Torrance, Calif.) in cryomolds and frozen on dry ice as fresh-frozen samples or placed in phosphate-buffered 4% paraformaldehyde at 4°C overnight and mounted in paraffin. Control rabbits received saline. Male New Zealand White rabbits (1.8 to 2.2 kg) were obtained from Western Oregon Rabbit Company (Philomath, Oreg.). The animals were euthanized by an overdose of sodium pentobarbital by intravenous injection. All studies were approved by The Scripps Research Institute Animal Care and Use Committee and comply with National Institutes of Health guidelines.
Immunohistochemistry.
Four- to six-μm-thick sections of fresh-frozen OCT-embedded tissue were stained with an anti-rabbit TF mouse monoclonal antibody (11F) (20 μg/ml) (29) or a goat anti-rabbit vWF polyclonal antibody (1:2,000 dilution) (kindly provided by J. Ware, The Scripps Research Institute) using a peroxidase-antiperoxidase technique. Endogenous peroxidase activity was inhibited by Peroxo-block (Zymed Laboratories, San Francisco, Calif.). The peroxidase activity was visualized with 3,3′-diaminobenzidine tetrahydrochloride. To assess nonspecific staining, an irrelevant-isotype-matched mouse monoclonal antibody (kindly provided by L. Curtiss, The Scripps Research Institute) was substituted for the anti-TF mouse monoclonal antibody and normal goat serum was substituted for the goat anti-rabbit vWF.
Dual staining of TF and vWF on fresh-frozen sections was performed with the anti-rabbit TF monoclonal antibody 11F and an anti-rabbit vWF goat antibody, with primary antibodies detected with a fluorescein isothiocyanate-labeled donkey anti-mouse antibody or a Texas Red-labeled rabbit anti-goat antibody, respectively. Briefly, sections were fixed in acetone for 3 min at −20°C. The slides were then rinsed twice in phosphate-buffered saline (PBS) followed by incubation with a blocking solution (10% normal horse serum and 1% bovine serum albumin in PBS) for 30 min. The blocking solution was aspirated, and the sections were incubated with 11F (20 μg/ml) or control monoclonal antibody (20 μg/ml). The sections were next incubated with a fluorescein isothiocyanate-labeled donkey anti-mouse antibody (1:150) (Jackson ImmunoResearch, West Grove, Pa.) for 45 min at room temperature (RT) in a darkened chamber. In all subsequent steps, the slides were protected from exposure to light. The slides were washed in PBS as described above, and the tissue sections were then incubated with the anti-rabbit vWF goat antibody (1:1,000) or normal goat serum (1:1,000) for 1 h at RT. The antibody solution was aspirated, and the slides were washed in PBS as described above and incubated with a Texas Red-labeled rabbit anti-goat antibody (1:150) (Vector Laboratories, Burlingame, Calif.) for 45 min at RT. The slides were washed in PBS as described above and mounted with Fluoro Mount (Vector Laboratories). The slides were viewed, and images were captured with a scanning confocal microscope (MR 1000; Bio-Rad, Hercules, Calif.). Alveolar macrophages were detected with a mouse monoclonal antibody, RAM11 (1:25) (Dako Corp., Carpinteria, Calif.), according to the recommended protocol.
In situ hybridization.
Radiolabeled TF riboprobes were made by using BamHI/PvuII (antisense) and EcoRI/SphI (sense) fragments of rabbit TF cDNA (1) as templates for in vitro transcription, employing SP6 polymerase in the presence of 35S-UTP (>1,200 Ci/mmol; Amersham Corp., Arlington Heights, Ill.). The templates were removed by digestion with RQ1 DNase for 15 min at 37°C, and the riboprobes were purified by phenol extraction and ethanol precipitation. In situ hybridization was performed on 5-μm-thick tissue sections fixed in 4% paraformaldehyde by using a 35S-UTP-labeled TF riboprobe as described previously (21). The slides were exposed in the dark at 4°C for 8 weeks. The slides were developed for 2 min in D19 developer (Kodak), fixed, washed in water, and counterstained with hematoxylin and eosin. Photomicrographs were taken by polarized light epiluminesence. To assess the specificity of radiolabeled TF mRNA antisense riboprobe, tissue sections from control or LPS-treated rabbits were hybridized with a 35S-UTP-radiolabeled sense TF riboprobe.
Northern blots.
Total RNA was isolated from frozen tissues by the guanidinium thiocyanate method (5), and its concentration was determined by measurement of absorbance at an optical density of 260 nm. Total RNA (10 μg) was analyzed for TF mRNA by Northern blotting with a 32P-labeled rabbit TF cDNA (an 821-bp SmaI-SacI fragment) as described previously (1). Autoradiography was performed at −80°C with Kodak XAR film with intensifying screens. Loading controls were performed by reprobing membranes with a 32P-labeled (330-bp) glyceraldehyde 3-phosphate dehydrogenase (G3PDH) probe. The level of TF mRNA in the samples was quantitated by densitometric analysis with a Personal Densitometer and ImageQuant software (Molecular Dynamics, Palo Alto, Calif.).
TF activity.
Brain acetone powder was prepared as described previously (3). TF activity was measured by a single-step clotting assay (25).
RESULTS
LPS induces TF expression in the brain.
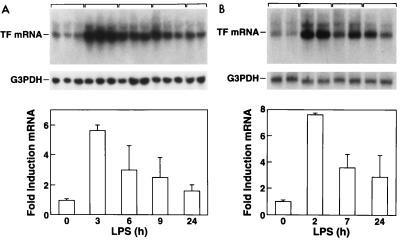
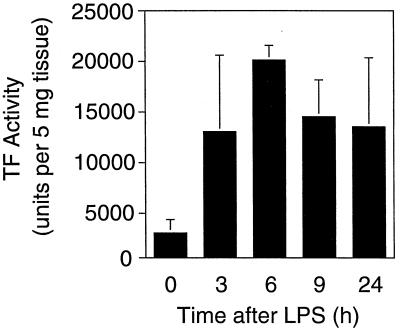
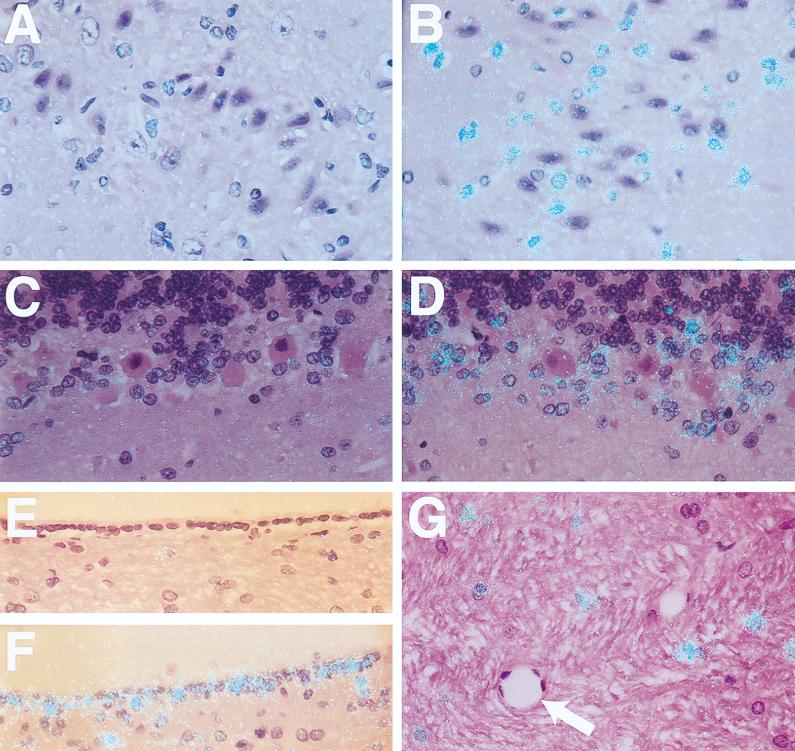
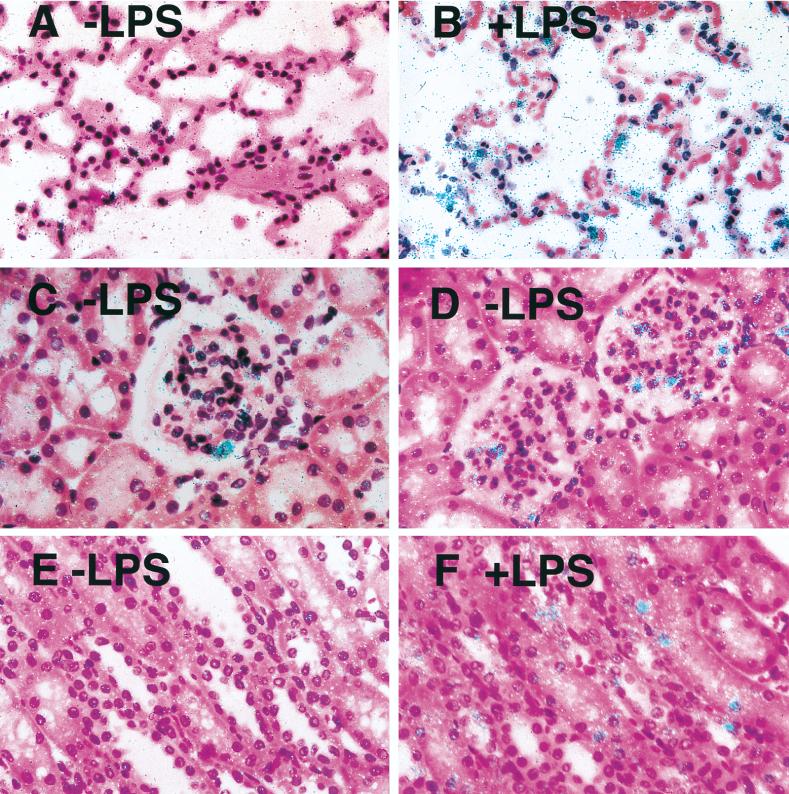
Northern blot analysis demonstrated high levels of TF mRNA in rabbit brains (Fig. 1A). Administration of three injections of LPS (10 μg/dose) over 24 h resulted in transient induction of TF mRNA, with maximal induction of 5.8-fold ± 0.3-fold (mean ± standard deviation [SD]) at 3 h. Subsequently, TF mRNA expression decreased at 6 and 9 h but remained above basal levels at 24 h. Administration of a single dose of 10 μg of LPS resulted in a similar induction of TF mRNA (7.6-fold induction) (Fig. 1B). LPS also increased TF activity in a time-dependent manner, with maximal levels observed at 6 h (Fig. 2). Dissection of the brains demonstrated LPS induction of TF mRNA expression in all regions (basal ganglia, thalamus, cerebellum, cortex, brain stem, and hippocampus) (data not shown), suggesting that the responsive cell type was widely distributed throughout the brain. In situ hybridization studies demonstrated that LPS induced TF mRNA expression in cells of the cerebral cortex and cerebellum (Fig. 3A to D). These cells exhibited large gray nuclei, no distinct nucleoli, and uncondensed chromatin, all morphological features typical of astrocytes (10). Previously, we showed that astrocytes and specialized astrocytes called Bergmann glia in mouse brains expressed TF mRNA (10, 21). The low level of signal observed in astrocytes and Bergmann glia in brains from control rabbits (Fig. 3A and C) does not represent different baseline levels of TF expression in different species but rather the low-level signal observed in this in situ hybridization experiment. We chose to show this experiment to demonstrate the dramatic induction of TF mRNA expression in astrocytes. Independent experiments demonstrated a stronger signal for TF mRNA in astrocytes in the brains of control rabbits (data not shown). In addition, LPS induced TF mRNA expression in ependymal cells lining the ventricles (Fig. 3E and F). TF mRNA expression was not detected in microvascular endothelial cells (Fig. 3G). No signal was observed with a radiolabeled TF sense probe (data not shown). With the single-injection model, the pattern of brain TF mRNA expression was similar to that described above for the three-injection model (data not shown). These results indicated that comparable results were obtained with the two models of LPS-induced sepsis. We chose to focus the majority of our subsequent studies on the simpler one-injection model, although similar results were observed with the three-injection model. Immunohistochemical analysis demonstrated TF protein expression in the brains of control rabbits and increased TF staining in brains from LPS-treated rabbits, consistent with induction of TF expression by astrocytes (data not shown).
FIG. 1.
LPS induction of TF mRNA in the brain. (A) TF mRNA expression in a three-injection model. Tissues were collected 3, 6, 9, and 24 h after the last dose of LPS. Quantitation of brain TF mRNA was performed by densitometric analysis of the Northern blots. TF mRNA levels were normalized by using G3PDH mRNA levels and expressed as fold induction ± SD. (B) TF mRNA expression in a single-injection model.
FIG. 2.
LPS induction of TF activity in the brain. Brain extracts were prepared from control and LPS-treated rabbits (three-injection model), and TF procoagulant activity was measured by a single-stage clotting assay. Data are presented as means ± SD.
FIG. 3.
Localization of LPS-inducible TF mRNA in the brain. In situ hybridization was performed on brain sections from control rabbits (A, C, and E) and from rabbits 3 h after the last LPS injection in a three-injection model (B, D, F, and G). The cerebral cortex (A and B), cerebellum (C and D), ependymal cell lining of the ventricles (E and F), and brain stem (G) are shown (the arrow indicates a blood vessel). All sections were hybridized with a radiolabeled antisense TF probe (magnification, ×368). Exposure time was 8 weeks.
LPS induces TF expression in the lung.
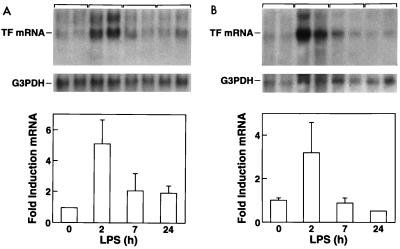
Northern blot analysis demonstrated that LPS increased TF mRNA expression in the lung, with maximal induction at 2 h (3.2-fold induction) (Fig. 4A). Increased TF mRNA expression was also observed by in situ hybridization (Fig. 5A and B). The morphologies and locations of these TF mRNA-positive cells indicated that they were mostly epithelial cells. A few TF mRNA-positive alveolar macrophages were identified by performing in situ hybridization for TF mRNA and by performing immunohistochemistry for a macrophage marker (RAM11) on adjacent sections (data not shown). Immunohistochemical analysis confirmed TF protein expression in epithelial cells (data not shown).
FIG. 4.
LPS induction of TF mRNA expression in the lung (A) and kidney (B). Northern blot analysis of TF mRNA and G3PDH mRNA from rabbits treated for different times with a single 10-μg dose of LPS. TF mRNA levels were normalized with G3PDH and expressed as fold induction ± SD.
FIG. 5.
Localization of LPS-inducible TF mRNA expression in lung and kidney. In situ hybridization for TF mRNA was performed on pulmonary and renal tissue collected from control rabbits (A, C, and E) and rabbits 2 h after treatment with a single dose of LPS (B, D, and F). The lung (A and B), glomeruli (C and D), and renal tubules (E and F) are shown. All sections were hybridized with a radiolabeled TF antisense probe (magnification, ×332). The exposure time was 8 weeks.
LPS induces TF expression in the kidney.
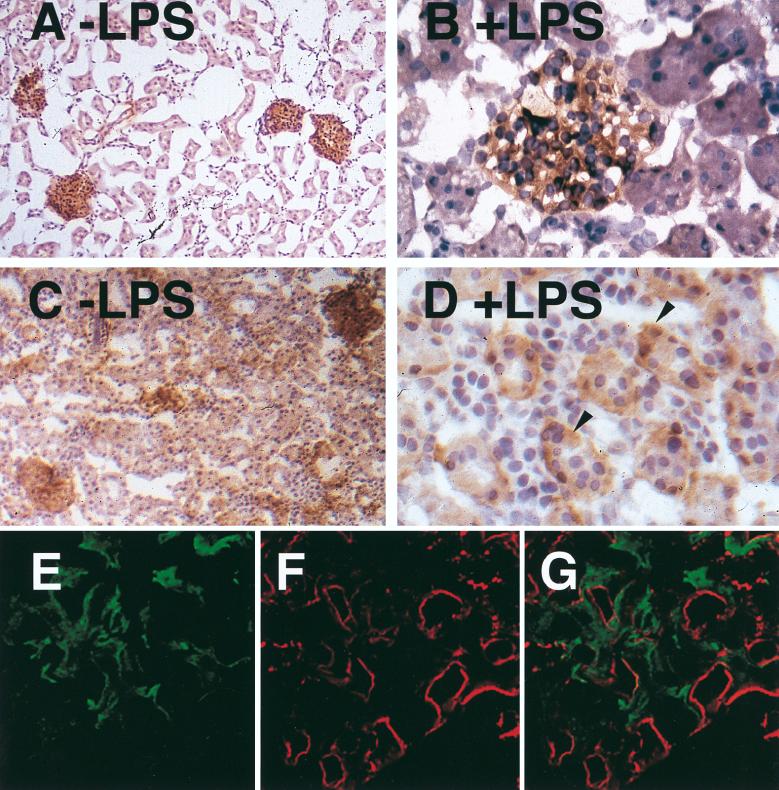
Northern blot analysis demonstrated TF mRNA expression in kidneys from normal rabbits (Fig. 4B). Administration of a single 10-μg dose of LPS resulted in maximal TF mRNA expression at 2 h (5.6-fold induction). In situ hybridization demonstrated TF mRNA in glomeruli but not tubules of kidneys from control rabbits (Fig. 5C and E). LPS increased TF mRNA expression in glomerular cells and induced TF mRNA expression in tubular epithelial cells (Fig. 5D and F). Immunohistochemical analysis demonstrated that LPS increased TF staining in glomerular cells and induced TF staining in the tubular epithelial cells (Fig. 6A to D). To determine if some of the TF-positive cells in the glomeruli were endothelial cells, dual immunofluorescence studies with imaging by confocal microscopy were performed. Dual staining for TF and vWF demonstrated that TF was not expressed by endothelial cells but was expressed by the epithelial and/or mesangial cells of the glomeruli (Fig. 6E to G).
FIG. 6.
Immunolocalization of TF in the kidney after administration of a single dose of LPS. Sections from control rabbits (A and B) and rabbits 7 h after a single dose of LPS (C and D) are shown. The sections were photographed at ×82 (A and C) or ×328 (B and D) magnification. The arrowheads (D) indicate TF-positive tubular cells. No staining was observed with a control antibody (data not shown). (E, F, and G) Immunofluorescence staining detected by confocal microscopy for TF (11F) and vWF (goat anti-rabbit vWF antibody) on histological sections from a rabbit 24 h after a single 10-μg dose of LPS. Green staining (TF) is observed in the mesangium but is not found on the endothelium of the glomerular capillary tuft. Red staining (vWF) marks the endothelium. (E) TF staining alone, (F) vWF staining alone; (G) combined confocal image of TF and vWF staining (magnification, ×328).
LPS does not induce TF expression in endothelial cells in the spleen.
A previous study reported LPS induction of TF protein expression in microvascular endothelial cells of the marginal zones of the spleens of baboons. For comparison, we examined LPS induction of TF expression in the microvasculature of the spleens of endotoxemic rabbits. LPS induced TF mRNA expression in specific cells of the spleen that by morphology appeared to be mononuclear cells (data not shown). To determine if some of the TF-positive cells were endothelial cells, dual immunofluorescence studies were performed. We observed TF staining in the adventitial fibroblasts of vessels from both control (data not shown) and LPS-treated rabbits by confocal microscopy (Fig. 7A). Importantly, TF-positive adventitial fibroblast cells were spatially separated from vWF-positive vascular endothelial cells (Fig. 7A to C).
FIG. 7.
TF expression in the spleen. Seven hours after a single dose of LPS, green-staining TF is observed on adventitial cells of the microvasculature but is not found on endothelial cells. Red staining (vWF) marks the endothelium. (A) TF staining alone; (B) vWF staining alone; (C) combined confocal image of TF and vWF staining (magnification, ×400).
DISCUSSION
LPS induction of TF expression has been studied in baboons, mice, rats, and rabbits. Taken together, these studies suggest that different endotoxemic models exhibit distinct patterns of TF expression (Table 1). For example, in LPS-treated rabbits we observed a dramatic induction of TF expression in the brain, in cells identified morphologically as astrocytes. In contrast to rabbits, no increase in cerebral TF expression was observed in LPS-treated mice (21) or in a lethal baboon model of E. coli sepsis (8). These differences appear to represent species-specific differences in TF expression. At present, the role of increased brain TF in endotoxemic rabbits is unclear and will require further investigation.
TABLE 1.
TF expression in different models of endotoxemiaa
| Model | Expression in:
|
Reference | |||
|---|---|---|---|---|---|
| Kidney | Lung | Spleen | Brain | ||
| Baboon | ↑ Epithelial cells in glomeruli | ↑ Epithelial cells | ↑ Mφ, ↑EC | No change | 8 |
| Mouse | ↑ Tubular cells, glomeruli negative | ↑ Epithelial cells | NA | No change | 21, 33 |
| Rat | NA | ↑ Monocytes, EC negative | NA | NA | 14 |
| Rabbit | ↑ Glomeruli, ↑ tubules | ↑ Macrophages | NA | NA | 4, 30 |
| ↑ Glomeruli, ↑ tubular cells | ↑ Epithelial cells | EC negative | ↑ Astrocytes | Present study | |
Expression assessed by immunohistochemistry (baboon and rat), in situ hybridization (mouse) or functional assay (rabbit). ↑, increased expression; NA, not assessed; EC, endothelial cells; Mφ, macrophages.
Inducible intrapulmonary TF expression, with consequent activation of coagulation and local fibrin deposition, has been suggested as an important mediator of pulmonary injury and loss of lung function in septic shock syndrome. In this study, administration of LPS to rabbits resulted in increased lung TF mRNA expression in epithelial cells and alveolar macrophages (Table 1). A recent study reported that LPS induced TF expression in alveolar macrophages (30). In rats, there is an increase in the number of TF-positive monocytes in the pulmonary microvasculature (14). Administration of E. coli to baboons (8) resulted in increased TF expression in alveolar epithelial cells. Mice administered LPS exhibit inducible TF expression in alveolar epithelial cells (21). Thus, it would appear that alveolar macrophages and alveolar epithelial cells both may contribute to upregulation of pulmonary procoagulant activity and resultant pulmonary dysfunction.
The kidney is one of the organs most sensitive to the pathologic effects of septic shock syndrome. Local fibrin deposition in glomeruli and in the renal interstitium may lead to reduced renal perfusion and loss of renal function. Previously, we have used the three-injection model to demonstrate glomerular fibrin deposits and tubular necrosis in the rabbit kidney (22). Here we show that TF expression is induced in both glomeruli and tubular epithelial cells of LPS-treated rabbits (Table 1). Dual staining for TF and vWF demonstrated that TF was not expressed in glomerular endothelial cells. The pattern of LPS-inducible TF mRNA and protein expression was consistent with previous studies reporting increased procoagulant activity in glomeruli and tubules of endotoxemic rabbits (4, 30). As in rabbits, LPS administration to mice resulted in an upregulation of renal tubular cell TF mRNA (33). However, TF is not expressed in murine glomeruli (18). In the baboon, TF was increased in epithelial cells of the renal glomeruli but not in tubular cells (8). The pattern of TF expression may determine if the glomeruli and tubules are more or less sensitive to fibrin deposition and subsequent renal dysfunction in different species. Further studies are required to determine the TF expression pattern in human patients with sepsis.
LPS induction of TF expression in endothelial cells in vivo is controversial. Drake et al. (8) reported, for septic baboons, TF expression by endothelial cells of the splenic microvasculature in vessels of the marginal zone by using double immunofluorescence staining for TF and vWF. In contrast, we and others have failed to observe TF expression in endothelial cells in LPS-treated mice and rats (14, 21). Dual staining for TF and vWF in spleens of endotoxemic rabbits did not demonstrate TF expression by splenic microvascular endothelial cells (Table 1) (8). The difference between our observations and those for septic baboons may represent the use of different species, differences between the models, or an advance in technology (confocal microscopy) that more accurately delineates the sites of fluorochrome localization. In vitro studies demonstrate LPS induction of TF expression in endothelial cells (20), suggesting the existence of a repression system that functions in vivo to prevent induction of TF in endothelial cells during sepsis. The mechanism of repression is unknown but may involve shear stress, since a recent study indicated that shear stress inhibits cytokine induction of TF in cultured endothelial cells (23).
The identification of a variety of cell types in different organs exhibiting inducible TF expression suggests that these cells may contribute to local fibrin deposition and DIC. Comparison of the results derived from different endotoxemic models indicates species-specific differences in the pattern of TF expression that may determine the distribution of fibrin deposition. The differences suggest that one must be cautious in extrapolating results from animal studies to the pattern of TF expression in humans.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant HL16411 (N.M.). Nigel Mackman is an established investigator of the American Heart Association. Jonathan Erlich is the recipient of a Don and Lorraine Jacquot traveling fellowship from the Royal Australasian College of Physicians.
We thank J. Robertson for assistance with preparing the manuscript. Technical support was provided by Y. Ko, W. Maske, and M. Smith. We thank M. Eddleston for dissection of the rabbit brains, and M. O’Connell and R. Santucci for critical reading of the manuscript.
REFERENCES
- 1.Andrews B S, Rehemtulla A, Fowler B J, Edgington T S, Mackman N. Conservation of tissue factor primary sequence among three mammalian species. Gene. 1991;98:265–269. doi: 10.1016/0378-1119(91)90184-d. [DOI] [PubMed] [Google Scholar]
- 2.Bach R R. Initiation of coagulation by tissue factor. Crit Rev Biochem. 1988;23:339–368. doi: 10.3109/10409238809082548. [DOI] [PubMed] [Google Scholar]
- 3.Broze G J, Leyham J E, Schwartz B D, Miletich J P. Purification of human brain tissue factor. J Biol Chem. 1985;260:10917–10920. [PubMed] [Google Scholar]
- 4.Brukman J, Wiggins R C. Procoagulant activity in kidneys of normal and bacterial lipopolysaccharide-treated rabbits. Kidney Int. 1987;32:31–38. doi: 10.1038/ki.1987.168. [DOI] [PubMed] [Google Scholar]
- 5.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 6.Contrino J, Hair G, Kreutzer D L, Rickles F R. In situ detection of tissue factor in vascular endothelial cells: correlation with the malignant phenotype of human breast disease. Nat Med. 1996;2:209–215. doi: 10.1038/nm0296-209. [DOI] [PubMed] [Google Scholar]
- 7.Dackiw A P B, McGilvray I D, Woodsie M, Nathens A B, Marshall J C, Rotstein O D. Prevention of endotoxin-induced mortality by antitissue factor immunization. Arch Surg. 1996;131:1273–1279. doi: 10.1001/archsurg.1996.01430240027003. [DOI] [PubMed] [Google Scholar]
- 8.Drake T A, Cheng J, Chang A, Taylor F B., Jr Expression of tissue factor, thrombomodulin, and E-selectin in baboons with lethal Escherichia coli sepsis. Am J Pathol. 1993;142:1–13. [PMC free article] [PubMed] [Google Scholar]
- 9.Drake T A, Morrissey J H, Edgington T S. Selective cellular expression of tissue factor in human tissues. Implications for disorders of hemostasis and thrombosis. Am J Pathol. 1989;134:1087–1097. [PMC free article] [PubMed] [Google Scholar]
- 10.Eddleston M, de la Torre J C, Oldstone M B A, Loskutoff D J, Edgington T S, Mackman N. Astrocytes are the primary source of tissue factor in the murine central nervous system—a role for astrocytes in cerebral hemostasis. J Clin Investig. 1993;92:349–358. doi: 10.1172/JCI116573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edgington T S, Mackman N, Brand K, Ruf W. The structural biology of expression and function of tissue factor. Thromb Haemost. 1991;66:67–79. [PubMed] [Google Scholar]
- 12.Fleck R A, Rao L V M, Rapaport S I, Varki N. Localization of human tissue factor antigen by immunostaining with monospecific, polyclonal anti-human tissue factor antibody. Thromb Res. 1990;57:765–781. doi: 10.1016/0049-3848(90)90148-6. [DOI] [PubMed] [Google Scholar]
- 13.Flössel C, Luther T, Müller M, Albrecht S, Kasper M. Immunohistochemical detection of tissue factor (TF) on paraffin sections of routinely fixed human tissue. Histochemistry. 1994;101:449–453. doi: 10.1007/BF00269495. [DOI] [PubMed] [Google Scholar]
- 14.Hara S, Asada Y, Hatakeyama K, Marutsuka K, Sato Y, Kisanuki A, Sumiyoshi A. Expression of tissue factor and tissue factor pathway inhibitor in rat lungs with lipopolysaccharide-induced disseminated intravascular coagulation. Lab Investig. 1997;77:581–589. [PubMed] [Google Scholar]
- 15.Lang I M, Mackman N, Kriett J M, Moser K M, Schleef R R. Prothrombotic activation of pulmonary arterial endothelial cells in a patient with tuberculosis. Hum Pathol. 1996;27:423–427. doi: 10.1016/s0046-8177(96)90118-5. [DOI] [PubMed] [Google Scholar]
- 16.Levi M, ten Cate H, Bauer K A, van der Poll T, Edgington T S, Büller H R, van Deventer S J H, Hack C E, Wouter ten Cate J, Rosenberg R D. Inhibition of endotoxin-induced activation of coagulation and fibrinolysis by pentoxifylline or by a monoclonal anti-tissue factor antibody in chimpanzees. J Clin Investig. 1994;93:114–120. doi: 10.1172/JCI116934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li A G, Chang A C K, Peer G T, Hinshaw L B, Taylor F B. Comparison of the capacity of rhTNF-alpha and escherichia coli to induce procoagulant activity by baboon mononuclear cells in vivo and in vitro. Shock. 1996;5:274–279. doi: 10.1097/00024382-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Luther T, Flössel C, Mackman N, Bierhaus A, Kasper M, Albrecht S, Sage E H, Iruela-Arispe L, Grossmann H, Ströhlein A, Zhang Y, Nawroth P P, Carmeliet P, Loskutoff D J, Müller M. Tissue factor expression during human and mouse development. Am J Pathol. 1996;149:101–113. [PMC free article] [PubMed] [Google Scholar]
- 19.Mackmann N. Regulation of the tissue factor gene. FASEB J. 1995;9:883–889. doi: 10.1096/fasebj.9.10.7615158. [DOI] [PubMed] [Google Scholar]
- 20.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78:747–754. [PubMed] [Google Scholar]
- 21.Mackman N, Sawdey M S, Keeton M R, Loskutoff D J. Murine tissue factor gene expression in vivo: tissue and cell specificity and regulation by lipopolysaccharide. Am J Pathol. 1993;143:76–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Mathison J C, Wolfson E, Ulevitch R J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Investig. 1988;81:1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsumoto Y, Kawai Y, Watanabe K, Sakai K, Murata M, Handa M, Nakamura S, Ikeda Y. Fluid shear stress attenuates tumor necrosis factor-alpha-induced tissue factor expression in cultured human endothelial cells. Blood. 1998;91:4164–4172. [PubMed] [Google Scholar]
- 24.Morrissey J H, Drake T A. Procoagulant response of the endothelium and monocytes. In: Schlag G, Redl H, editors. Pathophysiology of shock, sepsis and organ failure. Berlin, Germany: Springer-Verlag; 1993. pp. 564–574. [Google Scholar]
- 25.Morrissey J H, Fair D S, Edgington T S. Monoclonal antibody analysis of purified and cell-associated tissue factor. Thromb Res. 1988;52:247–261. doi: 10.1016/0049-3848(88)90084-9. [DOI] [PubMed] [Google Scholar]
- 26.Okajima K, Yang W-P, Okabe H, Inoue M, Takatsuki K. Role of leukocytes in the activation of intravascular coagulation in patients with septicemia. Am J Hematol. 1991;36:265–271. doi: 10.1002/ajh.2830360408. [DOI] [PubMed] [Google Scholar]
- 27.Rothberger H, Dove F B, Lee T K, McGee M P, Kardon B. Procoagulant activity of lymphocyte-macrophage populations in rabbits: selective increases in marrow, blood, and spleen cells during Shwartzman reactions. Blood. 1983;61:712–717. [PubMed] [Google Scholar]
- 28.Taylor F B, Jr, Chang A, Ruf W, Morrissey J H, Hinshaw L, Catlett R, Blick K, Edgington T S. Lethal E. coli septic shock is prevented by blocking tissue factor with monoclonal antibody. Circ Shock. 1991;33:127–134. [PubMed] [Google Scholar]
- 29.Tipping P G, Erlich J H, Apostolopoulos J, Mackman N, Loskutoff D J, Holdsworth S R. Glomerular tissue factor expression in crescentic glomerulonephritis. Correlations between antigen, activity, and mRNA. Am J Pathol. 1995;147:1736–1748. [PMC free article] [PubMed] [Google Scholar]
- 30.Ueda Y, Lee K H, Ito S, Saito K, Niesen N, Brentjens J R, Gans R O B. In vivo neutralization of tumor necrosis factor attenuates the generalized Shwartzman reaction in the rabbit. J Endotoxin Res. 1996;3:67–75. [Google Scholar]
- 31.Virca G D, Kim S Y, Glaser K B, Ulevitch R J. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem. 1989;264:21951–21956. [PubMed] [Google Scholar]
- 32.Warr T A, Rao L V M, Rapaport S I. Disseminated intravascular coagulation in rabbits induced by administration of endotoxin or tissue factor: effect of anti-tissue factor antibodies and measurement of plasma extrinsic pathway inhibitor activity. Blood. 1990;75:1481–1489. [PubMed] [Google Scholar]
- 33.Yamamoto K, Loskutoff D J. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Investig. 1996;97:2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Deng Y, Luther T, Müller M, Ziegler R, Waldherr R, Stern D M, Nawroth P P. Tissue factor controls the balance of angiogenic and antiangiogenic properties of tumor cells in mice. J Clin Investig. 1994;94:1320–1327. doi: 10.1172/JCI117451. [DOI] [PMC free article] [PubMed] [Google Scholar]